Abstract
The effect of disodium fumarate (DF) on the ruminal fermentation profiles, the accumulation of lipopolysaccharide (LPS) and bioamines, and the composition of the ruminal bacterial community was investigated by in vitro rumen fermentation. The addition of DF increased the total gas production; the concentrations of propionate, valerate, total volatile fatty acids, and ammonia–nitrogen; and the rumen pH after a 24 h fermentation. By contrast, DF addition decreased the ratio of acetate to propionate and the concentrations of lactate, lipopolysaccharide, methylamine, tryptamine, putrescine, histamine, and tyramine (P < 0.05). Principal coordinates analysis and molecular variance analysis showed that DF altered the ruminal bacterial community (P < 0.05). At the phylum level, DF decreased the proportion of Proteobacteria, and increased the proportions of Spirochaetae and Elusimicrobia (P < 0.05). At the genus level, DF decreased the percentage of Ruminobacter, while increasing the percentage of Succinivibrio and Treponema (P < 0.05). Overall, the results indicate that DF modified rumen fermentation and mitigated the production of several toxic compounds. Thus, DF has great potential for preventing subacute rumen acidosis in dairy cows and for improving the health of ruminants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In modern dairy production systems, the energy requirements of high-producing dairy cattle are usually met by feeding large amounts of rapidly fermentable carbohydrates. However, the inclusion of these carbohydrates can lead to an accumulation of volatile fatty acids (VFA), which causes a drop in the rumen pH. If the increase in VFA production rises above the absorptive capacity of the rumen epithelium, the pH will continue to drop, and this can result in subacute ruminal acidosis (SARA) [1, 2]. SARA, which is characterized by a ruminal pH below 5.6 for 3–5 h per day [3], can result in reductions in feed intake and fiber digestion, as well as decreases in milk yield and declines in the health of the animals [4].
During SARA, the accumulation of VFA decreases ruminal pH and causes a change in the rumen microbiota [5]. Concurrent with the shift in the microbial populations, the concentrations of potentially toxic and inflammatory compounds increase in the rumen. One compound of particular interest is lipopolysaccharide (LPS), a component of the cell walls of the gram-negative bacteria, as it can elicit an inflammatory response in the mammalian cells [6]. When animals are challenged with a SARA-inducing ration, the easy access to fermentable carbohydrates initially results in a logarithmic growth of bacteria. This is later followed by a release of LPS due to massive bacterial lysis that occurs in response to the reduced availability of substrates, the greatly reduced rumen pH, and the accumulation of the end products of rumen fermentation [7,8,9].
Other potentially harmful compounds produced during SARA, in addition to LPS, include biogenic amines, which are believed to associate with lameness [10]. Previous studies showed that, during SARA, a low ruminal pH together with high levels of LPS and biogenic amines, can reduce the barrier function of the rumen epithelium [10, 11]. This barrier damage potentially allows LPS and biogenic amines to translocate into the blood, where they stimulate a systemic inflammatory response [6]. This emphasizes the importance of preventing the accumulation of LPS and bioamines, as well as the decrease in the ruminal pH, in animals experiencing SARA.
Many strategies have been used to improve the ruminal pH during SARA. Ionophores, organic acids, and probiotics based on yeasts (e.g., Saccharomyces cerevisiae) have been explored as stabilizers of the ruminal pH and for improving milk production [12,13,14]. Among these ruminal modulators, fumarate has been proposed as a promising modifier of ruminal fermentation [15]. Fumarate is thought to increase the activity of the succinate–propionate metabolic pathway of several rumen bacteria, which results in increased lactic acid uptake and propionate production [16]. However, the potential for fumarate to serve as a mitigator of the concentration of ruminal LPS and bioamines remains to be established. Besides, previous studies have revealed the fumarate can stimulate the growth of fumarate-utilizing bacteria, including Fibrobacter succinogenes, Selenomonas ruminantium, Veillonella parvula, and Wolinella succinogenes in the rumen [12]; however, information is limited regarding the effects of fumarate on the composition of the ruminal bacterial community.
In this study, we hypothesized that fumarate could positively affect the composition of the ruminal bacterial community, thereby possibly mitigating the accumulation of LPS and bioamines in the rumen. The effects of disodium fumarate (DF) were examined on the changes of the in vitro rumen fermentation profile, the concentration of LPS and bioamines, and the composition of the ruminal microbial community.
Methods
Animals
The experimental design and procedures were approved by the Animal Care and Use Committee of Nanjing Agricultural University. Animals were cared for in accordance with the 1998 guidelines established by the Chinese Science and Technology Committee on Experimental Animal Care and Use.
Three ruminally cannulated Holstein cows (500 ± 23.5 kg BW) served as rumen fluid donors for these experiments. Cattle were fed a 60% alfalfa hay and 40% concentrate (corn grain-based) diet.
Experimental Design
The experimental procedure was as described by Martínez-Fernández et al. [17]. Ruminal contents were obtained immediately before the morning feeding and strained through four layers of cheesecloth. This filtered rumen fluid was mixed with a buffer solution at a ratio of 1:3 (v/v). A 40 mL volume of the mixture was dispensed into a 100-mL bottle containing 1 g substrate (0.49 g soybean meal, 0.21 g maize silage, 0.15 g Chinese wildrye, and 0.15 g alfalfa hay). This substrate was milled through a 1 mm screen before being weighed into the bottles. The final concentration of disodium fumarate (DF) (Sigma) in each group is 0, 4, 8, and 16 mmol/L. The bottles were sealed with rubber stoppers and then aluminum caps, and incubated for 24 h at 39 °C. Each group had four replicates.
Analytical Procedures
Gas production was measured at different time intervals using a pressure transducer and a calibrated syringe [18]. After a 24 h-incubation, the bottles were uncapped and the pH was determined immediately using a pH meter (Ecoscan pH 5, Singapore). The bottles were then immersed in an ice bath to stop the fermentation. The contents of each bottle were homogenized before withdrawing the samples. Short chain fatty acids were determined according to Jin et al. [19], by gas chromatography (GC-14B, Shimadzu, Japan) with a capillary column (Supelco, Bellefonte, USA) at a column temperature of 110 °C; injector temperature of 180 °C; detector temperature of 180 °C. Ammonia–nitrogen (NH3–N) was measured by the indophenol method [20]. Lactic acid was analyzed according to the method of Barker [21]. LPS was measured by a chromogenic end-point Tachypleus amebocyte lysate assay kit (Chinese Horseshoe Crab Reagent Manufactory, Xiamen, China). The concentration of biogenic amine was analyzed according to the method of Wang et al. [22].
DNA Extraction
DNA was extracted using a QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. A FastPrep®-24 Instrument (MP Biomedicals, South Florida, USA) was used for processing (bead beating) at a setting of 5 for 2 min. The DNA concentration was measured using a NanoDrop 1000 Spectrophotometer (Nyxor Biotech, Paris, France).
Illumina MiSeq Sequencing and Data Processing
The bacterial communities in the 0 and 16 mmol/L DF groups were determined according to the method of Liu et al. [23]. The V3–V4 regions of the bacterial 16S rRNA genes were amplified using primers 338F (5′-barcode-ACT CCT RCG GGA GGC AGC AG)-3′ and 806R (5′-GGA CTA CCV GGG TAT CTA AT-3′). PCR amplicons were sequenced on an Illumina MiSeq platform according to standard protocols [24], by Majorbio Bio-Pharm Technology Co., Ltd., Shanghai, China. The raw data were processed using the QIIME (version 1.70) software package [25]. The minimum quality score was 20. Sequences with two nucleotide mismatchs and ambiguous characters and those shorter than 50 bp were discarded. Only sequences that overlapped longer than 10 bp were assembled. OTUs were clustered with a 97% similarity cutoff using UPARSE (version 7.1 http://drive5.com/uparse/). Chimeric sequences were identified and removed using UCHIME [26]. The representative sequences were aligned against Greengenes 13.5 using PyNAST [27], with the default parameters set by QIIME. Sequences were classified using the Ribosomal Database Project classifier with a standard minimum support threshold of 80% [28]. Community diversity was estimated using the ACE, Chao1, and Shannon indexes. Principal coordinate analysis (PCoA) was performed by the unweighted UniFrac distance method [29]. The analysis of molecular variance (AMOVA) was conducted based on an unweighted distance to assess the significant differences among the samples using the MOTHUR program (version 1.29) [30].
Statistical Analysis
Fermentation data were analyzed using a one-way analysis of variance (ANOVA) with the statistical software package SPSS vs. 20.0 (SPSS Inc., Chicago, IL, USA). Linear and quadratic effects due to DF addition were determined using polynomial contrasts. The microbial data were analyzed using the nonparametric Kruskal–Wallis test. All P values from the nonparametric Kruskal–Wallis test for microbial data were adjusted by the false discovery rate. Differences were considered statistically significant at P < 0.05.
Results
Effect of DF on the Lipopolysaccharide and Biogenic Amines Contents and The Characteristics of In Vitro Rumen Fermentation
As shown in Table 1, DF addition linearly increased the total gas production, pH, and the concentrations of propionate, valerate, NH3–N, and total volatile fatty acids (TVFA) (P < 0.05), and linearly decreased the concentration of lactic acid and the ratio of acetate to propionate (P < 0.05). No significant differences were observed in the concentrations of acetate, butyrate, isobutyrate, and isovalerate between the control and the DF treatment groups (P > 0.05). DF addition also linearly decreased the concentrations of LPS, methylamine, tryptamine, putrescine, histamine, and tyramine (P < 0.05).
Effects of DF on The Ruminal Bacterial Community
The Illumina sequencing reads that passed the quality control tests included 133,520 from the control and 138,870 from the DF group. In total, 799 OTUs were formed from the control samples, and 804 OTUs were formed from the DF group samples. Rarefaction curves showed that the sequencing effort covered the majority of bacterial diversity (Supplemental Figure S1). The number of OTUs, Shannon index, Chao1 index, and ACE index did not differ significantly between the control and DF groups (P > 0.05, Supplemental Table S1). Principal coordinate analysis (PCoA) showed that the bacterial communities differed between the control and DF groups (Supplemental Figure S2), as confirmed by the molecular variance analysis (AMOVA, Fs = 2.74668, P = 0.031).
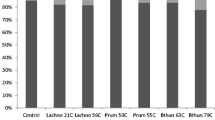
At the phylum level, DF decreased the proportion of Proteobacteria (P < 0.05), and increased the proportions of Spirochaetae and Elusimicrobia (P < 0.05) (Supplemental Table S2). At the genus level, DF addition decreased the proportions of Ruminobacter, Acetitomaculum, Moryella, Bacillus, Staphylococcus, unclassified Christensenellaceae, unclassified Succinivibrionaceae, and unclassified Defluviitaleaceae (P < 0.05), and it increased the proportions of Succinivibrio, Treponema, Blautia, Sutterella, Anaerovorax, Anaerosporobacter, Oligosphaeraceae, Schwartzia, unclassified Bacteroidales, and unclassified Elusimicrobia (P < 0.05, Fig. 1; Supplemental Table S3).
The Correlations Between The Dominant Bacterial Phyla and Rumen Fermentation Parameters
As shown in Supplemental Table S4, Pearson correlation analysis revealed that the presence of Proteobacteria was positively correlated with the concentrations of methylamine, tryptamine, and putrescine and negatively associated with the concentrations of acetate, propionate, isovalerate, valerate, and TVFA (P < 0.05). Lentisphaerae were positively correlated with propionate and TVFA and negatively correlated with lactate and putrescine (P < 0.05). Spirochaetae were positively correlated with ruminal pH, propionate, and NH3–N and negatively associated with lactate, putrescine, tyramine, and the ratio of acetate to propionate (P < 0.05). Elusimicrobia were negatively correlated with the level of LPS (P < 0.05). Tenericutes was positively correlated with methylamine and tryptamine (P < 0.05).
Discussion
The addition of DF modified the in vitro rumen fermentation. In general, DF decreased the ruminal lactate concentration and increased the propionate concentration and the pH value, in agreement with previous reports [16, 31]. Fumarate is an intermediate of the citric acid cycle. In the rumen, it is reduced to succinate through succinate–propionate pathway, and then decarboxylated to propionate [32, 33]. Fumarate can also increase the microbial lactate uptake by microbes such as Selenomonas ruminantium [34], which might partly explain the decrease in the lactate concentration observed in the current study. However, malate has been reported to increase lactate uptake by Selenomonas ruminantium, whereas fumarate had no effect [35]. The actual mechanism that leads to the decrease in the lactate concentration therefore remains unclear.
The concentration of the toxic LPS, a component of the cell walls of gram-negative bacteria, was decreased by DF addition. Previous studies showed that feeding high levels of grain to dairy cows led to a decline in the ruminal pH [4, 36]. This, in turn, caused the death and cell lysis of gram-negative bacteria, thereby increasing the concentration of free LPS in the rumen [4]. The concentration of LPS might therefore be closely related to the abundance of gram-negative bacteria. In the current study, the concentration of LPS was negatively correlated with the relative abundance of Elusimicrobia and a group of unclassified bacteria. The cultivated members of Elusimicrobia, namely Elusimicrobium minutum and Endomicrobium proavitum, were gram-negative bacteria [37, 38].
The addition of DF decreased the concentrations of biogenic amines and increased the pH value. Wang et al. [22] reported that the concentrations of biogenic amines were negatively correlated with the ruminal pH value. A decrease in the ruminal pH was considered to increase the activity of bacterial amino acid decarboxylases, as well as promoting the growth of some microbes, such as Streptococcus bovis and Lactobacillus spp., which generate biogenic amines [22, 39]. In the current study, no increase was observed in the relative abundance of Lactobacillus and Streptococcus, which suggested that other bacteria might be involved in the generation of biogenic amines. In the present study, the concentrations of biogenic amines, such as methylamine, tryptamine, and putrescine, were positively correlated with the relative abundance of Proteobacteria. The members of this bacterial phyla were considered to be involved in the ruminal proteolysis.
Disodium fumarate addition increased the percentages of the dominant genera Succinivibrio and Treponema. Succinivibrio spp. are involved in the metabolism of succinate and lactate in the rumen [40]. Mao et al. [31] also reported an increase in Succinivibrio dextrinisolvens in the rumen of goats fed DF. The non pathogenic Treponema spp. are commensal in the gastrointestinal tract of cows [41], and they are also involved in the metabolism of succinate and lactate in the rumen [42]. The relative abundance of Ruminobacter also decreased following the addition of DF. Ruminobacter spp., such as Ruminobacter amylophilus 70, are mainly known for the amylolytic activities [43], which suggests that the degradation rate of starch might decline.
In summary, DF has a beneficial effect on the rumen fermentation of high concentrate feeds by increasing pH and propionate production, enhancing lactate utilization, and reducing the concentrations of LPS and biogenic amines. DF therefore has great potential for use as a feed additive for preventing SARA in ruminants.
References
Fernando SC, Purvis HT 2nd, Najar FZ, Sukharnikov LO, Krehbiel CR, Nagaraja TG, Roe BA, Desilva U (2010) Rumen microbial population dynamics during adaptation to a high-grain diet. Appl Environ Microbiol 76(22):7482–7490
Hattori K, Matsui H (2008) Diversity of fumarate reducing bacteria in the bovine rumen revealed by culture dependent and independent approaches. Anaerobe 14(2):87–93
Alzahal O, Rustomo B, Odongo NE, Duffield TF, McBride BW (2007) Technical note: a system for continuous recording of ruminal pH in cattle. J Anim Sci 85(1):213–217
Plaizier JC, Krause DO, Gozho GN, McBride BW (2008) Subacute ruminal acidosis in dairy cows: the physiological causes, incidence and consequences. Vet J 176(1):21–31
Mao S, Zhang R, Wang D, Zhu W (2012) The diversity of the fecal bacterial community and its relationship with the concentration of volatile fatty acids in the feces during subacute rumen acidosis in dairy cows. BMC Vet Res 8:237
Gozho GN, Krause DO, Plaizier JC (2007) Ruminal lipopolysaccharide concentration and inflammatory response during grain-induced subacute ruminal acidosis in dairy cows. J Dairy Sci 90(2):856–866
Hespell RB (1979) Efficiency of growth by ruminal bacteria. Fed Proc 38(13):2707–2712
Mackie R, Gilchrist FM, Robberts AM, Hannah P, Schwartz HM (1978) Microbiological and chemical changes in the rumen during the stepwise adaptation of sheep to high concentrate diets. J Agric Sci 90(02):241–254
Russell JB, DiezGonzalez F (1998) The effects of fermentation acids on bacterial growth. Adv Microb Physiol 39:205–234
Nocek JE (1997) Bovine acidosis: implications on laminitis. J Dairy Sci 80(5):1005–1028
Liu JH, Xu TT, Liu YJ, Zhu WY, Mao SY (2013) A high-grain diet causes massive disruption of ruminal epithelial tight junctions in goats. Am J Physiol Regul Integr Comp Physiol 305(3):R232–R241
Castillo C, Benedito JL, Méndez J, Pereira V, López-Alonso M, Miranda M, Hernández J (2004) Organic acids as a substitute for monensin in diets for beef cattle. Anim Feed Sci Technol 115(1):101–116
Nagaraja TG, Avery TB, Bartley EE, Roof SK, Dayton AD (1982) Effect of lasalocid, monensin or thiopeptin on lactic acidosis in cattle. J Anim Sci 4(3):649–658
Newbold CJ, Wallace RJ, McIntosh FM (1996) Mode of action of the yeast Saccharomyces cerevisiae as a feed additive for ruminants. Brit J Nutr 76(2):249–261
Callaway TR, Martin SA (1996) Effects of organic acid and monensin treatment on in vitro mixed ruminal microorganism fermentation of cracked corn. J Anim Sci 74(8):1982–1989
Mao SY, Zhang G, Zhu WY (2007) Effect of disodium fumarate on in vitro rumen fermentation of different substrates and rumen bacterial communities as revealed by denaturing gradient gel electrophoresis analysis of 16S ribosomal DNA. Asian Austral J Anim 20(4):543–549
Martínez-Fernández G, Abecia L, Arco A, Cantalapiedra-Hijar G, Martín-García AI, Molina-Alcaide E, Kindermann M, Duval S, Yáñez-Ruiz DR (2014) Effects of ethyl-3-nitrooxy propionate and 3-nitrooxypropanol on ruminal fermentation, microbial abundance, and methane emissions in sheep. J Dairy Sci 97:3790–3799
Theodorou MK, Williams BA, Dhanoa MS, Mcallan AB, France J (1994) A simple gas-production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim Feed Sci Tech 48(3–4):185–197
Jin W, Meng Z, Wang J, Cheng Y, Zhu W (2017) Effect of nitrooxy compounds with different molecular structures on the rumen methanogenesis, metabolic profile, and methanogenic community. Curr Microbiol 74(8):891–898
Weatherburn MW (1967) Phenol-hypochlorite reaction for determination of ammonia. Anal Chem 39(8):971–974
Barker SBS, Summerson WH (1941) The colorimetric determination of lactic acid in biological material. J Biol Chem 138:535–554
Wang DS, Zhang RY, Zhu WY, Mao SY (2013) Effects of subacute ruminal acidosis challenges on fermentation and biogenic amines in the rumen of dairy cows. Livest Sci 155(2–3):262–272
Liu J, Zhang M, Zhang R, Zhu W, Mao S (2016) Comparative studies of the composition of bacterial microbiota associated with the ruminal content, ruminal epithelium and in the faeces of lactating dairy cows. Microb Biotechnol 9(2):257–268
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R (2012) Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6(8):1621–1624
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5):335–336
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26(19):2460–2461
Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R (2010) PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26(2):266–267
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73(16):5261–5267
Lozupone C, Knight R (2005) UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71(12):8228–8235
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75(23):7537–7541
Mao SY, Zhang G, Zhu WY (2008) Effect of disodium fumarate on ruminal metabolism and rumen bacterial communities as revealed by denaturing gradient gel electrophoresis analysis of 16S ribosomal DNA. Anim Feed Sci Technol 140(3–4):293–306
Asanuma N, Iwamoto M, Hino T (1999) Effect of the addition of fumarate on methane production by ruminal microorganisms in vitro. J Dairy Sci 82(4):780–787
Lopez S, Valdes C, Newbold CJ, Wallace RJ (1999) Influence of sodium fumarate addition on rumen fermentation in vitro. Br J Nutr 81(1):59–64
Nisbet DJ, Martin SA (1990) Effect of dicarboxylic acids and Aspergillus oryzae Fermentation extract on lactate uptake by the ruminal bacterium Selenomonas ruminantium. Appl Environ Microbiol 56(11):3515
Nisbet DJ, Martin SA (1993) Effects of fumarate, l-malate, and an Aspergillus oryzae fermentation extract on d-Lactate utilization by the ruminal bacterium Selenomonas ruminantium. Curr Microbiol 26:133–136
Khafipour E, Krause DO, Plaizier JC (2009) A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J Dairy Sci 92(3):1060–1070
Herlemann DPR, Geissinger O, Ikeda-Ohtsubo W, Kunin V, Sun H, Lapidus A, Hugenholtz P, Brune A (2009) Genomic analysis of “Elusimicrobium minutum”, the first cultivated representative of the phylum “Elusimicrobia” (formerly termite group 1). Appl Environ Microbiol 75(9):2841–2849
Zheng H, Dietrich C, Radek R, Brune A (2016) Endomicrobium proavitum, the first isolate of Endomicrobia class. nov. (phylum Elusimicrobia)—an ultramicrobacterium with an unusual cell cycle that fixes nitrogen with a Group IV nitrogenase. Environ Microbiol 18(1):191–204
Bailey SR, Baillon ML, Rycroft AN, Harris PA, Elliott J (2003) Identification of equine cecal bacteria producing amines in an in vitro model of carbohydrate overload. Appl Environ Microbiol 69:2087–2093
O’Herrin SM, Kenealy WR (1993) Glucose and carbon dioxide metabolism by Succinivibrio dextrinosolvens. Appl Environ Microbiol 59(3):748–755
Mao S, Zhang M, Liu J, Zhu W (2015) Characterising the bacterial microbiota across the gastrointestinal tracts of dairy cattle: membership and potential function. Sci Rep UK 5:16116
Stanton TB (1984) Glucose metabolism of Treponema bryantii, an anaerobic rumen spirochete. Can J Microbiol 30(5):526–531
Anderson KL (1995) Biochemical analysis of starch degradation by Ruminobacter amylophilus 70. Appl Environ Microbiol 61(4):1488–1491
Acknowledgements
The present study was supported by the Natural Science Foundation of Jiangsu Province of China (BK20151431) and the Natural Science Foundation of China (31372339).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jin, W., Xue, C., Liu, J. et al. Effects of Disodium Fumarate on In Vitro Rumen Fermentation, The Production of Lipopolysaccharide and Biogenic Amines, and The Rumen Bacterial Community. Curr Microbiol 74, 1337–1342 (2017). https://doi.org/10.1007/s00284-017-1322-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-017-1322-y