Abstract
Nejayote is an alkaline wastewater generated during the nixtamalization process. Nejayote contains high-value compounds such as ferulic acid (FA), which is widely employed as a substrate for the biotechnological production of flavors and aromas. In the present study, the isolation, identification, and characterization of a native strain of Bacillus megaterium were performed, and its capacity to produce 4-vinylguaiacol (4VG) from ferulic acid was evaluated by employing growing cell and resting cell systems. Growing cells of native B. megaterium biotransformed 6 mM crude FA in nejayote into 2.1 mM 4VG, reaching a productivity of 0.21 mM h−1 4VG, while nejayote enriched with FA at 10, 15, and 25 mM resulted in the formation of 2.4, 3.8, and 6.2 mM 4VG and productivities of 0.24, 0.38, and 0.51 mM h−1 4VG, respectively. In the resting cell system, from 6 and 25 mM pure FA, 3.5 mM 4VG was produced (0.18 mM h−1 4VG), while at 10 and 15 mM FA, 4.6 and 5.1 mM 4VG (average of 0.24 mM h−1 4VG) were obtained, respectively. The native B. megaterium strain, isolated from nejayote, showed great biotechnological potential to produce 4VG from crude FA contained in this wastewater, in which other Bacillus species, such as B. licheniformis and B. cereus, were unable to grow and biotransform FA into 4VG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ferulic acid (FA) is a hydroxycinnamic acid found as a component of the plant cell wall in free form and as a homodimer or esterified with lignocellulosic polysaccharides [1]. The extraction and purification of FA from natural sources is important due to its numerous applications in the pharmaceutical, cosmetic, and food industries [2]. The use of FA as a substrate for the biotechnological production of flavors and aromas such as vanillin and 4-vinylguaiacol (4VG) is well known [3, 4], and these products can be labeled as “natural flavors” according to USA and EU legislation [5, 6]. 4VG is a highly valued compound with a clove aroma that provides the desirable smoky aroma and flavor to Belgian and German-style wheat beers and soy sauce and contributes to sensory characteristics in food products [7]. The aroma compounds from the styrene family, such as 4VG, are principally synthesized chemically. Currently, consumers demand natural products obtained from plants or by biotechnological processes [4]. Biotechnological production of 4VG comprises the biotransformation of FA, with the Bacillus genus one of the most reported biotransforming agents, including B. coagulans B. cereus, B. licheniformis, B. aryabhattai, and B. methylotrophicus strains [8,9,10,11,12].
The mechanism proposed for the microbial production of 4VG is the side-chain cleavage of FA by nonoxidative decarboxylation by the enzyme FA decarboxylase (EC 4.1.1.102). FA decarboxylation could be part of a detoxification system that maintains metabolism-inhibitory compounds under a threshold concentration [13]. However, it has been reported that concentrations higher than 5 mM FA inhibit the detoxification system and the simultaneous bioconversion of FA to 4VG [14, 15]. This represents a considerable limitation for biotransformation processes that are relevant to the prospection of novel FA-tolerant strains to enhance the productivity of 4VG. An industrial source for recovering FA is nejayote [16], wastewater resulting from the nixtamalization process used in Mexico and other Latin American countries to produce tortillas and other byproducts [17]. In addition to FA, nejayote contains phenolic compounds [18], starch, non-amylaceous polysaccharides, proteins, and sugars [19, 20]. Due to its composition, nejayote has antimicrobial or growth-inhibitory properties [17], and some studies have focused on the isolation of native strains from nejayote for biotechnological applications [21, 22]. However, none of these studies have evaluated the tolerance of the isolated strains to high FA concentrations and their conversion of FA into highly valued compounds, such as 4VG.
In the current study, we report the isolation, identification, and characterization of the native strain Bacillus megaterium LBI001 from nejayote. The tolerance to high concentrations of FA and efficiency of biotransformation of crude and pure FA into 4VG in growing and resting cell systems were assessed.
Materials and Methods
Native Strain Isolation from Alkaline Maize Wastewater (Nejayote)
A native strain was isolated from nejayote provided by a local mill located in Hermosillo, Sonora Mexico. Nejayote pH was adjusted (6.5) with H2SO4 and incubated at 25 °C for 24–48 h. Then, samples were plated on nutrient agar containing 1 g L−1 of FA and incubated at 37 °C for 24 h. Single colonies were plated again on nutrient agar containing 2 and 3 g L−1 of FA, and plates that developed a clove aroma were stored at 4 °C for further analyses.
Morphological and Biochemical Characterization of the New Ferulic-Acid-Biotransforming Native Strain
The cell morphology of bacteria was examined using optical microscopy (Nikon Type 104c). Gram staining and biochemical characterization of the strain were carried out according to the procedure described by Tindall et al. [23]. The hydrolysis of ethyl ferulate activity was performed as described previously [24].
Genotypic Characterization and Sequence Analysis of the New Ferulic-Acid-Biotransforming Native Strain
After growth at 37 °C for 24 h, a single colony was used directly to amplify a 1.4 kb fragment of the 16S rRNA gene using primers 63F and 1387R at 500 nM in 20 µL [25]. Both strands of the PCR product were sequenced (Macrogen USA Corp), and the corresponding consensus sequence was assembled with CLC Main Workbench 5.5 (CLC BIO) and aligned with those available in GenBank using the BLAST algorithm [26]. The sequence was deposited in GenBank.
A phylogenetic tree was constructed using the UPGMA method [27]. The analysis involved 19 nucleotide sequences. The codon positions included were 1st + 2nd + 3rd + Noncoding. All positions containing gaps and missing data were eliminated. There were a total of 488 positions in the final dataset. Reference sequences were retrieved from GenBank under the accession numbers indicated on the trees.
Nejayote Production
2 kg of white maize was cooked at 95 °C for 45 min in 4 L of lime solution (Nixtacal® provided by Calidra). Supernatant (nejayote) was collected and treated according to Asaff and Reyes [18]. The effluent free of suspended solids, containing 6 mM crude FA, was used for biotransformation into 4VG in a growing cell system.
Inoculum Preparation for Biotransformation Assays
A 250-mL Erlenmeyer flask with 100 mL of TSB broth (BD Difco, USA) was inoculated using a single colony from freshly streaked agar plates containing bacterial cultures and incubated at 37 °C and 120 rpm. Samples were taken every 2 h, the microbial culture was stopped at 12 h when the stationary phase reached OD600nm = 2.136, and 4 mg mL−1 of biomass was obtained.
Resting Cell System
A 10 mL aliquot of the inoculum at the stationary phase was centrifuged (15,300×g, 4 °C, 20 min), and the recovered pellets were washed three times with 50 mM phosphate buffer (PB) at pH 7. The washed pellets were resuspended in 10 mL of 50 mM PB with 5, 10, 15, and 25 mM FA (Minkab), containing 4 mg cells mL−1, and then transferred to 20 mL glass bottles, which were incubated at 37 °C and 120 rpm for 12 h. Samples were taken every 2 h and stored at − 10 °C for further analysis.
Growing Cell System
Biotransformation was carried out in 250-mL Erlenmeyer flasks containing 100 mL of nejayote with 6 mM crude FA and enriched nejayote with pure FA to obtain concentrations of 10, 15, and 25 mM. The flasks were inoculated with 5 mL of inoculum of the B. megaterium LBI001 strain (~0.2 mg cells mL−1 solution) and incubated at 37 °C and 120 rpm for 12 h. Samples were taken every 2 h and stored at − 10 °C for further analysis.
For the biotransformation efficiency assays, 125-mL Erlenmeyer flasks containing 30 mL of nejayote were inoculated with 1 mL of inocula of B. megaterium LBI001, B. licheniformis FD36, and B. cereus MS15, cultured for 24 h. The flasks were incubated at 37 °C and 120 rpm for 24 h.
Analytical Techniques
Biomass
The biomass concentration during biotransformation was measured according to AOAC, 986.35 [28]. A Neubauer hemocytometer chamber was used for bacterial cell counting.
Quantitation of FA and 4VG
The biotransformation samples were centrifuged (15,300×g, 4 °C, 15 min). The supernatant was filtered with a 0.22-μm PTFE membrane. FA and 4VG were measured by HPLC Varian 920-LC and PDA detector. A Phenomenex ODS-25 μm 250 × 4.6 μ column at 35 °C was used with a linear gradient elution of acetonitrile:water (60:40) acidified with trifluoroacetic acid (0.01%) at a flow rate of 1 mL min −1 and wavelength 254 and 320 nm.
Biotransformation and Growth Parameters
The estimated biotransformation parameters were the molar yield (Yp/s) of product formation (Eq. 1), the molar substrate consumption (Eq. 2), and the productivity (Eq. 3).
where P and P0 are the final and initial 4VG molar concentrations; S and S0 are the final and initial FA molar concentrations, respectively. The molar productivity was expressed as the maximum molar concentration of 4VG over time (t).
The growth kinetic parameters (Xmax and µmax) were estimated by fitting the experimental data to three-parameter logistic models (Eq. 4).
where X represents the biomass concentration (g L−1), and Xo and Xmax are the initial and maximal biomass concentrations (g L−1), respectively, µ is the specific growth rate (h−1), and t is the biotransformation time.
Statistical Analysis
The biotransformations were performed in duplicate, and kinetic parameters were reported as the mean ± standard deviation and compared with the Tukey–Kramer test at the 95% confidence limit. The analysis was carried out using the statistical package NCSS version 7.0 (Number Cruncher Statistical System for Windows, Utah, USA).
Results
Isolation, Characterization, and Identification of the Native Strain
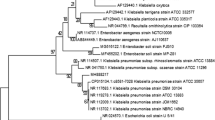
Axenic cultures had the same colonial macroscopic morphology with round to irregular colonies that were white in color and had undulate margins. The phenotypical and biochemical characterization of the isolated native strain is shown in Table 1. The 16S rRNA gene sequence of the strain LBI001 showed 99% similarity to B. megaterium GMB4002-a and B. megaterium GMB40025003 with NCBI Accession Numbers AB738968.1 and AB738973.1, respectively. Sequence data were deposited in GenBank under Accession Number MH729583. Figure 1 shows the phylogenetic tree.
Biotransformation of Crude FA by Three Bacillus Species
The crude FA in nejayote was biotransformed by B. licheniformis FD36, B. cereus MS15, and B. megaterium LBI001 into 4VG, achieving concentrations of 0.006, 0.012, and 2.4 mM, respectively, after 24 h, while the consumption of FA was 10.9, 19.5, and 96%, respectively. Only the culture of the B. megaterium LBI001 strain showed biomass growth (1.9 × 109 cells mL−1) because the B. licheniformis FD36 and B. cereus MS15 cultures maintained the cell number at the inoculum level (1 × 107 cells mL−1).
Biotransformation of FA into 4VG by B. megaterium LBI001 in a Growing Cell System
During the biotransformation of FA, the maximum biomass concentration (Xmax) was achieved at 12 h in all cases (Fig. 2a–d). The estimated values for Xmax were 3.15, 2.51, 1.40, and 1.12 mg mL−1 at 6, 10, 15, and 25 mM FA, respectively, but the last two values were statistically similar (α < 0.05). The values for μmax at 6, 10, 15, and 25 mM FA were 0.51, 0.44, 0.32, and 0.22 h−1, respectively; however, nonstatistically significant differences were found among them (α < 0.05). The correlation coefficients (R2) for the experimental values obtained at 6, 10, 15, and 25 mM FA fitted to the three parameters logistic model were 0.95, 0.97, 0.95, and 0.92, respectively.
During the first 2 h of culture, FA was not consumed, nor was 4VG detected, as this time corresponded to the adaptation phase of B. megaterium in nejayote. Between 2 and 4 h, at 6 and 10 mM FA (Fig. 2a, b, respectively), the FA concentration diminished but without 4VG production. At 15 and 25 mM (Fig. 2c, d), a similar phenomenon was observed between 2 and 6 h, suggesting that at these periods, cells started to internalize FA. During the exponential phase of growth and stationary phase, similar profiles of FA consumption and 4VG production were observed at any initial FA concentration.
The biotransformation parameters estimated at the maximal 4VG concentrations (10 h for 6, 10, and 15 mM FA and 12 h for 25 mM) are shown in Fig. 3a–d. The production of 4VG was 2.1, 2.4, 3.8, and 6.2 mM 4VG at 6, 10, 15, and 25 mM FA, respectively (Fig. 3a), while the percentage of FA consumption was 97, 90, 71, and 32%, respectively, at those concentrations (Fig. 3b). Molar yields of 4VG (Yp/s) were statistically similar at 6, 10, and 15 mM FA (average 33%) but increased up to 76% at 25 mM (Fig. 3c). Finally, at 6 and 10 mM FA, 4VG productivities were statistically similar (average of 0.23 mM h−1), increasing up to 0.38 and 0.51 mM h−1 at 15 and 25 mM FA, respectively (Fig. 3d).
Biotransformation of FA into 4VG by B. megaterium LBI001 in a Resting Cell System
Unlike the growing cell system in which biotransformation did not start immediately, in the resting cell system, biotransformation of FA into 4VG at any FA concentration started immediately, followed by an exponential decay of FA and formation of 4VG until 12 h, stabilizing at 16 to 20 h, as depicted in Fig. 4a–d.
The biotransformation parameters estimated at the maximal 4VG concentrations (20 h) are shown in Fig. 5a–d. At 6 and 25 mM FA (Fig. 5a), 4VG production was statistically similar (average of 3.5 mM), while at 10 and 15 mM FA, higher concentrations (4.6 and 5.1 mM 4VG, respectively) were achieved, although those values were statistically similar.
The FA consumption (%) at 6, 10, 15, and 25 mM FA (Fig. 5b) was 88, 64, 25, and 24%, respectively. The highest yield of 4VG (Yp/s) (Fig. 5c) was obtained at 6 mM FA with a value of 82%, while at 10 and 15 mM FA, the yields were lower (80%), although statistically similar. The lowest yield (51%) was obtained at 25 mM FA.
Regarding productivity (Fig. 5d), the highest values were obtained at 10 and 15 mM FA (average of 0.24 mM h−1). At 6 and 25 mM FA, the productivities were lower (average of 0.18 mM h−1).
Discussion
Isolation, Characterization, and Identification of the Native Strain
The phenotypical and biochemical characteristics of the LBI001 strain isolated from nejayote correspond to the Bacillus genus [29]. Phylogenetic analysis of the 16S rRNA gene sequence of the strain LBI001 (1196 bp) indicated that its closest relatives were Bacillus megaterium GMB4002-a (99% similarity), Bacillus megaterium GMB5003 (99% similarity), and Bacillus aryabhattai B8W22 (99%). However, the degree of similarity between the amino acid sequence of LBI001 (data not shown) indicated that its closest relative was Bacillus megaterium GMB4002-a (100% similarity). UPGMA phylogenetic tree (Fig. 1) and the maximum-parsimony tree (not shown) revealed that the strain LBI001 clustered most closely with these strains.
Biotransformation of Crude FA by Three Bacillus Species
Although nejayote is reported as a suitable substrate for some bacterial growth [21, 30], its high phenolic content [17, 31] usually inhibits the growth of other bacteria. Indeed, the ability of the strain LBI001 to grown on nejayote and transform crude FA into 4VG suggests an adaptive tolerance to FA and other phenolics because other Bacillus species, such as B. licheniformis and B. cereus, were unable to grow despite both species being reported as effective agents for biotransforming FA from other sources into 4VG. [10, 22].
The sequence analysis of ferulic acid decarboxylases of B. pumilus (AC050701.1), B. subtilis (ARW33292.1), B. licheniformis (AVI46886.1), and B. amyloliquefaciens (ARW40653.1) from NCBI and alignment with Clustal W 2.1 showed that the sequences are highly conserved with approximately 83% similarity or higher. Despite the homology between them, some variations at the level of the catalytic site could cause sufficient changes to modify the efficiency of FA conversion among species [32]. Matte et al. [33] reported the crystal structure of phenolic decarboxylase from Bacillus pumilus UI 670, which showed high overall similarity to the enzyme from L. plantarum and B. subtilis. A conformational change in the β1–β2 loop between the B. pumilus and L. plantarum structures was observed; this region influences the shape and size of the cavity that defines the active site. On the other hand, the broad range of specificity shown by phenolic acid decarboxylases could be explained by the presence of certain amino acid sequence motifs that are absent in enzymes showing more restricted substrate specificity [34]. The highest amino acid sequence variability among the bacterial phenolic acid decarboxylases was found in a region of 18 amino acids adjacent to the C-terminal region, proposed to be involved in enzyme–substrate specificity [34]. Since nejayote contains several phenolic acids, the ability of the strain LBI001 to grow on nejayote and transform crude FA suggests that several phenolic acid decarboxylases could be involved in its detoxification system.
Biotransformation of FA into 4VG by B. megaterium LBI001 in a Growing Cell System
The Xmax at 15 and 25 mM FA was lower than the Xmax at 6 and 10 mM FA (Fig. 2). This finding suggests that there is an inhibitory effect of FA and/or 4VG on bacterial growth. Lee et al. [35] reported that the FA decarboxylase of B. pumilus was inhibited at concentrations higher than 3 mM 4VG. Additionally, during the production of 4-vinylphenol by E. coli expressing a decarboxylase from L. plantarum, inhibition was observed at concentrations higher than 4 mM [36]. As depicted in Fig. 3a, at 15 and 25 mM FA, 3.8 and 6.2 mM 4VG, respectively, were obtained; this concentration is higher than those of the inhibitory limits. On the other hand, the μmax of B. megaterium at any initial FA concentration was statistically similar, showing that FA had no inhibitory effect on growth until 4VG accumulation occurred. The μmax of B. megaterium grown on nejayote (0.22–051 h−1) was higher than the μmax of B. subtilis grown on minimal medium with several carbon sources (0.05–0.20 h−1) [37], revealing that nejayote is an appropriate culture medium for the B. megaterium LBI001 strain.
The molar yields (YP/S) and productivities (P) of 4VG at 6 and 10 mM FA (Fig. 3c, d) suggest that FA was consumed as a carbon source by the strain LBI001. This phenomenon was also reported in Amycolatopsis sp. ATCC 39116, which can use vanillin, the product of the FA biotransformation process, as a carbon source for cell growth [3]. In the case of 4VG, the mechanism proposed for its incorporation into metabolism involves the excision of the aromatic ring and its incorporation into the β-ketoadipate pathway for the formation of aliphatic molecules [38], maintaining inhibitory compounds under a minimal concentration [39]. The improvement of 4VG productivities at 15 and 25 mM FA suggests that the LBI001 strain acts as a whole-cell biocatalyst, avoiding the use of FA and 4VG as carbon sources. Similar behavior was reported by Laforgue and Lonvaud-Funel [40] in the yeast B. bruxellensis, which retains decarboxylase activity during wine fermentation. However, more studies are necessary to thoroughly understand the changes in the metabolism of B. megaterium cultivated at high concentrations of FA. Additionally, the bioconversion parameters (YP/S and P) obtained at the highest FA concentrations (0.38 and 0.51 mM h−1 4VG at 15 and 25 mM FA, respectively) show that the strain LBI001 converts FA into 4VG as the main biotransformation product (Supplementary Figure), as one of the most efficient members of the Bacillus genus for biotransforming FA into 4VG. For instance, a productivity of 0.03 mM h−1 4VG from 5 mM pure FA, with vanillin as a byproduct, was reported employing B. aryabhattai [39]. The strain B. methylotrophicus showed a 0.09 mM h−1 concentration of 4VG from 2.5 mM FA, and trace amounts of vanillin, vanillic acid, and protocatechuic acid were also produced. Mishra et al. [10] reported a 0.044 mM h−1 4VG productivity from 2.5 mM FA employing B. cereus SAS-3006. Low 4VG productivities are mostly associated with multiple FA conversion pathways and the degradation of 4VG into other phenolic compounds, such as vanillin, vanillic acid, and protocatechuic acid [41]. Thus, the regulation and expression of the microbial enzymes involved in FA degradation can modulate the production of several metabolites and reduce the efficiency of 4VG production. However, the absence of the formation of byproducts may not be sufficient since even when 4VG is obtained as the only product, very low productivities were obtained, possibly due to the low efficiency of ferulate decarboxylase. For instance, Adamu et al. [14] obtained a productivity of 0.001 mM h−1 4VG from FA employing Lactobacillus farciminis, which was 510 times lower than the highest productivity reported in the current study.
Although our results demonstrate that the strain LBI001 converts FA into 4VG at elevated FA concentrations (15 and 25 mM FA) acting as a whole-cell biocatalyst, it is evident that the system must be optimized to avoid the inhibition phenomenon by the product (4VG) since the amount of residual FA was very high (e.g., 69% at 25 mM FA).
Biotransformation of FA into 4VG in a Resting Cell System
Unlike the growing cell system in which 4VG production is growth-associated (Fig. 2), in the resting cell system, the lag phase was not observed (Fig. 4). In this case, bioconversion started very quickly once FA was internalized in the cells present at a high concentration (4 mg mL−1). Similar results were obtained by Sun et al. [42] with immobilized cells of B. licheniformis.
In the resting cell system, at 6 and 25 mM FA, the amounts of 4VG produced were statistically similar (3.5 mM), increasing slightly up to 4.85 mM 4VG at 10 and 15 mM FA, whereas in the growing cell system, 4VG increased from 2.1 to 6.2 mM as the concentration of FA increased. The YP/S of the resting cell system at concentrations of 6, 10, and 15 mM FA remained above 83% (Fig. 5), whereas in growing cell system at the same concentrations, the YP/S values were below 36% (Fig. 2), while at 25 mM FA, the YP/S increased up to 75% (Fig. 3d). However, at the highest FA concentration in the resting cell system, the YP/S decreased (51%) (Fig. 5c). As shown in Fig. 4d, the maximum concentration of 4VG was reached at 8 h of culture and remained almost constant, while FA decreased linearly. This finding could be related to the formation of other molecules from FA and 4VG that were not excreted and were employed to generate maintenance energy. It is possible that a dynamic equilibrium between the formation and the degradation speeds of 4VG was achieved. Chai et al. [43] reported the capacity of the strain Cupriavidus sp. B-8 to biotransform FA; however, the produced 4VG was rapidly metabolized into vanillic and protocatechuic acids.
Finally, productivity (P) in the resting cell system was between 0.20 and 0.25 mM h−1 4VG, whereas in growing cell system, the productivity was between 0.38 and 0.51 h−1 mM 4VG, more than twice that observed in the resting cell system. This suggests that a nutrient-rich environment such as nejayote allows the generation of enough maintenance energy to support some metabolic pathways properly [39], particularly those related to the biotransformation of FA into 4VG, making it more efficient. Additionally, it has been reported that phenolic decarboxylases are inducible enzymes [44, 45]. Nonetheless, the ferulic acid decarboxylase of B. megaterium LBI001 showed constitutive expression of the enzyme, although it was five times lower than that under induction conditions (data not shown), which would explain the lower productivity of 4VG in the resting cells compared to growing cells.
In conclusion, the current study shows that the native B. megaterium LBI001 strain, isolated from the biological niche of the wastewater “nejayote,” possesses great biotechnological potential to produce 4VG, a highly valued product in the aroma and flavor industries. The FA and other phenolics contained in nejayote suggest that the native strain developed an adaptive mechanism to support high concentrations of phenolics and an efficient detoxification system that drives 4VG formation from crude FA and could reduce production costs by avoiding expenses related to the purification of raw materials.
References
Tilay A, Bule M, Kishenkumar J, Annapure U (2008) Preparation of ferulic acid from agricultural wastes: its improved extraction and purification. J Agr Food Chem 56:7644–7648
Kumar N, Pruthi V (2014) Potential applications of ferulic acid from natural sources. Biotechnol Rep 4:86–93
Ma X, Daugulis AJ (2014) Transformation of ferulic acid to vanillin using a fed-batch solid–liquid two-phase partitioning bioreactor. Biotechnol Prog 30:207–214
Mishra S, Sachan A, Vidyarthi AS, Sachan SG (2014) Microbial production of 4-vinylguaiacol from ferulic acid by Bacillus cereus SAS-3006. Biocatal Biotransform 32:259–266
Council Directive 88/388/EEC (1988) On the approximation of the laws of the Member States relating to flavourings for use in foodstuffs and to source materials for their production. Off J Eur Union L 184:61
US Code of Federal Regulations (1985) Food and Drug Administration Washington. 21, 101.22a.3
Langos D, Granvogl M, Schieberle P (2013) Characterization of the key aroma compounds in two Bavarian wheat beers by means of the sensomics approach. J Agric Food Chem 61:11303–11311
Hu H, Li L, Ding S (2015) An organic solvent-tolerant phenolic acid decarboxylase from Bacillus licheniformis for the efficient bioconversion of hydroxycinnamic acids to vinylphenol derivatives. Appl Microbiol Biotechnol 99:5071–5081
Karmakar B, Vohra RM, Nandawar H, Sharma P, Gupta KG, Sobti RC (2000) Rapid degradation of ferulic acid via 4-vinylguaiacol and vanillin by a newly isolated strain of Bacillus coagulans. J Biotechnol 80:195–202
Mishra S, Sachan A, Vidyarthi AS, Sachan SG (2014) Transformation of ferulic acid to 4-vinylguaiacol as a major metabolite: a microbial approach. Rev Environ Sci Biotechnol 13:377–385
Paz A, Carballo J, Pérez MJ, Domínguez JM (2016) Bacillus aryabhattai BA03: a novel approach to the production of natural value-added compounds. World J Microbiol Biotechnol 32:159
Zhang Y, Wang XJ, Chen SY, Guo LY, Song ML, Feng H, Bai JG (2015) Bacillus methylotrophicus isolated from the cucumber rhizosphere degrades ferulic acid in soil and affects antioxidant and rhizosphere enzyme activities. Plant Soil 392:309–321
Baqueiro-Peña I, Rodríguez-Serrano G, González-Zamora E, Augur C, Loera O, Saucedo-Castañeda G (2010) Biotransformation of ferulic acid to 4-vinylguaiacol by a wild and a diploid strain of Aspergillus niger. Bioresour Technol 101:4721–4724
Adamu HA, Iqbal S, Chan KW, Ismail M (2012) Biotransformation of ferulic acid to 4-vinylguaiacol by Lactobacillus farciminis. Afr J Biotechnol 11:1177–1184
Liu J, Guo T, Yang T, Xu J, Tang C, Liu D, Ying H (2017) Transcriptome analysis of Clostridium beijerinckii adaptation mechanisms in response to ferulic acid. Int J Biochem Cell Biol 86:14–21
Asaff T, Reyes V (2015) Method and system for the integral treatment of wastewater from the maize industry. US Patent 3,681,38A1, 2015
Gutiérrez-Uribe JA, Rojas-García C, García-Lara S, Serna-Saldivar SO (2010) Phytochemical analysis of wastewater (nejayote) obtained after lime-cooking of different types of maize kernels processed into masa for tortillas. J Cereal Sci 52:410–416
Asaff T, Macias-Ochoa R, De la Torre M (2004) Method for recovery ferulic acid. US Patent WO 2004/110975A1
Ayala-Soto FE, Serna-Saldívar SO, García-Lara S, Pérez-Carrillo E (2014) Hydroxycinnamic acids, sugar composition and antioxidant capacity of arabinoxylans extracted from different maize fiber sources. Food Hydrocoll 35:471–475
Rojas-García C, García-Lara S, Serna-Saldivar SO, Gutiérrez-Uribe JA (2012) Chemopreventive effects of free and bound phenolics associated to steep waters (nejayote) obtained after nixtamalization of different maize types. Plant Foods Hum Nutr 67:94–99
Sánchez-González M, Blanco-Gámez A, Escalante A, Valladares AG, Olvera C, Parra R (2011) Isolation and characterization of new facultative alkaliphilic Bacillus flexus strains from maize processing waste water (nejayote). Lett Appl Microbiol 52:413–419
Sun LH, Lv SW, Yu F, Li SN, He LY (2018) Biosynthesis of 4-vinylguaiacol from crude ferulic acid by Bacillus licheniformis DLF-17056. J Biotechnol 281:144–149
Tindall BJ, Sikorski J, Smibert RA, Krieg NR (2007) Phenotypic characterization and the principles of comparative systematics. In: Gerhardt P (ed) Methods for general and molecular microbiology. American Society of Microbiology, Washington, pp 330–393
Bacame-Valenzuela FJ, Rodríguez-González JA, Mateos-Díaz JM, Kirchmayr M, Valenzuela-Soto E, Reyes-Vidal Y, Esqueda M, Asaff-Torres A (2015) Screening of sonoran desert fungal strains for feruloyl esterase activity. J Pure Appl Microbiol 9:131–138
Marchesi JR, Sato T, Weightman AJ, Martin TA, Fry JC, Hiom SJ, Wade WG (1998) Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol 64:795–799
Altschul SF, MaddenTL Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Kumar S, Dudley J, Nei M, Tamura K (2008) MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform 9:299–306
AOAC (2000) Official methods of analysis 986.35, 16th edn. Association of Official Analytical Chemists, Maryland
Logan NA, Vos PD (2015) Bacillus. In: Whitman W (ed) Bergey’s manual of systematics of archaea and bacteria. Wiley, Hoboken, pp 1–163
Ramírez-Romero G, Reyes-Velázquez M, Cruz-Guerrero A (2013) Study of nejayote as culture medium for probiotics and production of bacteriocins. Rev Mex Ing Quim 12:463–471
Niño-Medina G, Carvajal-Millan E, Lizardi J, Rascón-Chu A, Márquez- Escalante J, Gardea A (2009) Maize processing wastewater arabinoxylans: gelling capability and crosslinking content. Food Chem 115:1286–1290
Landete JM, Rodríguez H, Curiel JA, de las Rivas B, Mancheño JM, Muñoz R (2010) Gene cloning, expression and characterization of phenolic acid decarboxylase from Lactobacillus brevis RM84. J Ind Microbiol Biotechnol 37:617–624
Matte A, Grosse S, Bergeron A, Abokitse K, Lau P (2010) Structural analysis of Bacillus pumilus phenolic acid decarboxylase, a lipocalin-fold enzyme. Acta Cryst 66:1407–1414
Barthelmebs L, Divis C, Cavin JF (2001) Expression in Escherichia coli of native and chimeric phenolic acid decarboxylases with modified enzymatic activities and method for screening recombinant E. coli strains expressing these enzymes. Appl Environ Microbiol 67:1063–1069
Lee IY, Volm TG, Rosazza JP (1998) Decarboxylation of ferulic acid to 4-vinylguaiacol by Bacillus pumilus in aqueous-organic solvent two-phase systems. Enzym Microb Technol 23:261–266
Salgado JM, Rodríguez-Solana R, Curiel JA, de las Rivas B, Munoz R, Domínguez JM (2014) Bioproduction of 4-vinylphenol from corn cob alkaline hydrolysate in two-phase extractive fermentation using free or immobilized recombinant E. coli expressing pad gene. Enzyme Microb Technol 58:22–28
Mageshwaran V, Inmann F, Holmes LD (2014) Growth kinetics of Bacillus subtilis in lignocellulosic carbon sources. Int J Microbiol Res 6:570–574
Fuchs G, Boll M, Heider J (2011) Microbial degradation of aromatic compounds—from one strategy to four. Nat Rev Microbiol 9:803–816
Oliver JD (2005) The viable but non culturable state in bacteria. J Microbiol 43:93–100
Laforgue R, Lonvaud-Funel A (2012) Hydroxycinnamic acid decarboxylase activity of Brettanomyces bruxellensis involved in volatile phenol production: relationship with cell viability. Food Microbiol 32:230–234
Mabinya LV, Mafunga T, Brand JM (2010) Bioconversion of ferulic acid and 4-vinylguaiacol by a white-rot fungus isolated from decaying wood. Afr J Biotechnol 9:1955–1958
Salmerón-Alcocer A, Rodríguez-Mendoza N, Pineda-Santiago V, Cristiani-Urbina E, Juárez-Ramírez C, Ruiz-Ordaz N, Galíndez-Mayer J (2003) Aerobic treatment of maize-processing wastewater (nejayote) in a single-stream multi-stage bioreactor. J Environ Eng Sci 2:401–406
Chai L, Zhang H, Yang W, Zhu Y, Yang Z, Zheng Y, Chen Y (2013) Biodegradation of ferulic acid by newly isolated strain of Cupriavidus sp. B-8. J Cent South Univ 20:1964–1970
Cavin JF, Dartois V, Diviès C (1998) Gene cloning, transcriptional analysis, purification, and characterization of phenolic acid decarboxylase from Bacillus subtilis. Appl Environ Microbiol 64:1466–1471
Tran NP, Gury J, Dartois V, Nguyen TK, Seraut H, Barthelmebs L, Cavin JF (2008) Phenolic acid-mediated regulation of the padC gene, encoding the phenolic acid decarboxylase of Bacillus subtilis. J Bacteriol 190:3213–3224
Acknowledgements
This work is financially supported by the Mexican Council of Science and Technology (CONACyT), Grant No. 2017-01-6267. V. Contreras-Jácquez would like to thank CONACYT for the scholarship awarded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baqueiro-Peña, I., Contreras-Jácquez, V., Kirchmayr, M.R. et al. Isolation and Characterization of a New Ferulic-Acid-Biotransforming Bacillus megaterium from Maize Alkaline Wastewater (Nejayote). Curr Microbiol 76, 1215–1224 (2019). https://doi.org/10.1007/s00284-019-01726-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-019-01726-4