Abstract
The bioactivity spectrum of fungal endophytes isolated from Zingiber officinale was analyzed against clinical pathogens and against the phytopathogen Pythium myriotylum, which causes Pythium rot in ginger. One of the isolates GFM13 showed broad bioactivity against various pathogens tested including P. myriotylum. The spore suspension as well as the culture filtrate of the endophytic fungal isolate was found to effectively protect ginger rhizomes from Pythium rot. By molecular identification, the fungal endophyte was identified as Paraconiothyrium sp. The bioactive compound produced by the isolate was separated by bioactivity-guided fractionation and was identified by GC–MS as danthron, an anthraquinone derivative. PCR amplification showed the presence of non-reducing polyketide synthase gene (NR-PKS) in the endophyte GFM13, which is reported to be responsible for the synthesis of anthraquinones in fungi. This is the first report of danthron being produced as the biologically active component of Paraconiothyrium sp. Danthron is reported to have wide pharmaceutical and agronomic applications which include its use as a fungicide in agriculture. The broad-spectrum antimicrobial activity of danthron and the endophytic origin of Paraconiothyrium sp. offer immense applications of the study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungal endophytes are rich source of bioactive metabolites. Though all plants are considered to harbor endophytes, host plants try to strictly limit their growth [29]. This selection is likely to favor the establishment of endophytic association by those microorganisms which secrete secondary metabolites to protect the plant from phytopathogens and also promote plant growth. Interactions between the endophyte, host, and pathogen are suggested to be essential for the endophytic production of bioactive metabolites which may be an antibiotic, inducer or a regulator [18]. Most of the bioactive metabolites isolated from fungal endophytes have demonstrated an array of activity including antibacterial [4], antifungal [34], cytotoxic [36], anticancerous [4], immunosuppressive, anti-inflammatory [5], and antioxidant activities [38]. Interestingly, antifungal chemical scaffolds of endophytic origin may be expected to have anticancerous activity, like in the case of anticancerous molecules such as taxol and leucinostatin A. These are primarily antifungal compounds produced by endophytic fungi to protect their host plants from oomyceteous phytopathogenic fungi like Pythium [30, 31].
Zingiber officinale Rosc. (Ginger) is one of the most important members of the family Zingiberaceae and has been grown over thousands of years for culinary and medicinal needs. India is the largest producer of ginger in the world with its state Kerala being one of the major producers in the country. Asians have for long used ginger as a remedy from diarrhea, indigestion, motion sickness, fever, chills, flu, cough, sore throat, infectious diseases, arthritis, and muscular aches [21]. More than a hundred compounds have been reported from rhizome of ginger which includes gingerols and diarylheptanoids [12]. The crude extract and metabolites of ginger are known for their antibacterial [13], antifungal [1], anti-inflammatory [12], antitumorigenic, antiapoptotic [23], and antiangiogenic effects [15]. Thus an endophyte surviving in the complex chemical environment of ginger rhizome may be considered to exhibit various chemical adaptations to survive in its host. Also as Pythium rot is a major disease of ginger, these endophytes may be expected to produce antifungal compounds against Pythium which may possess broad range of bioactivity. Among the various bioactive secondary metabolites produced by fungi, polyketides are highly remarkable due to their structural and bioactive diversity. Polyketide biosynthesis is mediated by large multifunctional enzymes known as polyketide synthases (PKS) and the structural variations in polyketides, which are responsible for their diverse bioactivity, are implemented during synthesis by these enzymes. Fungal iterative type І PKS can be of non-reducing, partially reducing, or highly reducing types which depends on reduction that occur during polyketide chain formation [7]. The biosynthetic complexities of these enzymes could be expected to result in the production of polyketides with a broad range of bioactivity. However, there are only limited reports on the characterization of PKS-mediated bioactive compounds from endophytic fungi. In the current study, fungal endophytes of ginger with inhibitory effect towards Pythium myriotylum were investigated for the presence of compounds with broad-spectrum activity, and also for the presence PKS gene with promising applications in agricultural and pharmaceutical fields.
Materials and Methods
Fungal Endophyte Isolation
Zingiber officinale Rosc. (var. Mahima) rhizomes were procured from Kerala Agricultural University, Kerala, around May 2015. The rhizome samples were surface-sterilized as per previous report [25]. The last wash of surface sterilization was plated onto PDA (Potato dextrose agar) plates for confirming the accuracy of the sterilization procedure. Rhizome was then cut into small pieces (0.5 × 0.5 cm) and placed on PDA plates containing streptomycin sulfate (250 µg L− 1). The plates were kept at room temperature for 30 days. The mycelia emerging from the rhizome pieces were subcultured on to PDA plates and stored for further studies.
Screening for Activity Against Pythium myriotylum
Dual culture technique was done to screen endophytic fungi with activity against Pythium myriotylum [16]. The endophytic fungi were inoculated on a PDA plate following which it was incubated for seven days at room temperature for the fungus to grow. P. myriotylum was then inoculated on the edge of the culture plate. These plates were then incubated at room temperature for three to five days. P. myriotylum cultured on PDA plate served as control. Percentage inhibition of fungal growth was calculated using the formulae:
where R1 and R2 are the radial diameters of the phytopathogenic fungal colony in the control plate and dual culture plate, respectively [22].
Screening Activity Against Clinical Pathogens
The endophytic fungi isolated were cultured on PDA plates for 7 days at room temperature. Agar blocks (0.5 × 0.5 cm) containing the endophyte was cut from PDA plates and was added into 250 mL potato dextrose broth (PDB) which was further kept at room temperature for 21 days with shaking. Culture broth was subsequently filtered and extracted with ethyl acetate to obtain crude fungal extract which was finally dissolved in 1 mL methanol. The antibacterial activity of the fungal extracts (40 µL) was checked against Bacillus subtilis (MTCC 121), Escherichia coli (MTCC 723), Staphylococcus aureus (MTCC 96), Salmonella enterica typhimurium (MTCC 1251), and Klebsiella pneumoniae (MTCC 109) by well diffusion assay. The fungal isolate showing broad antagonism towards P. myriotylum and the pathogenic bacteria was selected for further studies.
Bioactivity of the Crude Extract Against Various Phytopathogens
The antifungal activity of the fungal extract of the selected endophyte was also analyzed against various fungal phytopathogens by well diffusion method. Bioactivity of GFM13 crude extract was screened against Pythium myriotylum, Sclerotium rolfsii, Colletotrichum acutatum, Corynespora cassiicola, Rhizoctonia solani, Fusarium oxysporum, and Phytophthora infestans. To PDA plates inoculated with phytopathogen, 40 μL of the crude extract was added while methanol was added in the control plate. The plates were kept at room temperature and observed for any growth inhibition of phytopathogens.
Rhizome Protective Effect of GFM13 from P. myriotylum Infection
The protective effect of the endophytic fungi GFM13, from P. myriotylum infection on ginger rhizomes, was studied by treating it with both endophytic fungal spore suspension as well as fungal culture filtrate. Endophytic fungal spore suspension was prepared by adding 20 mL of sterile distilled water to a fully grown fungal culture plate. The spores were then scrapped out carefully using a sterile spatula. Meanwhile endophyte grown in PDB for 21 days was filtered to obtain the culture broth. For the treatment, ginger rhizome pieces were surface-sterilized according to Shultz et al. [25]. Surface-sterilized rhizome pieces were divided into three sets. The first set was soaked in spore suspension, second in fungal culture filtrate, and the set kept as control was soaked in sterile distilled water for 30 min. The treated rhizomes pieces were separately placed in sterile petri plates, three pieces each, and were inoculated with a growing culture of P. myriotylum on agar disc, with uniform size. The sealed plates were incubated at room temperature and were observed for any infection. The experiment was done in triplicate.
Morphological and Molecular Identification of the Selected Endophytic Fungi
For morphological identification of the fungus, microscopic study was done by slide culture technique. Trypan blue was used to stain the slide culture. The culture was then observed using a bright field microscope. QImaging software was used to process the images. ITS (Internal transcribed spacer) region of the fungal genome was amplified by Polymerase Chain Reaction (PCR) and was sequenced for the molecular identification of the fungus. For this, genomic DNA was isolated from the selected fungus by CTAB method [33]. ITS1 (5′-TCC gTAggTgAA CCT gCg g-3′) and ITS4 (5′-TCC TCC gCT TAT TgA TAT gC-3′) were used as primers to PCR amplify the ITS regions [35]. Sure cycler 8800 (Agilent technologies) was used for the PCR with conditions as follows: an initial denaturation for 5 min at 95 °C, 35 cycles of denaturation for 1 min at 94 °C, annealing for 30 s at 55.5 °C, followed by extension for 2 min at 72 °C. Finally an extension for 10 min at 72 °C was done. Agarose gel electrophoresis was done to confirm the PCR product formation. The PCR product was used as the template for sequencing PCR using BigDye Terminator Sequence Reaction Ready Mix (Applied Biosystem). BLAST (Basic Local Alignment Search Tool) analysis of the sequence was done to identify similarity to reported sequences.
Large-Scale Culture and Isolation of Bioactive Metabolite Produced by the Selected Fungus
The endophytic fungus, GFM13, was cultured in 100 mL of different media like Wickerham medium, Czapek Dox medium, potato dextrose medium, and solid rice medium for the optimization of culture media for large-scale culture. As extracts prepared from solid rice media showed maximum bioactivity, GFM13 was grown on large scale in solid rice media for isolation of the bioactive metabolite. Fermentation was done in ten 500 mL Erlenmeyer flasks. Each flask contained 100 g rice soaked overnight in 100 mL distilled water prior to autoclaving. To the sterilized media, an agar piece (0.5 × 0.5 cm) of GFM13 fungus grown on PDA was added. This was incubated at room temperature for 15 days. The culture was then extracted using ethyl acetate. Dried extract was obtained by evaporation of the culture extract on a vacuum rotary flash evaporator at 40 °C. TLC of the extract was performed using TLC plates precoated with Silica gel 60 F254. TLC was done using various solvents to standardize the solvent system for column chromatography. For isolation of the active compound, column-based chromatography was done using a hexane–ethyl acetate gradient on a 60–120 mesh silica gel column. Various fractions collected from the column were further pooled on the basis of TLC profile and its bioactivity was subsequently checked.
Bioactive Metabolite Identification
Both the extract and bioactive fraction were subjected to GC–MS (Gas Chromatography–Mass Spectrometry) analysis. GC–MS analysis was done with Agilent Technologies-7890 GC System. One microliter of the sample solution was introduced into the GC system provided with a 30 m × 0.25 mm inner diameter, 0.25-μm film thickness Agilent 190913-433 column. Carrier gas used was Helium. For GC–MS the temperature of the GC oven was raised from 100 to 250 °C at 5 °C min− 1. The chromatogram and mass spectra were recorded and analyzed. The MS of each peak was analyzed by comparing with the NIST (National Institute of Standards and Technology) library to identify the compounds.
Screening for NR-PKS Gene
Degenerate primers LC1/LC2c were used to amplify NR-PKS gene from GFM13 [3]. The thermal cycling program used was an initial denaturation for 5 min at 94 °C, 35 cycles of denaturation for 1 min at 94 °C, annealing for 1 min at 55 °C, extension for 1 min at 72 °C. A final extension was done for 10 min at 72 °C. Following agarose gel electrophoresis, the purified PCR product was sequenced. BLAST analysis of the sequence obtained was done to identify similarity to reported PKS sequences. Phylogenetic analysis of sequence obtained and sequences retrieved from NCBI was done using MEGA 6 with neighbor joining method using 1000 bootstrap replicates.
Results
Fungal Endophyte Isolation and Screening for its Bioactivity
Eight fungal endophytes were isolated from rhizome pieces of ginger. Absence of any growth on control plate confirmed the fungi isolated as endophytes. An isolate designated as GFM13 showed highest antifungal activity against the P. myriotylum showing a percentage inhibition of 85% (Fig. 1). The isolate also showed significant antibacterial activity against clinical pathogens like B. subtilis (MTCC 121), S. aureus (MTCC 96), and S. typhimurium (MTCC 1251) (Fig. 2a). Due to its considerable bioactivity against P. myriotylum and clinical pathogens, GFM13 was selected for further studies. Crude extract of the selected endophyte showed activity against P. myriotylum, and also against other phytopathogens like Sclerotium rolfsii, Colletotrichum acutatum, Corynespora cassiicola, and Rhizoctonia solani, in well diffusion assay (Fig. 2b).
Inhibition of Pythium myriotylum growth by GFM13, analyzed by dual culture technique. a Control plate with P. myriotylum only. b Dual culture plate with P. myriotylum and the isolate GFM13. Here the growth of P. myriotylum has been restricted to the agar plug (marked) used for inoculation due to the antagonistic activity of GFM13. Scale bar represents 9.7 mm
Broad-spectrum antimicrobial activity analysis of the crude extract prepared from GFM13 by well diffusion method. a Activity of GFM13 extracts prepared from (1) solid rice and (2) PDB culture against bacterial pathogens. a1, a2, and a3 represent the test organisms S. aureus, B. subtilis, and S. enterica typhimurium, respectively. b Activity of GFM13 against phytopathogens. (a) and (b) are the methanol control and GFM13 extract in methanol. b1, b2, b3, b4, and b5 represent the phytopathogens P. myriotylum, S. rolfsii, C. acutatum, C. cassiicola, and R. solani respectively. Scale bars represent 8 mm
Rhizome Protective Effect of GFM13 from P. myriotylum Infection
After 24 h of incubation it was observed that, on all control rhizome pieces inoculated with P. myriotylum agar plugs, the pathogen rapidly spread across the rhizome, infecting the ginger pieces. But on rhizomes treated with spore suspension and culture extracts of endophytic fungi GFM13 there was no P. myriotylum infection observed after 24 h of incubation. After 96 h, the P. myriotylum was found to be grown profusely on all the control rhizome pieces, while on endophytic fungal culture broth-treated and spore suspension-treated rhizome pieces, still there was complete absence of P. myriotylum infection (Fig. 3). In rhizome pieces treated with GFM13 spore suspension, the endophyte was found to grow on the rhizome surfaces after 96 h of incubation. The spore suspension as well as the culture filtrate was able to effectively protect rhizomes from P. myriotylum infection which indicated the presence of potent antimicrobial metabolite in the culture filtrate.
Identification of the Selected Isolate
On PDA plate, isolate GFM13 appeared peach colored with reverse yellow, exuding crystalline yellow substance to the medium which also formed root-like structures as culture aged. Under microscope, hyphae appeared septate with walls of older mycelia turning dark brown (Fig. 4). BLAST analysis of the ITS sequence obtained showed 99% identity to Paraconiothyrium sp. The sequence was deposited in GenBank with accession number KX247121.
Isolation and Identification of the Bioactive Compound
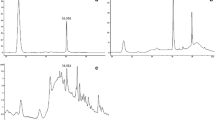
The extraction of solid rice media with ethyl acetate yielded 4 g of crude extract. For isolation of the bioactive compound, 2 g of crude extract was separated by column chromatography using hexane–ethyl acetate gradient. Fractions collected were pooled into 19 fractions on basis of their TLC profile. The bioactivity of these fractions were analyzed (Fig. 5) and the bioactive fraction was identified. GC–MS analysis of this fraction identified the most abundant peak in the total ion chromatogram (TIC) to be at RT 27.445 min (Fig. 6). The mass spectrum of this peak showed a mass of m/z 240 with fragments 212, 184, 155, 138, 120, and 92 (Fig. 7) which exhibited 94% identity to danthron in NIST library search. The mass and its fragments obtained were also in agreement with the GC–mass spectra of standard danthron in previous reports [17]. The crude extract also showed the presence of the same compound in GC–MS analysis.
Broad antimicrobial activity of column purified fraction (32 mg/mL) obtained from crude extract of GFM13, by well diffusion analysis. The fraction is shown to maintain the activity shown by the crude extract against a P. myriotylum, b S.enterica typhimurium, and c S. aureus. Scale bars represents 12.5 mm
Mass spectra of the most prominent compound (at RT 27.445 min) identified in the bioactive fraction of GFM13 by GC–MS. The compound is shown to have a mass of m/z 240 with the fragmentation masses 212, 184, 155, 138, 120, and 92. The obtained mass spectra are the chemical signature of compound danthron as identified through NIST library search. Fragmentation pattern of danthron is shown in the inset
Screening for PKS Gene
While screening for NR-PKS gene, the primer pair LC1/LC2c gave a product with nearly 700 bp size (Fig. 8) corresponding to partial gene sequence of NR-PKS [3]. The PCR product was sequenced and the BLAST result of the sequence showed maximum similarity of 86% to the ketoacyl synthase gene of NR-PKS obtained from a fungus Trypethelium eluteriae. The partial sequence obtained was deposited at GenBank with accession number KX247126.
Discussion
Current study reports the isolation of Paraconiothyrium sp. as endophytic fungi from Zingiber officinale for the first time. Endophytic Paraconiothyrium sp. producing isopimarane diterpene glycosides has been previously reported from beech tree stem [26]. P. brasiliense isolated from branches of Acer truncatum was reported to produce bergamotane sesquiterpenoids [10], and the same fungi isolated from Cinnamomum camphora was found to produce potential antifungal metabolites against various phytopathogens [11]. Endophytic Paraconiothyrium sp. isolated by Khan et al. [14] showed broad bioactivity due to the production of ascotoxin. Paraconiothyrium sp. isolated from various Taxus sp. have been found to possess the ability to produce the host metabolite, taxol [19, 28]. Host-specific Paraconiothyrium variable isolated from Cephalotaxus harringtonia produced 13-oxo-9,11-octadecadienoic acid during co-culture with phytopathogen, which reduced the production of mycotoxin by the pathogen during competition with the endophyte [6]. This endophyte was later found to alter the metabolome of its host plant for producing metabolites, which favored its own growth and survival [32]. A recent study by Soliman et al. [27] found taxol-producing endophytic Paraconiothyrium sp. of Yew trees, to migrate to pathogen entry sites of the plant for creating extracellular barriers laced with taxol to prevent the entry of phytopathogens. These findings suggest that the presence of Paraconiothyrium sp. as endophytes in many host plants is not a momentary occurrence and they may have a long history of co-evolution with their hosts, protecting them from phytopathogens. Paraconiothyrium minitans is a commercially produced biocontrol agent and is highly efficient in controlling Sclerotinia diseases [39]. Thus, isolation of Paraconiothyrium sp. as endophyte from ginger with activity against various phytopathogens was found to seem significant. In the rhizome protection study, the isolate was found to effectively protect ginger rhizomes from P. myriotylum infection. Both the spore suspension and the culture filtrate resisted the growth of phytopathogen which indicated the antimicrobial constituents produced by the isolate to be responsible for the biocontrol property shown by the endophyte, and hence the isolate was further investigated for the identification of its bioactive metabolites.
The GC–MS analysis of both the crude extract and the bioactive fraction identified danthron as the bioactive constituent of GFM13. Danthron (1,8-dihydroxyanthraquinone), also called as chrysazin, is used as a stimulant laxative in some countries. It is reported to have antibacterial, antifungal [2], and anticancerous activity [20]. Danthron is an anthraquinone derivative and its synthesis is mediated by the enzymes polyketide synthases. Anthraquinones are produced by many fungal genera [8]. Danthron was found as an antifungal constituent produced by a marine-derived fungus, Beauveria bassiana [37]. Chaetomium globosum isolated from rhizosphere soil of cucumber showed a wide range of bioactivity, where one of the bioactive constituents produced was danthron [2]. Though danthron is reported in certain plants, fungus, and insects, this is the first report of danthron being produced as the bioactive constituent of Paraconiothyrium sp. Bioactive anthraquinones are suggested to undergo auto-oxidation to release hydrogen peroxide which can degrade fungal cell wall and also mediate oxidation of other anthraquinones to produce more efficient antimicrobials. In Trichoderma harzianum, anthraquinones with higher oxidation number was found to show better antifungal activity [19]. Similar mechanisms may be considered to be the basis of broad antimicrobial effect of danthron which can have wide applicability. In addition, anthraquinones can enhance plant’s systemic resistance by inducing hypersensitive responses, increasing the total peroxidase activity, and by the induction of synthesis of stilbenes. Defence mechanism in grape wine leaves was found to be induced by anthraquinone-rich plant extracts and also by pure emodin, an anthraquinone, thus conferring them resistance against downy mildew [9]. As danthron is used as a fungicide against powdery mildew, production of this compound by an endophyte can have tremendous agronomic applications. Presence of NR-PKS gene provides the possible indication for the biosynthetic basis for danthron production, as anthraquinones in fungi are produced specifically by NR-PKS [8]. Some biosynthetic pathways involving PKS genes are silent when fungi are cultured in the laboratory and would be only expressed in their natural habitat [24]. So there is the chance of production of more diverse and bioactive anthraquinones by the isolated fungi during their endophytic lifestyle.
Paraconiothyrium sp. isolated in the current study may be considered as the natural biocontrol agent harbored by the ginger rhizomes for its protection against phytopathogens including Pythium sp. Danthron biosynthesis by the endophytic Paraconiothyrium sp. observed in the study offers its agricultural applications to limit plant diseases.
References
Agarwal M, Walia S, Dhingra S, Khambay BPS (2001) Insect growth inhibition, antifeedant and antifungal activity of compounds isolated/derived from Zingiber officinale Roscoe (ginger) rhizomes. Pest Manag Sci 57:289–300. https://doi.org/10.1002/ps.263
Awad NE, Kassem HA, Hamed MA, El-Naggar MAA, El-Feky AMM (2014) Bioassays guided isolation of compounds from Chaetomium globosum. J Mycol Med 24:e35–e42. https://doi.org/10.1016/j.mycmed.2013.10.005
Bingle LE, Simpson TJ, Lazarus CM (1999) Ketosynthase domain probes identify two subclasses of fungal polyketide synthase genes. Fungal Genet Biol 26:209–223. https://doi.org/10.1006/fgbi.1999.1115
Budhiraja A, Nepali K, Sapra S, Gupta S, Kumar S, Dhar KL (2013) Bioactive metabolites from an endophytic fungus of Aspergillus species isolated from seeds of Gloriosa superba Linn. Med Chem Res 22:323–329. https://doi.org/10.1007/s00044-012-0032-z
Chapla VM, Zeraik ML, Ximenes VF, Zanardi LM, Lopes MN, Cavalheiro AJ, Silva DH, Young MC, Fonseca LM, Bolzani VS, Araujo AR (2014) Bioactive secondary metabolites from Phomopsis sp., an endophytic fungus from Senna spectabilis. Molecules 19:6597–6608. https://doi.org/10.3390/molecules19056597
Combès A, Ndoye I, Bance C, Bruzaud J, Djediat C, Dupont J, Nay B, Prado S (2012) Chemical communication between the endophytic fungus Paraconiothyrium Variabile and the phytopathogen Fusarium oxysporum. PLoS ONE 7:e47313. https://doi.org/10.1371/journal.pone.0047313
Cox RJ, Simpson TJ (2009) Fungal type I polyketide synthases. Methods Enzymol 459:49–78. https://doi.org/10.1016/S0076-6879(09)04603-5
Gessler NN, Egorova AS, Belozerskaya TA (2013) Fungal anthraquinones. Appl Biochem Microbiol 49:85–99. https://doi.org/10.1134/s000368381302004x
Godard S, Slacanin I, Viret O, Gindro K (2009) Induction of defence mechanisms in grapevine leaves by emodin- and anthraquinone-rich plant extracts and their conferred resistance to downy mildew. Plant Physiol Bioch 47:827–837. https://doi.org/10.1016/j.plaphy.2009.04.003
Guo Z, Ren F, Che Y, Liu G, Liu L (2015) New bergamotane sesquiterpenoids from the plant endophytic fungus Paraconiothyrium brasiliense. Molecules 20:14611–14620. https://doi.org/10.3390/molecules200814611
Han M, Liu T, Cai X, Chen K, Liu C, Brian K, Xue Y, Gu Y (2012) A new endophytic Paraconiothyrium brasiliens LT161 shows potential in producing antifungal metabolites against phytopathogens. African J Microbiol Res 6:7572–7578
Jolad SD, Lantz RC, Chen GJ, Bates RB, Timmermann BN (2005) Commercially processed dry ginger (Zingiber officinale): composition and effects on LPS-stimulated PGE2 production. Phytochemistry 66:1614–1635. https://doi.org/10.1016/j.phytochem.2005.05.007
Karuppiah P, Rajaram S (2012) Antibacterial effect of Allium sativum cloves and Zingiber officinale rhizomes against multiple-drug resistant clinical pathogens. Asian Pac J Trop Biomed 2:597–601. https://doi.org/10.1016/s2221-1691(12)60104-x
Khan AL, Hamayun M, Hussain J, Kang S-M, Lee I-J (2012) The newly isolated endophytic fungus Paraconiothyrium sp. LK1 produces ascotoxin. Molecules 17:1103–1112. https://doi.org/10.3390/molecules17011103
Kim EC, Min JK, Kim TY, Lee SJ, Yang HO, Han S, Kim YM, Kwon YG (2005) [6]-Gingerol, a pungent ingredient of ginger, inhibits angiogenesis in vitro and in vivo. Biochem Biophys Res Commun 335:300–308. https://doi.org/10.1016/j.bbrc.2005.07.076
Kumar S, Kaushik N (2013) Endophytic fungi isolated from oil-seed crop Jatropha curcas produces oil and exhibit antifungal activity. PloS ONE 8:e56202. https://doi.org/10.1371/journal.pone.0056202
Kunze A, Witteb L, Aregullinc M, Rodriguez E, Proksch P (1996) Anthraquinones in the Leaf Beetle Trirhabda geminata (Chrysomelidae). Z Naturforsch. 51c: 249–252. https://doi.org/10.1515/znc-1996-3-417
Kusari S, Hertweck C, Spiteller M (2012) Chemical ecology of endophytic fungi: origins of secondary metabolites. Chem Biol 19:792–798. https://doi.org/10.1016/j.chembiol.2012.06.004
Liu K, Ding X, Deng B, Chen W (2009) Isolation and characterization of endophytic taxol-producing fungi from Taxus chinensis. J Ind Microbiol Biotechnol 36:1171–1177. https://doi.org/10.1007/s10295-009-0598-8
Lu HF, Wang HL, Chuang YY, Tang YJ, Yang JS, Ma YS, Chiang JH, Lu CC, Yang JL, Lai TY, Wu CC, Chung JG (2009) Danthron induced apoptosis through mitochondria- and caspase-3-dependent pathways in human brain Glioblastoma multiforms GBM 8401 cells. Neurochem Res 35:390–398. https://doi.org/10.1007/s11064-009-0067-9
Ma X, Gang DR (2006) Metabolic profiling of in vitro micropropagated and conventionally greenhouse grown ginger (Zingiber officinale). Phytochemistry 67:2239–2255. https://doi.org/10.1016/j.phytochem.2006.07.012
Rabha AJ, Naglot A, Sharma GD, Gogoi HK, Veer V (2014) In vitro evaluation of antagonism of endophytic Colletotrichum gloeosporioides against potent fungal pathogens of Camellia sinensis. Indian J Microbiol 54:302–309. https://doi.org/10.1007/s12088-014-0458-8
Radhakrishnan EK, Bava SV, Narayanan SS, Nath LR, Thulasidasan AK, Soniya EV, Anto RJ (2014) [6]-Gingerol induces caspase-dependent apoptosis and prevents PMA-induced proliferation in colon cancer cells by inhibiting MAPK/AP-1 signaling. PloS ONE 9:e104401. https://doi.org/10.1371/journal.pone.0104401
Schroeckh V, Scherlach K, Nutzmann HW, Shelest E, Schmidt-Heck W, Schuemann J, Martin K, Hertweck C, Brakhage AA (2009) Intimate bacterial-fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans. Proc Natl Acad Sci 106:14558–14563. https://doi.org/10.1073/pnas.0901870106
Schulz B, Wanke U, Draeger S, Aust HJ (1993) Endophytes from herbaceous plants and shrubs: effectiveness of surface sterilization methods. Mycol Res 97:1447–1450. https://doi.org/10.1016/s0953-7562(09)80215-3
Shiono Y, Kikuchi M, Koseki T, Murayama T, Kwon E, Aburai N, Kimura K (2011) Isopimarane diterpene glycosides, isolated from endophytic fungus Paraconiothyrium sp. MY-42 Phytochemistry 72:1400–1405. https://doi.org/10.1016/j.phytochem.2011.04.016
Soliman Sameh SM, Greenwood John S, Bombarely A, Mueller Lukas A, Tsao R, Mosser Dick D, Raizada Manish N (2015) An endophyte constructs fungicide-containing extracellular barriers for its host plant. Curr Biol 25:2570–2576. https://doi.org/10.1016/j.cub.2015.08.027
Somjaipeng S, Medina A, Kwaśna H, Ordaz Ortiz J, Magan N (2015) Isolation, identification, and ecology of growth and taxol production by an endophytic strain of Paraconiothyrium variabile from English yew trees (Taxus baccata). Fungal Biol 119:1022–1031. https://doi.org/10.1016/j.funbio.2015.07.007
Strobel G, Daisy B (2003) Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol 67:491–502. https://doi.org/10.1128/mmbr.67.4.491-502.2003
Strobel GA (2003) Endophytes as sources of bioactive products. Microbes Infect 5:535–544. https://doi.org/10.1016/s1286-4579(03)00073-x
Strobel GA, Torczynski R, Bollon A (1997) Acremonium sp.—a leucinostatin A producing endophyte of European yew (Taxus baccata). Plant Sci 128:97–108. https://doi.org/10.1016/s0168-9452(97)00131-3
Tian Y, Amand S, Buisson D, Kunz C, Hachette F, Dupont J, Nay B, Prado S (2014) The fungal leaf endophyte Paraconiothyrium variabile specifically metabolizes the host-plant metabolome for its own benefit. Phytochemistry 108:95–101. https://doi.org/10.1016/j.phytochem.2014.09.021
Voigt K, Cigelnik E, O’Donnell K (1999) Phylogeny and PCR identification of clinically important Zygomycetes based on nuclear ribosomal-DNA sequence data. J Clin Microbiol 37:3957–3964
Wang J, Wang G, Zhang Y, Zheng B, Zhang C, Wang L (2014) Isolation and identification of an endophytic fungus Pezicula sp. in Forsythia viridissima and its secondary metabolites. World J Microbiol Biotechnol 30:2639–2644. https://doi.org/10.1007/s11274-014-1686-0
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322
Wu L-S, Hu C-L, Han T, Zheng C-J, Ma X-Q, Rahman K, Qin L-P (2012) Cytotoxic metabolites from Perenniporia tephropora, an endophytic fungus from Taxus chinensis var. mairei. Appl Microbiol Biotechnol 97:305–315. https://doi.org/10.1007/s00253-012-4189-7
Yamazaki H, Rotinsulu H, Kaneko T, Murakami K, Fujiwara H, Ukai K, Namikoshi M (2012) A new dibenz[b,e]oxepine derivative, 1-hydroxy-10-methoxy-dibenz[b,e]oxepin-6,11-dione, from a marine-derived fungus, Beauveria bassiana TPU942. Mar Drugs 10:2691–2697
Yuan Y, Tian JM, Xiao J, Shao Q, Gao JM (2014) Bioactive metabolites isolated from Penicillium sp. YY-20, the endophytic fungus from Ginkgo biloba. Nat Prod Res 28:278–281. https://doi.org/10.1080/14786419.2013.850686
Zeng W, Wang D, Kirk W, Hao J (2012) Use of Coniothyrium minitans and other microorganisms for reducing Sclerotinia sclerotiorum. Biol Control 60:225–232. https://doi.org/10.1016/j.biocontrol.2011.10.009
Acknowledgements
We are thankful to KSCSTE for providing facilities under KSCSTE-SARD Programme. We are also grateful to DBT, Govt of India, for providing instrumentation facility under DBT-MSUB and DBT-RGYI support scheme. The ginger samples were provided by Kerala Agricultural University, Thrissur, Kerala. The authors also thank the Department of Applied Chemistry, CUSAT, for GC–MS analysis.
Funding
This work was supported by DST, Government of India, under DST-PURSE program (Order No.: SR/S9/Z-23/2010/22).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Anisha, C., Sachidanandan, P. & Radhakrishnan, E.K. Endophytic Paraconiothyrium sp. from Zingiber officinale Rosc. Displays Broad-Spectrum Antimicrobial Activity by Production of Danthron. Curr Microbiol 75, 343–352 (2018). https://doi.org/10.1007/s00284-017-1387-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-017-1387-7