Abstract
Wolbachia is gram negative obligate endosymbiont known for reproductive manipulation in the host. It is important to study the presence of natural Wolbachia in mosquitoes which can later help in understanding the effect of transfected strain on indigenous strain. With this view, the present study is undertaken to focus on the prevalence, diversity, infection frequencies, phylogeny and density of indigenous Wolbachia strains in wild mosquito species of Odisha. Our study confirms Wolbachia presence in Ae. albopictus, Cx. quinquefasciatus, Cx. vishnui, Cx. gelidus, Ar. subalbatus, Mn. uniformis, and Mn. indiana. Wolbachia in the above mosquitoes were separated into two supergroups (A and B). Ae. albopictus, the major vector of dengue and chikungungunya had both super-infection and mono-infection. The ovaries of Ae. albopictus were highest in density of Wolbachia as compared to midguts or salivary glands. wAlBA and wAlbB density were variable in mosquitoes of F1 generation for both the sex and at different age. We also found that Wolbachia super-infection in females tends to increase whereas wAlbA density reduced completely as compared to wAlbB in males when they grew old. Giemsa stained squashed ovaries revealed pink pleomorphic Wolbachia cells with different shapes and forms. This study is unique in its kind covering the major aspects of the endosymbiont Wolbachia and focusing on its potential as a biocontrol agent in arboviral outbreaks. Knowledge on potential of the indigenous strain and interactions between Wolbachia and viruses can be utilized further to reduce the global burden of vector borne diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mosquitoes are vectors gaining medical importance throughout the globe due to transmission of numerous diseases like malaria, filaria, dengue, chikungunya, Japanese encephalitis, etc. A brief update on their population structure and distribution prototype is required to evaluate their roles in pathogen transmission and development of their control strategies. Vector borne diseases are expanding its horizon to newer geographical regions due to progressive urbanization, globalization and climatic changes. Vector control remains the only choice to reduce the disease burden. Emergence of insecticide resistance and efforts in developing effective vaccines has urged scientists to develop alternate tools to combat vector borne diseases [20, 23]. In this scenario, sterile male technique, Wolbachia-infected replacement, [17, 22] and radiation are needed to be implemented to combat the dengue and other vector populations in endemic areas [13, 51].
Wolbachia, a gram negative, obligate, endosymbiont resides mainly in reproductive tissues of insects. They are known for both maternal and horizontal transmission [32, 34, 45]. They are known to infect 40–75% of insect species [55]. To facilitate their spread in the host providing them fitness benefit they cause reproductive manipulations including feminization, parthenogenisis, male killing and cytoplasmic induction (CI) [35, 46]. The main theme behind the use of Wolbachia as a biological control is its ubiquity in insect vectors, widespread distribution, reproductive parasitism, antiviral protection which has been explored by researchers in the world, and thus successful outcomes have also been demonstrated in field trials [12, 18, 24, 30]. 16Sr RNA sequences revealed seven (A–H) supergroups with Wolbachia in arthropods belonging to A and B supergroups [25, 46]. Multiple or single strain of Wolbachia may infect single or multiple hosts which may provide a complement or interference for transfected strains [27, 41].
It is highly crucial to identify natural occurrence of Wolbachia in mosquitoes for purposes of medical importance. For this, a critical assessment on the incidence of Wolbachia in natural populations of mosquitoes is essential that may aid in a thorough understanding on the effect of transfection of new Wolbachia strains to mosquitoes already inhabited by indigenous Wolbachia strain within.
There have been a few studies in India till date to detect the presence of Wolbachia in wild populations of mosquitoes [29] and Drosophila [28]. India is a hub for various vector borne diseases claiming health and lives in lacs. The present study will focus on the prevalence, diversity, and infection frequencies of indigenous Wolbachia strains in wild mosquito species collected from different locations of an Indian state, Odisha through the surface protein gene, wsp, a quick evolving gene. An investigation on phylogeny of wsp sequences for Wolbachia strains similarities and variations was also done with an attempt to discuss the existing subgroups. The density distribution study in organs and sex was done with the aim to further analyze the potential of indigenous Wolbachia in viral inhibition. This study will provide a base for controlling arboviral diseases in many parts of Odisha. We used standard and quantitative PCR based techniques along with staining protocol to detect Wolbachia differential distribution in somatic tissues and germlines.
Materials and Methods
Selection of Study Area
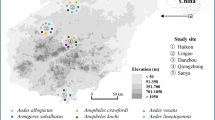
The districts of Odisha with repeated outbreaks of vector borne diseases were selected for the study. Samples were collected from four distinct physiographical regions; coastal plains (Jagatsinghpur and Kendrapara), central tableland (Angul), northern plateau (Keonjhar and Mayurbhanj), and eastern ghats (Kalahandi) (Fig. 1). Aquatic stages were collected from urban and rural areas of each district.
Sampling and Rearing
Adult samples were collected from the above mentioned districts through mechanical aspirator. Larvae and pupae collection was made through dip method or by Pasteur pipette [47] from peri-domestic water collections (used tires, discarded small and large wastes, tree holes etc.) and domestic containers (cement tanks, earthen pots, plastic buckets), labeled and brought to laboratory for eclosion. Larvae and pupae were reared to adults at room temperature (29–30 °C) and relative humidity 70 ± 5% with 16:8 light:dark cycle. Larvae were fed on yeast powder and adults on 10% sucrose and rabbit blood. The eclosed adults were identified based on the standard keys described by Barraud, Christophers, and Harbach [3, 5, 11]. The identified samples were immediately processed or subjected to storage at − 80 °C for future use.
Primer Designing
Strain specific primers (new) were designed from sequences obtained from Genbank, NCBI for Ae. albopictus. The designing was carried out using PRIMER 3 software. The designed primers are wspFN/wAlbAN for strain A and wspFN/wAlbBN for strain B (wspFN-5′-GAAGATATGCCTATCACTCC-3′; wAlBAN-5′GTATGTCAGCACTCCTTT-3′; wAlBBN-5′-CCCAGAAATCAAGCTTTATGC-3′).
Molecular Analysis
DNA was extracted using HiPura™ insect DNA purification kit (Himedia) from adults of Aedes, Culex, Armigeres, Anopheles, and Drosophila species (as positive control). DNA was also extracted from ovaries, salivary glands and midguts of female mosquitoes every alternate day till day 30 of survival. Along with this DNA extraction was done for individual male Ae albopictus on each day till day 20 of their survival. Extracted DNA was stored at − 20 °C for future use.
Standardization, Optimization, and Evaluation of PCR
Screening for Wolbachia in mosquito and Drosophila were done by wsp gene (81F/691R) specific PCR. Samples positive for wsp primers were genotyped using newly designed strain specific primers wspFN/wAlbAN wspFN/wAlbBN (for Ae. albopictus). Wolbachia supergroup and subgroup primers were later used for further confirmation [53]. The reaction mixture for PCR included 0.25 mM dNTPs, 2.5 mM primers and 1 U Taq DNA polymerase following cycling conditions: 95 °C for 5 min, followed by 35 cycles of 95 °C for 1 min, 56 °C for 1 min and 72 °C for 2 min, followed by 72 °C for 10 min. 10 µl of the PCR products were run 1.5% agarose gel.
Sequencing and Phylogenetic Analysis
1.5% Agarose gel was used for PCR product visualization under UV transilluminator and subjected to purification by HiPura™ PCR product purification kit (Himedia). EDTA/ethanol was used for the precipitation of PCR product after which 10 µl of Hi-Di-formamide was used for pellet resuspension. The suspension was then subjected to direct sequencing following the manufacturer’s instructions in a 16 capillary (90 cm) automated DNA sequencer (Applied Biosystems, Foster City, CA, USA). The sequencing mixture contained 2.5 × sequencing buffer, 5 × big dye terminator, 20 mM of either forward or reverse primers of wsp genes and 50 ng of purified PCR product. The cycling parameters used were as follows: 96 °C for 1 min followed by 25 cycles of 96 °C for 10 s, 50 °C for 5 s, and 60 °C for 4 min. The sequences obtained were later edited and analyzed through the Sequencing analysis software (Applied Biosystems, Foster City, CA, USA).
The wsp gene sequences generated from the study were deposited in GenBank. Multiple sequence alignment was done using Clustal Omega and phylogenetic analysis were performed using partial wsp gene sequences of the mosquito isolates obtained from Odisha and other regions of Asia through MEGA (Version 7.0.26) (Arizona State University, Tempe, AZ, USA) using the maximum-likelihood method [36]. 500 bootstrap replications under the nearest-neighbor-interchange procedure were used to confirm the robustness of each node.
Wolbachia q-PCR
The densities of Wolbachia were quantified in whole body of wild strain of Ae. albopictus at day 0, 5, 18 and 30 post eclosion. Further densities were also quantified in wild male and female of Ae. albopictus. Along with quantifying Wolbachia from whole body of mosquitoes, Wolbachia was also quantified from salivary gland, midgut and ovary. The primers exclusively concentrated on Wolbachia surface protein (wsp) of wAlbA and wAlbB. The Wolbachia genome copy number was normalized using the mosquito actin gene. Ae. albopictus actin gene was amplified with the forward primer actAlb-dir (GCAAACGTGGTATCCTGAC) and reverse primer actAlb-rev (GTCAGGAGA ACTGGGTGCT). A standard curve mentioned by Tortosa et al. [38] was used as the reference. q-PCR reactions were performed in a 20 µl total volume containing 2 ng of genomic DNA, 0.5 µM of each primer and 3 µl of FastStart Universal SYBR Green Master (Roche). Cycling was performed using a Light-Cycler480 Instrument (Roche) for 45 amplification cycles of 94 °C for 4 s, 65 °C for 15 s and 72 °C for 25 s.
Microscopic Analysis
Commercially prepared Giemsa stain (SRL-45881) was used to stain ovaries of wild female Ae. albopictus and Cx. quinquefasciatus. A standardized staining condition (taking into account varying staining time, dilution, and buffer pH) as mentioned by Wright [50] was used to visualize mosquito Wolbachia in ovary smears.
Statistical Analyses
Wolbachia positivity in frequency between ovaries and midgut/salivary gland in Ae. albopictus, Ar. subalbatus and Cx. quinquefasciatus was calculated using one-way ANOVA. The density of Wolbachia between ovaries, midgut and salivary gland of Ae. albopictus was also calculated using one-way ANOVA. A comparison between male and female infection status from Ae. albopictus collected from four physiographical regions was calculated using Fisher’s exact test.
GenBank Submissions
MF805773, MF805774, MF805775, MF805776, MF805777, MH753508, MH753510, MH765637, MH765639, MH765641, MH765643, MH765645, MH765647, MH765649, MH765651.
Results
Distribution of Mosquito Species in Odisha
A total of 1634 mosquitoes were collected during the period May 2017 to Feb 2018. Following the standard keys of identification the mosquitoes included the species like Anopheles culicifacies, Anopheles stephensi, Anopheles barbirostris, Anopheles subpictus, Anopheles vagus, Anopheles minimus, Anopheles fluviatilis, Aedes aegypti, Aedes albopictus, Aedes vittatus, Culex quinquefasciatus, Culex vishnui, Culex gelidus, Armigeres subalbatus, Armigeres theobaldi, Mansonia indiana, Mansonia uniformis, and Mansonia annulifera. Along with mosquitoes 27 Drosophila simulans were also collected for reference as positive control.
Wolbachia Distribution in Mosquito Species
Wolbachia was detected in 7 of 18 species of mosquitoes collected. The details are mentioned in Table 1. Anopheles sp, Ae. aegypti, Ae. vittatus, Ar. theobaldi, and Mn. annulifera were completely devoid of Wolbachia. A standard PCR was performed using Wolbachia specific primer (81F/691R) that amplified at 610 bp. A semi nested PCR using the amplified product from standard PCR produced bands at 577 bp and 449 bp for supergroups A and B respectively. Further A and B sub grouping primers amplified around 379 bp and 501 bp respectively. Newly designed strain specific primers for Ae. albopictus amplified at 65 bp for subgroup A and 164 bp for subgroup B as depicted in Fig. 2.
Gel picture amplifying wAlbA and wAlbB from Wolbachia positive Ae. albopictus. Lane 1 and 2 represents super-infection in females (65 bp for wAlbA and 164 bp for wAlbB). Lane 3 and 4 represents mono-infection in males (65 bp for wAlbA). Lane 5 is positive control Wolbachia DNA (610 bp; primers of Zhou et al. [53]) and lane 6 is negative control containing reaction mixture without Wolbachia DNA
On screening for subgroups, it was found that 80% of Ae. albopictus were super-infected (wAlbA + wAlbB), 5% were mono-infected with wAlbA and 10% were mono-infected with wAlbB. Along with this, 58.3% of Mn. uniformis and 38.8% of Mn. indiana were found to be superinfected; 85% of Cx. quinquefasciatus and 68.4% of Cx. vishnui were found to be mono-infected with wAlbB; 38.4% of Cx. gelidus and 65% of Ar. subalbatus were found to be mono-infected with wAlbA. D. simulans taken as control was found to be 100% mono-infected with wAlbA.
Sub-grouping and Phylogenetic Analysis
Sequences of wsp gene generated from the study were subjected to alignment using Clustal Omega. This included 15 sequences from this study and 14 sequences derived from NCBI. The phylogenetic analysis revealed separate clusters A and B belonging to two supergroups A and B of Wolbachia.
Maximum-Likelihood Method based on Tamura–Nei model [36] with a bootstrap value of 500 was employed to study the evolutionary history. Wsp gene sequences of Ae. albopictus are found to be present in both the groups A and B. Cx. quinquefasciatus, Cx vishnui. Mn indiana, Mn. uniformis, and D. simulans are clustered under supergroup B whereas Ar. subalbatus, Cx. gelidus, and D. simulans are clustered under supergroup A. D. simulans (positive control) had A infection. Stars indicate sequences with accessions generated from studies in different parts of Asia.
Three wsp sequences from Ae. albopictus and two sequences from Ar. subalbatus were found in a clade where Wolbachia AlbA sequences of Ae. albopictus (AF020058, AF397411 and JX476002) and Cx. gelidus (HM007831) were clustered together along with positive D. simulans (AF020067). Other sequences from Ae. albopictus, Cx. quinquefasciatus belonged to the clade where Wolbachia wAlbB (Pip) sequences from Ae. albopictus (HM007829, AF397412), Cx. quinquefasciatus (AF020060), Cx. vishnui (HM007825, KY71015), Mn. uniformis (KY523674), and Mn. indiana (AF317492) are clustered. The details of the sequences generated from the study are mentioned in Table 2.
Thus, a monophyletic group with representative members of Wolbachia from arthropods were clearly distinguished under groups A and B (Fig. 3).
Organ Specific Wolbachia Screening in Mosquito Species
On screening Wolbachia in different organs of female mosquitoes of Ae. albopictus, Cx. quinquefasciatus and Ar. subalbatus, it was found that ovaries were the highest to be infected followed by salivary glands and midguts (Fig. 4). One-way ANOVA revealed that there was a significant difference in Wolbachia positivity between ovaries and midguts/salivary glands in all the above mentioned species at P < 0.05. However, there was no significant difference in Wolbachia positivity between respective organs of three species studied at P < 0.05.
Based on these results, we tried to quantify Wolbachia in ovaries, midguts and salivary glands of Ae. albopictus. It was found that the number of wsp gene per host actin gene was highest in ovaries followed by salivary glands and midguts (n = 5 for each organ). Further one-way ANOVA suggested a significant difference between densities of all the tested organs at P < 0.05 (Fig. 5).
Sex-Specific Wolbachia Infection Pattern in Ae. albopictus
The density of Wolbachia in wild male and female Ae. albopictus was compared. There was 1.8 and 18.1 copies of wsp A and wsp B per host actin gene in male as different against 7.8 and 13 copies in female. The standard PCR in male Ae. albopictus, revealed no wAlbA mono-infection at day 3 of their survival suggesting the complete clearance of this strain by day 3 of survival. The prevalence of wAlbA and wAlbA + wAlbB differed significantly between males and females for four physiographical regions of Odisha (Fisher’s exact test, P < 0.05) (Fig. 6).
On quantifying Wolbachia at day 0, 5, 15, and 30 post eclosion, it was found that Wolbachia super-infection in females tend to increase whereas wAlbA density reduced completely as compared to wAlbB in males when they grew old (Fig. 7).
Microscopic Identification
Giemsa stained microscopic images of squashed ovary of wild caught Ae. albopictus and Cx. quinquefasciatus showed the presence of pink pleomorphic cells of Wolbachia with shapes ranging from cocci, comma to bacillus and chain forms. Wolbachia could not be observed in the ovaries of Ae. aegypti (Fig. 8).
a Giemsa stained smear of squashed ovary of Ae. aegypti without Wolbachia. b Giemsa stained smear of squashed ovary of Ae. albopictus with Wolbachia colonies. c Giemsa stained smears showing pleomorphic forms (red rounds) of Wolbachia in Cx. quinquefasciatus. d Giemsa stained ovary of Ae. aegypti without Wolbachia. e Purple stained Wolbachia on the follicle cells of ovary in Ae. albopictus
Discussion
Ever since the discovery of Wolbachia in Culex pipiens in 1936 [14], the importance of this endosymbiont has reached its peak. The common arthropods with natural infection of Wolbachia include D. melanogaster, Cnaphalocrocis medinalis, Lutzomyia sp. and Ae. albopictus [4, 6, 33, 40]. The general and strain differentiating primers [53] amplified wsp gene from Wolbachia-infected insects thus making wsp helpful in determining the evolutionary relationships between strains. On account of variability of wsp, more studies on biological aspects of Wolbachia can be done in future.
In this study a survey on vectors and non-vectors in Odisha was done to determine the extent of indigenous Wolbachia infections. The result indicates the presence of indigenous Wolbachia in Ae. albopictus, Ar. subalbatus, Mn. uniformis, Mn. indiana, Cx. quinquefasciatus, Cx. vishnui, and Cx. gelidus. D. simulans was taken as a positive control for Wolbachia. None of the field collected Anopheles species, Ae. aegypti and Mn. annulifera had Wolbachia in them. This is in concordance with the results of Hughes et al. [16], Kittayapong et al. [21], de Oliveira et al. [7], Wiwatanaratanabutr [48], and Van den Berg et al. [42]. Molecular analysis revealed that Cx. quinquefasciatus, Cx vishnui. Mn indiana, Mn. uniformis and D. simulans belong to supergroup B (wPip-group B) whereas Ar. subalbatus, Cx. gelidus, D. simulans, and D. melanogaster belong to supergroup A (wAlbA-group A).
Ae. albopictus, an efficient vector of dengue and chikungunya, had either super-infection (wAlbA-group A and wPip-group B) or mono-infection with wPip-group B. Kittyapong et al. [21] reported the same results. Both Mn. uniformis and Mn. indiana were also found to be superinfected. This shows that vector and non-vector species are natural niche to strain A and strain B of Wolbachia. Our results also confirm D. simulans to be infected with strain A of Wolbachia. Many studies have shown D. melanogaster to be naturally infected with wMel strain of Wolbachia. The induction of cytoplasmic incompatibility in females can be attributed to multiple Wolbachia strain infections [45]. The results from PCR screening were further co-evaluated/co-confirmed with phylogenetic analysis of the wsp sequences. Wolbachia sub-grouping is of utmost importance in tracing the evolutionary significance between the host and the symbiont [44].
The phylogenetic analysis also established the co-infection of wAlbA and wAlbB in Ae. albopictus mosquitoes collected from different regions. As observed, Wolbachia strains are distributed in both group A and group B, similar to those reported by Zhou et al. [53], Van Meer et al. [43], and Ruang-Areerate et al. [31]. High homology between wAlbA and wAlbB strains of Ae. albopictus indicates the stability of these two strains. Moreover, the high homology of Wolbachia from Ae. albopictus, Cx. quinquefasciatus and Ar. subalbatus may predict its origin from the same ancestral bacterial strain. A further study on comparison between Wolbachia strains can estimate a vector’s capacity to acquire, replicate and transmit a novel pathogen.
Wolbachia quantitative study also indicated that ovaries of tested mosquitoes were infected from day 0 of emergence while midgut and salivary glands acquired Wolbachia infection at 8 and 10 days post eclosion respectively. This might indicate the gradual spread of Wolbachia within organs when a female mosquito grows old. The high density of Wolbachia in the ovaries in the present study justifies its transmission during oogenesis as evident from Giemsa staining in the ovarian cells. This affirms its presence in germ cell cytoplasm, follicular cells, nurse cells, and future oocytes. Since Wolbachia is transmitted transovarially from infected mother to offspring, the early stages of oogenesis or embryogenesis concentrates itself mainly in the germ-line of arthropods and nematodes [26, 37, 45]. Quantitative studies in other organs indicated that the density of Wolbachia is lower in salivary gland and lowest in the midgut which is similar to other reports where Wolbachia presence was observed in salivary glands, midguts, and ovaries of Ae. albopictus [40, 54]. Wolbachia infection in midgut and salivary glands might explain the reduction in replication and transmission of dengue virus in Ae. albopictus. Many authors described Wolbachia presence in various somatic tissues along with germ-line tissues in hosts making Wolbachia a potential organism incorporating disease resistant gene products [8].
Studies on density variance of individual Wolbachia strains showed a decrease in wAlbA density and increase in wAlbB density when males grow old; but super-infected females had an overall increase in density for both wAlbA and wAlbB. The study was conducted on laboratory reared F1 generation. wAlBA and wAlbB density were variable in mosquitoes of F1 generation for both the sex and at different age. This was further confirmed by standard PCR wherein there was no wAlBA infection in males of 3 day old; thereby confirming very low density of wAlbA that cannot be detected by standard PCR. The sensitivity of q-PCR made its detection even at 5 day of post eclosion. This explains the fact of only wAlbB infection existence in males. Females are thus considered to be a repertoire of Wolbachia prevalence in field population because of vertical transmission. This phenomenon has been clarified by studies of Tortosa et al. [39] which states that males receive super-infection at birth from their mothers but lose wAlbB when they grow old showing immunity to cytoplasmic incompatibility induced embryonic mortality. Here females show a virtual fixation of super-infection. Our study confirms a difference in total density of Wolbachia in wild male and female. Females tend to have a higher density than males. Studies have shown that density vary from region to region. Heterologous hosts encounter higher Wolbachia densities than native hosts, rendering more robustness for antiviral effects than native ones [10]. A decrease in density of Wolbachia occurred at high temperature and low nutrition [49].
We also performed microscopic studies on distribution of Wolbachia in ovaries of Ae. albopictus and later confirmed that PCR to be more sensitive and specific. Though microscopic method using Giemsa staining protocol helped in readily identifying morphology of Wolbachia present in Ae. albopictus and Cx. quinquefasciatus in laboratory, it could not confirm the distinction between two strains i.e., wAlbA and wAlbB inhabiting the mosquitoes. However, the PCR technique helped in strain typing of Wolbachia that may assist in further determining the competitive strain that can in future help in transfection to vectors, thereby reducing the transmission of diseases.
The study provides evidences on potential vectors of dengue, chikungunya (Ae. aegypti) and malaria (An. culicifacies and An. fluviatilis) that are inefficient to harbor Wolbachia and they hardly encounter Wolbachia horizontal transmission. So, further studies in Odisha can be made by transfecting a foreign strain into dengue and malaria vectors to analyze their pathogen replication capabilities. Future studies can help identify the interaction between host, virus and Wolbachia. Many studies reported that Wolbachia diminishes viral replication [1, 2] and reduces the life span of the host [19]. This fact makes Wolbachia an alternate choice for vector control leading to a massive reduction of the disease transmission. Since Wolbachia is not naturally present in wild Ae. aegypti, transfected Ae. aegypti has been successfully incorporated in field trials to maximize their spread in wild populations [9, 15, 24, 52], thereby reducing vector competence. The mass releases of wMel transfected Ae. aegypti in Australia has attempted success in reducing dengue burden in the released site with no adverse effects. With this, WHO along with various public health entities have advocated the use of Wolbachia-based strategies to control spread of arboviral diseases. Ample data on indigenous Wolbachia in mosquito may serve as a gateway for Wolbachia-based vector control approaches. It is compulsive to screen mosquito species for indigenous Wolbachia strains for its selection and future application in biocontrol strategies when conventional vector control strategies are less valuable. The current study is a preliminary effort to collect basic information regarding the extent of indigenous infections of Wolbachia in vectors and non-vectors of Odisha, India. Since Odisha is endemic for dengue and chikungunya, the study may be helpful to initiate vector control programs making a potential use of Wolbachia.
Wolbachia has been used as a driver for spread of disease resistant gene in mosquito being implemented worldwide. Our study provides a brief insight on the indigenous strain of Wolbachia circulating in mosquito species of Odisha. This study is unique in its kind covering the major aspects of the endosymbiont Wolbachia and focusing on its potential as a biocontrol agent in arboviral outbreaks. A robust knowledge on potential of the indigenous strain and further experiments including interactions between Wolbachia and viruses can be utilized further to reduce the global burden of vector borne diseases.
References
Aliota MT, Walker EC, Yepes AU, Velez ID, Christensen BM, Osorio JE (2016) The wMel strain of Wolbachia reduces transmission of chikungunya virus in Aedes aegypti. PLoS Negl Trop Dis 10:e0004677
Aliota MT, Peinado SA, Velez ID, Osorio JE (2016) The wMel strain of Wolbachia Reduces Transmission of Zika virus by Aedes aegypti. Sci Rep 6
Barraud PJ (1934) The fauna of British India, including Ceylon and Burma. In: Diptera V (ed) Family Culicidae, Tribe Megharini and Culicini. Taylor and Francis, London, pp 217–246
Chai HN, Du YZ, Qiu BL, Zhai BP (2011) Detection and phylogenetic analysis of Wolbachia in the Asiatic rice leafroller, Cnaphalocrocis medinalis, in Chinese populations. J Insect Sci 11:123
Christophers SR (1993) Family Culicidae. Tribes Anophelini: The fauna of British India, including Ceylon and Burma –Diptera, vol 4. Taylor and Francis, London, pp 1–271
Das B, Satapathy T, Kar SK, Hazra RK (2014) Genetic structure and Wolbachia genotyping in naturally occurring populations of Aedes albopictus across contiguous landscapes of Orissa, India. PLoS ONE 9:e94094
de Oliveira CD, Gonçalves DS, Baton LA, Shimabukuro PHF, Carvalho FD, Moreira LA (2015) Broader prevalence of Wolbachia in insects including potential human disease vectors. Bull Entomol Res 105:305–315
Dobson SL, Bourtzis K, Braig HR, Jones BF, Zhou W, Rousset F, O’Neill SL (1999) Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem Mol Biol 29:153–160
Dutra HL, DosSantos LM, Caragata EP, Silva JB, Villela DA, Maciel-de- Freitas R, Moreira LA (2015) From lab to field: The influence of urban landscapes on the invasive potential of Wolbachia in Brazilian Aedes aegypti mosquitoes. PLoS Negl Trop Dis 9:e0003689
Glaser R, Meola MA (20100 The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile Virus infection. Plos ONE 5: e11977
Harbach RE, Howard TM (2007) Index of currently recognized mosquito species (Diptera: Culicidae). Eur Mosq Bull 23:1–66
Hedges LM, Brownlie JC, O’Neill SL, Johnson KN (2008) Wolbachia and virus protection in insects. Science 322:702
Helinski ME, Parker AG, Knols BG (2006) Radiation-induced sterility for pupal and adult stages of the malaria mosquito Anopheles arabiensis. Malar J 5:41
Hertig M (1936) The rickettsia, Wolbachia pipientis (Gen. Et SP.N.) and associated inclusions of the mosquito Culex pipiens. Parasitol 28:453–486
Hoffmann AA, Iturbe-Ormaetxe I, Callahan AG, Phillips BL, Billington K, Axford JK, Montgomery B, Turley AP, O’Neil SL (2004) Stability of the wMel Wolbachia infection following invasion into Aedes aegypti populations. PLoS Negl Trop Dis 8:e3115
Hughes GL, Ren X, Ramirez JL, Sakamoto JM, Bailey JA, Jedlicka AE, Rasgon JL (2011) Wolbachia infections in Anopheles gambiae cells: transcriptomic characterization of a novel host-symbiont interaction. PLoS Pathog 7:e1001296
Iturbe-Ormaetxe I, Walker T, O’ Neill SL (2011) Wolbachia and the biological control of mosquito-borne disease. EMBO Rep 12:508–518
Jeffries CL, Walker T (2015) The potential use of Wolbachia-based mosquito biocontrol strategies for Japanese encephalitis. PLoS Negl Trop Dis 9:e0003576
Kambris Z, Blagborough AM, Pinto SB, Blagrove MS, Godfray HCJ, Sinden RE, Sinkins SP (2010) Wolbachia stimulates immune gene expression and inhibits Plasmodium development in Anopheles gambiae. PLoS Pathog 6:e1001143
Karunamoorthi K, Sabesan S (2013) Insecticide resistance in insect vectors of disease with special reference to mosquitoes: a potential threat to global public health. Health Scope 2:4–18
Kittayapong P, Baisley KJ, Baimai V, O’Neill SL (2000) Distribution and diversity of Wolbachia infections in Southeast Asian mosquitoes (Diptera: Culicidae). J Med Entomol. 37:340–345
Lambrechts L, Ferguson NM, Harris E, Holmes EC, McGraw EA, O’Neill SL, Ooi EE, Ritchie SA, Ryan PA, Scott TW, Simmons CP, Weaver SC (2015) Assessing the epidemiological effect of Wolbachia for dengue control. Lancet Infect Dis 15:862–866
Mnzava AP, Knox TB, Temu EA, Trett A, Fornadel C, Hemingway J, Renshaw M (2015) Implementation of the global plan for insecticide resistance management in malaria vectors: progress, challenges and the way forward. Malar J 14:173
Nguyen TH, Nguyen HL, Nguyen TY, Vu SN, Tran ND, Le TN, Vien QM, Bui TC, Le HT, Kutcher S, Hurst TP, Duong TTH, Jeffery JAL, Darbro JM, Kay BH, Iturbe-Ormaetxe I, Popovici J, Montgomery BL, Turley AP, Zigterman F, Cook H, Cook PE, Johnson PH, Ryan PA, Paton CJ, Ritchie SA, Simmons CP, O’Neill SL, Hoffmann AA (2015) Field evaluation of the establishment potential of wMelPop Wolbachia in Australia and Vietnam for dengue control. Parasit Vectors 8:563
O’Neill SL, Giordano R, Colbert AM, Karr TL, Robertson HM (1992) 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc Natl Acad Sci USA 89:2699–2702
O’Neill SL, Gooding RH, Aksoy S (1993) Phylogenetically distant symbiotic microorganisms reside in Glossina midgut and ovary tissues. Med Vet Entomol 7:377–383
Osei-Poku J, Han C, Mbogo CM, Jiggins FM (2012) Identification of Wolbachia strains in mosquito disease vectors. PLoS ONE 7:e49922
Ravikumar H, Prakash BM, Sampathkumar S, Puttaraju HP (2011) Molecular subgrouping of Wolbachia and bacteriophage WO infection among some Indian Drosophila species. J Genet 90(3):507–510
Ravikumar H, Ramachandraswamy N, Sampathkumar S, Prakash BM, Huchesh HC, Uday J, Puttaraju HP (2010) A preliminary survey for Wolbachia and bacteriophage WO infections in Indian mosquitoes (Diptera: Culicidae). Trop Biomed 27(3):384–393
Ritchie S (2014) Rear and release: a new paradigm for dengue control. Austral Entomol 53:363–367
Ruang Areerate T, Kittayapong P, Baimai V, O’Neill SL (2003) Molecular phylogeny of Wolbachia endosymbionts in Southeast Asian mosquitoes (Diptera:Culicidae) based on wsp gene sequences. J Med Entomol 40:1–5
Schilthuizen MO, Stouthamer R (1997) Horizontal transmission of parthenogenesis inducing microbes in Trichogramma wasps. Proc R Soc Biol Sci 264:361–366
Serpa LL, Marques GR, de Lima AP, Voltolini JC, de Arduino M, Barbosa GL, Andrade VR, Lima de VL (2013) Study of the distribution and abundance of the eggs of Aedes aegypti and Aedes albopictus according to the habitat and meteoro-logical variables, municipality of Sao Sebastiao, Sao Paulo State, Brazil. Parasit Vectors 6:321
Skinner SW (1982) Maternally inherited sex ratio in the parasitoid wasp Nasonia vitripennis. Science 215:1133–1134
Stouthamer R, Breeuwer JA, Hurst GD (1990) Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu Rev Microbiol 53:71–102
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Taylor MJ, Hoerauf A (1999) Wolbachia bacteria of filarial nematodes. Parasitol Today 15:437–442
Tortosa P, Courtiol A, Moutailler S, Failloux AB, Meil W (2008) Chikungunya-Wolbachia interplay in Aedes albopictus. Insect Mol Biol 17(6):677–684
Tortosa P, Charlat S, Labbe P, Dehecq JS, Barre H, Weill M (2010) Wolbachia age-sex-specific density in Ae. albopictus: A host evolutionary response to cytoplasmic incompatibility? PLoS ONE 5:e9700
Tsai KH, Lien JC, Huang CG, Wu WJ, Chen WJ (2004) Molecular (sub)grouping of endosymbiont Wolbachia infection among mosquitoes of Taiwan. J Med Entomol 41(4):677–683
Valette V, Essono PYB, Le Clec’h W, Johnson M, Bech N, Grandjean F (2013) Multi-infections of feminizing Wolbachia strains in natural populations of the terrestrial isopod Armadillidium vulgare. PLoS ONE 8:e82633
Van den Berg H, Velayudhan R, Ejov M (2013) Regional framework for surveillance and control of invasive mosquito vectors and re-emerging vector-borne diseases, 2014–2020. World Health Organ 26
VanMeer MM, Witteveldt J, Stouthamer R (1999) Phylogeny of the arthropod endosymbiont Wolbachia based on the wsp gene. Insect Mol Biol 8:399–408
Wang Z, Shen ZR, Song Y, Liu HY, Li ZX (2009) Distribution and diversity of Wolbachia in different populations of the wheat aphid Sitobion miscanthi (Hemiptera: Aphididae) in China. Eur J Entomol 106(1):49–55
Werren JH (1997) Biology of Wolbachia. Annu Rev Entomol 42:587–609
Werren JH, Zhang W, Guo LR (2005) Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc R Soc London B 261:55–71
WHO (1975) Manual on practical entomology in malaria part-II, methods tech. Offset Publication 13 World Health Organization, Geneva
Wiwatanaratanabutr I, Kittayapong P (2009) Effects of crowding and temperature on Wolbachia infection density among life cycle stages of Aedes albopictus. J Invertebr Pathol 102:220–224
Wiwatanaratanabutr I (2013) Geographic distribution of wolbachial infections in mosquitoes from Thailand. J Invertebr Pathol 114:337–340
Wright JD, Barr AR (1981) Wolbachia and the normal and incompatible eggs of Aedes polyneslensis (Diptera:Culicidae). J Invert Pathol 38:409–418
Yamada H, Parker AG, Oliva CF, Balestrino F, Gilles JRL (2014) X-Ray-induced sterility in Aedes albopictus (Diptera: Culicidae) and male longevity following irradiation. J Med Entomol 51:811–816
Yeap H, Axford JK, Popovici J, Endersby NM, Iturbe-Ormaetxe I, Ritchie SA, Hoffmann AA (2014) Assessing quality of life-shortening Wolbachia-infected Aedes aegypti mosquitoes in the field based on capture rates and morphometric assessments. Parasit Vectors 7:58
Zhou W, Rousset F, O’Neill SL (1998) Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc R Soc B 265:509–515
Zouache K, Voronin D, Tran-Van V, Mousson L, Failloux AB, Mavingui P (2009) Persistent Wolbachia and cultivable bacteria infection in the reproductive and somatic tissues of the mosquito vector Aedes albopictus. PLoS ONE 4:e6388
Zug R, Hammerstein P (2012) Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE 7:e38544
Acknowledgements
We are grateful to the Director, RMRC for providing a platform for this study. We thank Director, NVBDCP, Bhubaneswar and staff for sharing data for this study. We thank insectarium staff Ms Santoshini Dash and Ms Jyotiprabha Garanayak of RMRC, Bhubaneswar for technical help. We are extremely delightful to thank Lady Tata Memorial Trust, Mumbai, for providing scholarship for PhD to Miss Ipsita Mohanty.
Funding
This work is funded by Lady Tata Memorial Trust, Mumbai, India and Indian Council of Medical Research, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical Approval
All procedures performed in the study involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Mohanty, I., Rath, A., Swain, S.P. et al. Wolbachia Population in Vectors and Non-vectors: A Sustainable Approach Towards Dengue Control. Curr Microbiol 76, 133–143 (2019). https://doi.org/10.1007/s00284-018-1596-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-018-1596-8