Abstract
CodY is a pleiotropic regulator commonly found in Gram-positive bacteria and regulates various biological processes during the stringent response in a nutrient-limiting environment. CodY also participates in virulence factor expression in many low G+C Gram-positive pathogens, as observed in Bacillus anthracis. However, the mechanism by which B. anthracis CodY regulates metabolism and virulence factors in response to environmental changes is unclear. Here, we attempted to identify the link between CodY and B. anthracis regulation with codY-deficient and codY-overexpressing mutants using high-throughput transcriptional analysis. Growth pattern analyses of codY mutants in both rich and minimal media showed defects in early cell proliferation, with opposite patterns in the early stationary phase: CodY overexpression prolonged bacterial growth, whereas deletion inhibited growth. RNA sequencing of codY-deficient B. anthracis showed both positive and negative changes in the gene expression of proteases and virulence factors as well as genes related to stringent response-related metabolism and biosynthetic processing. We also found that changes in codY expression could alter virulence gene expression of B. anthracis, suggesting modes of regulation in its virulence in a CodY concentration-dependent manner. Collectively, we conclude from these results that CodY can both positively and negatively regulate its regulon via direct and/or indirect approaches, and that its mode of regulation may be concentration dependent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over its life cycle, a microbe is exposed to various conditions that hinder normal growth, such as nutrition depletion and suboptimal temperatures, and must adapt to survive. An important microbial adaptation mechanism is the stringent response, which can be observed in various bacteria, including Gram-positive bacteria. The stringent response involves activation of a diverse set of promoters activated at the beginning of the stationary phase to enhance viability and adapt to an unfriendly environment [1, 2]. In Firmicutes, a global transcription repressor, CodY, plays an essential role in the stringent response. The regulatory function of CodY is dictated by two effector molecules: the branched-chain amino acids (BCAAs) and guanosine triphosphates (GTPs). BCAA- and GTP-bound CodY binds to specific motifs, which leads to transcriptional repression [3]. A reduction in the levels of GTP and BCAA releases CodY from its binding site, enabling the transcription of stringent response genes [4]. In B. subtilis, CodY has been shown to play a role in regulating metabolism, biomolecule synthesis, transcription, and translation [4, 5].
In addition to its role in regulating metabolism, CodY contributes to the virulence of pathogenic Gram-positive bacteria [6]. Several studies have shown that CodY plays a significant regulatory role in virulence factor expression in organisms such as Clostridium botulinum [7], Streptococcus pyogenes [8, 9], and B. anthracis [10]. B. anthracis produces unique virulence factors—namely, a tripartite toxin and a poly-γ-D-glutamate capsule [11, 12]. These virulence factors are expressed at high levels at the onset of the stationary phase, in which the stringent response is activated. CodY is known to affect mRNA expression of the tripartite anthrax toxin and the accumulation of the activator AtxA, ultimately regulating the virulence of B. anthracis [10].
In this study, we used RNA-seq to analyze the global transcription regulated by CodY and to identify genes affected by CodY deregulation. Transcriptomic data revealed expression changes in metabolism- and translation-related genes. The levels of potential virulence factors in B. anthracis were also correlated with CodY deregulation. Supporting previous reports, we identified expression patterns of genes with putative CodY-binding sites. The data presented here provide a global view of CodY regulation in gene transcription through interaction with other regulators and the level of its active form.
Materials and Methods
Bacterial Strains, Plasmids, and Media
The bacterial strains created and used in this work are listed in Table S1. All E. coli strains were grown in Luria-Bertani medium [13] supplemented with ampicillin (200 μg/ml), kanamycin (Km, 50 μg/ml), erythromycin (Em, 100 μg/ml), or tetracycline (Tet, 20 μg/ml) when necessary. All B. anthracis strains were grown in brain heart infusion (BHI) medium, modified G medium (MGM) [14], or Ristroph medium supplemented with sodium bicarbonate (R + CO2) [15] with the following antibiotics when needed: Em (5 μg/ml), Km (100 μg/ml), Tet (20 μg/ml), or chloramphenicol (Cm, 50 μg/ml).
Construction of B. anthracis Mutant and Complementation Strains
The primers used in this work are listed in Table S2. The CodY-overexpressing B. anthracis strain HYC was constructed as follows. A full-length open reading frame (ORF) of codY was amplified using primers CodYORF-F and CodYORF-R. The amplified fragment was then inserted into pHT08 that had been digested with BamHI and XbaI (Fig. S1a). The resulting plasmid pHT-HisCodY was transformed into the DNA methylase-null E. coli strain ER2925 for unmethylated plasmids and then transformed into B. anthracis Sterne 34F2 by electroporation using the method described previously [16] with modifications in electroporation pulse setting (2.5 kV, 25 μF, 100 Ω). For its empty vector control strain SpHT, pHT08 was transformed into B. anthracis Sterne.
The deletion mutant B. anthracis strain BCD22 was constructed by replacing the codY gene open reading frame with a kanamycin resistance cassette (Fig. S2a). A gene fragment containing 500 bp upstream of codY and another fragment containing 500 bp downstream of codY were amplified with primer sets 500CodYUP and 500CodYDN, respectively. The upstream fragment was inserted into pKS1 that had been digested with EagI and PstI, and the downstream fragment was inserted after digesting with HindIII and KpnI. The constructed plasmid was designated as pKSCodY, and it was introduced into B. anthracis Sterne. Replacement of the codY ORF was performed according to the method described previously [17] with modifications in the usage of antibiotics. The loss of codY was confirmed by sequencing and immunoblotting using an anti-CodY antibody (Fig. S2d).
Complementation for CodY was achieved as follows (Fig. S3a): the shuttle vector pHY300PLK and a PCR fragment amplified with primers CompleCY-F and CompleCY-R were digested with BamHI and EcoRI and ligated, resulting in complementation vector pHYCompCodY. The plasmid was electroporated into the codY-deficient strain BCD22, resulting in a CodY-complemented strain, BCC7. CodY expression was confirmed by immunoblotting.
Protein Techniques
Secreted proteins from the parental Sterne and the mutant strains were collected using trichloroacetic acid (TCA) precipitation as follows: Cells were grown in BHI overnight, inoculated into fresh R + CO2 medium, and grown to the late-exponential growth phase (OD600 = 1.4–1.5). Samples were centrifuged to separate the supernatant from cells and further filtered to remove any potential spores. Filtered media were supplemented with TCA (final 10 %) and kept at 4 °C overnight. Protein pellets were collected by extensive centrifugation, washed with ice-cold acetone four times, and dried to remove all residual acetone. Dried samples were subjected to sodium dodecyl sulfate polyacrylamide electrophoresis (SDS-PAGE) and Western blotting. Antibodies used here are antilethal factor (ab13814; Abcam, Cambridge, United Kingdom), antiedema factor (ab21267; Abcam), and antiprotective antigen (ab1988; Abcam).
RNA Techniques
RNA samples from the wild-type and the mutant B. anthracis Sterne strains were extracted as follows: cells were grown in BHI overnight, inoculated into fresh R + CO2 media, and grown until they reached the late-exponential phase (OD600 = 1.4–1.5). Cells were collected by centrifugation and homogenized using RNAiso Plus (TaKaRa BIO), and RNA samples were purified according to the manufacturer’s instructions. Extracted RNA with purity (A 260/A 280) more than 1.9 was subjected to either ribosomal RNA (rRNA) depletion for RNA-seq or cDNA synthesis for quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR).
rRNAs from RNA samples were removed using the RiboMinus™ Transcriptome Isolation Kit (Invitrogen, Carlsbad, CA). These depleted RNA samples were used to construct paired-end transcriptome libraries using the NEBNext® Ultra™Directional RNA library Prep Kit for Illumina® following the manufacturer’s instructions (New England BioLabs (NEB), Ipswich, MA). Two biological replicates were prepared from each condition and were sequenced using HiSeq® 2500 (Illumina, San Diego, CA, USA). The raw reads were deposited in the Small Read Archive repository at NCBI (http://www.ncbi.nlm.nih.gov/sra) under the study accession number SRP051683.
First-strand cDNAs for qRT-PCR were synthesized using SuperScript II Reverse Transcriptase (Invitrogen). qRT-PCR was performed with synthesized cDNAs, SYBR® Premix Ex Taq™ II (TaKaRa BIO), and the appropriate primers (Table S2) using the ABI 7500 Real-Time PCR system (Applied Biosystems, Carlsbad, CA, USA) as previously described [18]. The threshold cycle (C t) of each gene was normalized to that of the housekeeping gene gyrB [19, 20]. The relative expression differences were calculated using the \( 2^{{ - \Delta \Delta C_{\text{t}} }} \) method [21].
Growth Pattern Analysis
Wild-type and mutant cells were grown in BHI overnight and inoculated into fresh BHI, MGM, or R + CO2 media (OD600 = 0.01), and grown at 37 °C for 24 h. For codY overexpression, IPTG was added at a final concentration of 1 μg/ml at two time points: early- and late-exponential growth phases. Absorbance was measured every hour in triplicate; a representative graph is shown in Fig. 1.
Growth pattern analysis of codY-mutated B. anthracis Sterne. a Growth patterns of SpHT (red circle, without IPTG; green triangle, with IPTG) and HYC (white square, without IPTG; blue rhombus, with IPTG) cultured in BHI (left), MGM (middle), or in R+CO2 (right) measured at the noted time periods. b Growth patterns of Sterne (white square) and BCD22 (black triangle) cultured in BHI (left), MGM (middle), or in R + CO2 (right) measured for noted time periods. Black arrows indicate the time point of sample collection. The experiments were performed in triplicate, and representative graphs are shown
Data Analysis
The expression profiling of the B. anthracis parental and mutant strains was determined using Rockhopper version 1.30 according to the author’s instructions [22]. Genes were considered differentially expressed, if the q-value was equal to or below 0.05 (q < 0.05) and if the log2-fold change was greater than 1 or less than −1. Differentially expressed genes (DEGs) from the dataset were functionally annotated using Database for Annotation, Visualization and Integrated Discovery (DAVID) version 6.7 [23] and the Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway version 72.1 [24, 25]. The quantitative PCR data were statistically analyzed using SPSS 21.0 (SPSS Inc., Chicago, IL). All data were tested in triplicate and presented as the mean ± standard error. The data were tested by one-way ANOVA followed by Tukey’s HSD post hoc test.
Results
CodY Deregulation Leads to Growth and Virulence Factor Expression Defects in B. anthracis Sterne
To determine the effect of CodY deregulation in B. anthracis, we constructed a B. anthracis Sterne strain (HYC) that overexpresses histidine hexamer-tagged (6XHis) CodY (Fig. S1a). Interestingly, overexpression of 6XHis-CodY led to the inhibition of toxin component expression, as tripartite toxin expression was greatly inhibited (Fig. S1b). We also constructed a codY-null B. anthracis mutant (BCD22) by introducing a kanamycin resistance cassette in place of the codY open reading frame, completely replacing it with the antibiotic marker (Fig. S2a). The loss of codY was confirmed by PCR and by immunoblotting against CodY and anthrax toxin components (Fig. S2b, c, d), as previous reports demonstrated that deletion of codY disrupts anthrax toxin production [10].
We then assessed the changes in growth due to CodY deregulation in various media. In rich BHI medium, early induction of 6XHis-CodY in the HYC strain delayed its growth (Fig. 1a, left panel), yet induction at the entry to stationary phase had no significant effects (data not shown). Delayed growth initiation was also observed in the codY-deficient mutant in BHI medium but the inhibition was minor (Fig. 1b, left panel). Under nutrient-limiting conditions, such as in MGM and R + CO2, both mutant B. anthracis strains showed diminished growth compared with their control strain (Fig. 1). HYC with or without IPTG induction showed significant growth defects in MGM, requiring up to 40 h for growth. These growth patterns showed that both overexpression and deletion of CodY may affect the phenotype of B. anthracis.
Deletion of codY Affects the Transcription of Genes for Metabolic Regulation, Precursor Biosynthesis, and Virulence
To obtain a global view of B. anthracis gene expression in a host-mimicking environment, we performed RNA-seq with the wild-type and mutant Sterne strains grown in R + CO2 at 37 °C. Samples were taken during the late-exponential phase [absorbance at 600 nm (OD600) = 1.4–1.5] when anthrax toxin production is at its peak. For the HYC strain, the expression profiles were omitted, because the quality of the raw reads and processed RNA-seq data were too poor to identify any significant changes. As for BCD22, a total of 405 genes were differentially expressed, with 83 genes upregulated and 322 genes downregulated compared with the parental strain (Table S3). A large portion of the upregulated genes were hypothetical, whereas the majority of downregulated genes were predicted to be membrane transport proteins (BAS0581, BAS3988), metabolism-related proteins (BAS4420, BAS4661), cell wall and spore coat proteins (BAS0579, BAS1287, BAS2359), and potential virulence factors (BAS0386, BAS1812).
To address the function of the genes and pathways affected by codY deletion, DEGs from the RNA-seq data were further analyzed using DAVID and KEGG pathway analysis. Although numerous genes were upregulated, no significant correlation or common pathways between these genes were found. Several upregulated genes related to the stress response and membrane transporters were found, indicating that codY deletion resulted in metabolic stress. Downregulated genes affected by the knockout were related to metabolic processes, such as glycolysis, the transport of peptides and amino acids, the tricarboxylic acid cycle, oxidative phosphorylation, and pyruvate metabolism (Table 1), similar to that of a previous transcriptomic study using B. anthracis Sterne 7702 [10]. Other downregulated cellular processes were closely related to the synthesis of biomolecules, such as purine, pyrimidine, and various amino acids.
Validation of RNA-Seq Using qRT-PCR
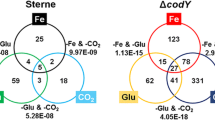
To confirm the differential expression of genes affected by codY deletion, we selected genes from RNA-seq and measured their mRNA expression using qRT-PCR. We measured the expression levels of two highly regulated genes in the transcriptomic profiles. The expression pattern of selected genes from RNA-seq showed a correlation with qRT-PCR values (Fig. 2).
Confirmation of transcriptomic and secretomic profiling of codY-deficient B. anthracis using qRT-PCR. Expression patterns of DEGs selected from transcriptome profiling data of Sterne (white) and BCD22 (gray) measured using qRT-PCR performed in triplicate. The values are presented as the means ± standard error. The asterisk symbols represent significance of the presented data determined by one-way ANOVA followed by Tukey’s HSD post hoc test (**P < 0.01)
Discussion
In this work, we studied the role of CodY in B. anthracis in a host-mimicking environment using high-throughput sequencing. Although we only performed biological duplicate experiments, we addressed this issue by limiting genes with fold-change values and triplicate qRT-PCR confirmation to determine genes with meaningful expression changes. Poor quality of the strain HYC RNA-seq may have been caused by excessive repression by overexpressed CodY. The whole transcriptomic profiling of codY-deficient B. anthracis revealed several putative genes that were suggested as potential virulence factors. BAS1812, a putative NlpC/P60 cell wall peptidase, is considered to be involved in the hydrolysis of peptidoglycan during bacterial proliferation [26]. It has been previously identified as an immunogenic protein in host system [27]. Several NlpC/P60 genes were identified to be involved in host invasion by L. monocytogenes [28], suggesting that BAS1812 may be involved in survival and pathogenesis during infection. BAS0386 (yceD), a stress response gene from the tellurite resistance operon yceCDEFGH, showed significant changes in expression without CodY-binding motifs. It was reported previously that reduced virulence and increased sensitivity to host defense mechanisms were observed in yceGH-deficient B. anthracis in a Caenorhabditis elegans model [29]. The results from this work indicate that yceD expression was reduced by codY deletion and that no CodY-binding sites were identified [30], suggesting the intervention of other regulators that are controlled by CodY in yceD regulation.
CodY generally acts as a transcriptional repressor to its target genes, binding to their promoters and/or within genes to block RNA polymerase binding. Our sequencing data, however, showed that CodY largely activates transcription of several enzymes and transcriptional factors, both with and without CodY-binding motifs. This complex transcriptional changes caused by codY disruption may be due to several factors: Firstly, changes in expression of CodY-regulated transcriptional regulators may have affected the transcriptome. One example is CodY-dependent accumulation of anthrax toxin activator AtxA [10]. As various putative regulators were shown to be depressed by codY deletion in this study, it is likely that CodY positively regulates gene regulation indirect approaches. Secondly, gene depression in BCD22 may reflect its overall growth defect in R + CO2 medium, which is highly related to other regulatory effects in B. anthracis. Third, the reduction in nutrient and biomolecules toward the end of exponential growth may hamper gene expression, which could be further exacerbated by codY deletion. CodY plays a role as a central metabolic regulator in biomolecule precursor synthesis and starvation-related metabolic pathways [5, 31]. Deregulation in the tricarboxylic acid cycle, amino acid biosynthesis processes, and oligopeptide transporters by codY mutation could have reduced the availability of amino acids, hindering transcription and/or translation of various proteins required for proper gene expression. Finally, it is possible that CodY has a positive regulatory function during growth transition that is overshadowed by its defined negative regulatory role. Recently, CodY’s role as a positive regulator has been addressed in B. subtilis ackA regulation [32], and in transcriptome profiles of BCAA-starved Listeria monocytogenes [33], supporting our findings in B. anthracis. However, its mechanism for positive regulation is yet unclear.
One of the interesting findings of this work was that aberrations in codY expression could change the phenotypes of B. anthracis, particularly virulence factor expression. Decrease in expression of anthrax toxin components were observed in both codY overexpression and null B. anthracis (Figs. S1 and S2). Change in virulence factor expression by CodY expression may imply that the level of intracellular CodY concentration determines the mode of regulation, at least for anthrax toxin components. In the process of anthrax toxin expression, B. anthracis CodY interacts with the atxA promoter region [30] and positively regulates the posttranslational accumulation of AtxA, as its deletion study showed no significant impact on the atxA transcription [10]. Based on the previous and the current observations, it is tempting to speculate that CodY acts as a weak repressor for unknown regulatory machinery that positively regulates the atxA expression. During rapid growth, multiple active CodY molecules interact with the atxA promoter and inhibits the atxA transcription, possibly by blocking RNA polymerase. However, this CodY binding would enhance recruitment of regulatory complex to the atxA promoter region, forming a poised promoter. After reaching stationary growth phase or upon exposure to nutrient-limiting environment, inactive CodY molecules detach from the atxA promoter region, allowing the atxA gene expression. As for codY deletion, atxA gene transcription is not affected, but its posttranslational accumulation is hampered by proteases (or proteasomes) that would be repressed by CodY. As for CodY overexpression, excessive CodY binding may have impeded transcription machinery that would inhibit atxA transcription. This premise, however, requires validation on the topological effect of CodY on B. anthracis chromosome, identification of the atxA expression activator(s) and enzyme(s) that would target and degrade AtxA.
In this work, we described the effect of codY mutation on global gene regulation of B. anthracis, and suggested that CodY may positively regulate its regulon via direct and indirect approaches. We also surmised that different levels of active CodY could influence the onset of virulence. As a pleiotropic regulator, CodY has a global regulatory impact on various biological processes, and disruption of its regulatory activity could cause substantial metabolic stress in the organism, curtailing its ability to adapt in environments with limited resources. The pathogenic phenotype in B. anthracis is also a CodY-mediated process, yet the exact mechanism remains unclear. Further studies of CodY-regulated transcription factors and proteases that could affect toxin activation might provide information for understanding the complex regulation in anthrax and novel therapeutic target for anthrax prophylaxis.
References
Barker MM, Gaal T, Josaitis CA, Gourse RL (2001) Mechanism of regulation of transcription initiation by ppGpp. I. Effects of ppGpp on transcription initiation in vivo and in vitro. J Mol Biol 305(4):673–688. doi:10.1006/jmbi.2000.4327
Barker MM, Gaal T, Gourse RL (2001) Mechanism of regulation of transcription initiation by ppGpp. II. Models for positive control based on properties of RNAP mutants and competition for RNAP. J Mol Biol 305(4):689–702. doi:10.1006/jmbi.2000.4328
Belitsky BR (2011) Indirect repression by Bacillus subtilis CodY via displacement of the activator of the proline utilization operon. J Mol Biol 413(2):321–336. doi:10.1016/j.jmb.2011.08.003
Ratnayake-Lecamwasam M, Serror P, Wong K-W, Sonenshein AL (2001) Bacillus subtilis CodY represses early-stationary-phase genes by sensing GTP levels. Genes Dev 15(9):1093–1103. doi:10.1101/gad.874201
Sonenshein AL (2007) Control of key metabolic intersections in Bacillus subtilis. Nat Rev Micro 5(12):917–927
Sonenshein AL (2005) CodY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr Opin Microbiol 8(2):203–207. doi:10.1016/j.mib.2005.01.001
Zhang Z, Dahlsten E, Korkeala H, Lindström M (2014) Positive regulation of botulinum neurotoxin gene expression by CodY in Clostridium botulinum ATCC 3502. Appl Environ Microbiol 80(24):7651–7658. doi:10.1128/aem.02838-14
Malke H, Ferretti JJ (2007) CodY-affected transcriptional gene expression of Streptococcus pyogenes during growth in human blood. J Med Microbiol 56(Pt 6):707–714. doi:10.1099/jmm.0.46984-0
Malke H, Steiner K, McShan WM, Ferretti JJ (2006) Linking the nutritional status of Streptococcus pyogenes to alteration of transcriptional gene expression: the action of CodY and RelA. Int J Med Microbiol 296(4–5):259–275. doi:10.1016/j.ijmm.2005.11.008
van Schaik W, Chateau A, Dillies MA, Coppee JY, Sonenshein AL, Fouet A (2009) The global regulator CodY regulates toxin gene expression in Bacillus anthracis and is required for full virulence. Infect Immun 77(10):4437–4445. doi:10.1128/iai.00716-09
Moayeri M, Leppla SH (2004) The roles of anthrax toxin in pathogenesis. Curr Opin Microbiol 7(1):19–24. doi:10.1016/j.mib.2003.12.001
Mock M, Fouet A (2001) Anthrax. Annu Rev Microbiol 55:647–671. doi:10.1146/annurev.micro.55.1.647
Bertani G (1951) Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol 62(3):293–300
Kim HU, Goepfert JM (1974) A sporulation medium for Bacillus anthracis. J Appl Bacteriol 37(2):265–267
Ristroph JD, Ivins BE (1983) Elaboration of Bacillus anthracis antigens in a new, defined culture medium. Infect Immun 39(1):483–486
Koehler TM, Dai Z, Kaufman-Yarbray M (1994) Regulation of the Bacillus anthracis protective antigen gene: CO2 and a trans-acting element activate transcription from one of two promoters. J Bacteriol 176(3):586–595
Shatalin KY, Neyfakh AA (2005) Efficient gene inactivation in Bacillus anthracis. FEMS Microbiol Lett 245(2):315–319. doi:10.1016/j.femsle.2005.03.029
Choi MR, Jung KH, Park JH, Das ND, Chung MK, Choi IG, Lee BC, Park KS, Chai YG (2011) Ethanol-induced small heat shock protein genes in the differentiation of mouse embryonic neural stem cells. Arch Toxicol 85(4):293–304. doi:10.1007/s00204-010-0591-z
Tu WY, Pohl S, Summpunn P, Hering S, Kerstan S, Harwood CR (2012) Comparative analysis of the responses of related pathogenic and environmental bacteria to oxidative stress. Microbiology 158(3):636–647. doi:10.1099/mic.0.057000-0
Pflughoeft KJ, Sumby P, Koehler TM (2011) Bacillus anthracis sin locus and regulation of secreted proteases. J Bacteriol 193(3):631–639. doi:10.1128/JB.01083-10
Baik SY, Jung KH, Choi MR, Yang BH, Kim SH, Lee JS, Oh DY, Choi IG, Chung H, Chai YG (2005) Fluoxetine-induced up-regulation of 14-3-3zeta and tryptophan hydroxylase levels in RBL-2H3 cells. Neurosci Lett 374(1):53–57. doi:10.1016/j.neulet.2004.10.047
McClure R, Balasubramanian D, Sun Y, Bobrovskyy M, Sumby P, Genco CA, Vanderpool CK, Tjaden B (2013) Computational analysis of bacterial RNA-Seq data. Nucleic Acids Res 41(14):e140. doi:10.1093/nar/gkt444
Huang DW, Sherman BT, Lempicki RA (2008) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4 (1):44–57. http://www.nature.com/nprot/journal/v4/n1/suppinfo/nprot.2008.211_S1.html
Kanehisa M, Goto S, Sato Y, Kawashima M, Furumichi M, Tanabe M (2014) Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res 42(Database issue):D199–D205
Kanehisa M, Goto S (2000) KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28(1):27–30
Anantharaman V, Aravind L (2003) Evolutionary history, structural features and biochemical diversity of the NlpC/P60 superfamily of enzymes. Genom Biol 4(2):R11
Chitlaru T, Altboum Z, Reuveny S, Shafferman A (2011) Progress and novel strategies in vaccine development and treatment of anthrax. Immunol Rev 239(1):221–236. doi:10.1111/j.1600-065X.2010.00969.x
Schmidt RL, Filak HC, Lemon JD, Potter TA, Lenz LL (2011) A LysM and SH3-domain containing region of the Listeria monocytogenes p60 protein stimulates accessory cells to promote activation of host NK cells. PLoS Pathog 7(11):e1002368. doi:10.1371/journal.ppat.1002368
Franks SE, Ebrahimi C, Hollands A, Okumura CY, Aroian RV, Nizet V, McGillivray SM (2014) Novel role for the yceGH tellurite resistance genes in the pathogenesis of Bacillus anthracis. Infect Immun 82(3):1132–1140. doi:10.1128/iai.01614-13
Château A, van Schaik W, Joseph P, Handke LD, McBride SM, Smeets FMH, Sonenshein AL, Fouet A (2013) Identification of CodY targets in Bacillus anthracis by genome-wide in vitro binding analysis. J Bacteriol 195(6):1204–1213. doi:10.1128/jb.02041-12
Geiger T, Wolz C (2014) Intersection of the stringent response and the CodY regulon in low GC Gram-positive bacteria. Int J Med Microbiol 304(2):150–155. doi:10.1016/j.ijmm.2013.11.013
Shivers RP, Dineen SS, Sonenshein AL (2006) Positive regulation of Bacillus subtilis ackA by CodY and CcpA: establishing a potential hierarchy in carbon flow. Mol Microbiol 62(3):811–822. doi:10.1111/j.1365-2958.2006.05410.x
Lobel L, Herskovits AA (2016) Systems level analyses reveal multiple regulatory activities of CodY controlling metabolism, motility and virulence in Listeria monocytogenes. PLoS Genet 12(2):e1005870. doi:10.1371/journal.pgen.1005870
Acknowledgments
We thank Dr. Konstantin Shatalin for providing an integration vector pKS1, and Dr. Abraham Sonenshein for anti-CodY antibody. This research was supported by Global Ph. D. Fellowship Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2012-003722), NRF Grant funded by Korean Government (MSIP 2011-0030049) and Agency of Defense Development (UC13002320).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, S.K., Jung, K.H., Yoon, S.N. et al. Late-Exponential Gene Expression in codY-Deficient Bacillus anthracis in a Host-Like Environment. Curr Microbiol 73, 714–720 (2016). https://doi.org/10.1007/s00284-016-1120-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-016-1120-y