Abstract
Growing evidence suggests that components of the innate immune system play a crucial role in regulating metabolic homeostasis. Macrophages were the primary immune cells to be described in both the white adipose tissue and the pancreatic islets. Therein, their functions, beneficial or detrimental, are extending under steady state and in the context of obesity-induced type 2 diabetes. Other populations, including innate lymphoid cells, are emerging as key sentinels of metabolic tissues and privileged partners of macrophages. The present review will thus explore the phenotype and the role of innate immune cells in metabolic physiology and dysfunction. Discussion will tackle pending questions and future perspectives in the field of immunometabolism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In physiology, immune cells reside in all organs, including metabolic tissues. The immune system is now recognized as a major regulator of metabolism, a function not related to classical immune responses of defense. As the major immune cells inside metabolic tissues, macrophages remain the most important cells in these novel immunometabolic interactions. At steady state, they represent around 30–50% of total immune cells in the white adipose tissue and up to 80–90% in the pancreatic islets, depending on the mouse strains. In the liver, macrophages predominate as Kupffer cells a specialized macrophage population, which role in liver physiology and pathology has been reviewed elsewhere [1].
For two decades now, experimental and clinical data have clearly established that intra-abdominal white adipose tissue is a site of inflammation during obesity and type 2 diabetes. Since the seminal discovery of increased production of tumor necrosis factor (TNF)-α in adipose tissue of obese mice, specific upregulation of genes encoding inflammatory factors have become key features of enlarged adipose tissue [2]. This local inflammation is associated with a marked accumulation of macrophages within the adipose tissue, especially in the intra-abdominal depot, during the course of obesity [3, 4]. More recently, macrophages were also shown to reside inside pancreatic islets, wherein an inflammatory response develops during obesity and type 2 diabetes [5]. However, the events that initiate metabolic inflammation in these tissues are unclear and could involve different, but synergetic, mechanisms including cell death of hypertrophic adipocytes or insulin-secreting β cells, lipotoxicity and glucotoxicity, endoplasmic reticulum and oxidative stress, and local hypoxia [6, 7]. A link between inflammation, macrophage accumulation, and metabolic dysfunction is suggested, whether it is at the level of insulin resistance, i.e., impaired cell response to insulin, or β cell dysfunction, i.e., impaired insulin secretion. However, this association is more and more challenged. The alternative view proposes that immune cells beneficially contribute to the maintenance of tissue integrity and that macrophage accumulation may be one body’s protective mechanism to face metabolic stress. Consequently, the study of macrophage influence on metabolism during physiology and pathology is still a relevant question.
The present review will thus explore the phenotype and the role of innate immune cells in metabolic physiology and dysfunction, focusing on macrophages and their privileged immune partners, the newly described innate lymphoid cells (ILCs). Special attention will be given to the most up-to-date publications on innate immune regulation of the white adipose tissue and the pancreatic islet functions in mice. Discussion will tackle pending questions and future perspectives in the field of immunometabolism.
Innate immune cell phenotypes and interactions in metabolic tissues
Spectrum of macrophage activation
Macrophages are well known to be versatile cells that can adopt specialized functions at particular tissue locations and actively respond to the local microenvironment. In metabolism, macrophages were initially described based on the M1/M2 paradigm of macrophage activation, extrapolated from in vitro polarization studies [8]. Classically activated M1 macrophages, characterized by a pro-inflammatory secretory profile, are polarized by stimulation with bacterial stimuli (e.g., lipopolysaccharide) and type 1 immune cytokines such as interferon (IFN)-γ. These macrophages are pro-inflammatory, expressing cytokines such as TNF-α and interleukin(IL)-1β, and are typically associated with pathogen clearance. In contrast, type 2 immune signals such as IL-4 and IL-13 induce an alternatively activated M2 macrophage subset. They secrete regulatory factors such as IL-10 and transforming growth factor (TGF)-β and promote angiogenesis, tissue homeostasis, and wound healing. However, recent studies suggest that macrophages residing inside metabolic tissues do not recapitulate the M1/M2 classification in vivo. Macrophages may rather adopt a metabolic activation state during weight gain and that the term “macrophages” represent heterogeneous subpopulations that differ in their origin, tissue localization, and function. Moreover, macrophages are also highly involved in interacting with other innate immune cells, including innate lymphoid cells (ILCs) and eosinophils that coordinate their polarization and viability under steady state and during metabolic stress.
Macrophage heterogeneity in white adipose tissue
Since 2003, macrophages are known to reside inside adipose tissue and accumulate during the course of obesity through circulating monocyte recruitment or in situ proliferation [3, 4, 9, 10]. In mice, manipulation of the chemotactic monocyte chemoattractant protein (MCP)-1/C-C chemokine receptor (CCR)2 system affects macrophage recruitment and/or proliferation in some [10,11,12,13] but not all models [14, 15]. These observations leave debated the pathophysiological relevance of MCP-1 to promote adipose tissue inflammation. Adipose tissue macrophage number significantly correlates with adipocyte size and body mass in obese mouse and subjects [3]. In mice, adipose tissue macrophages (ATM) express pan macrophage markers including F4/80, cluster of differentiation (CD)64, and MERTK. Extensive studies have focused on defining the identity and phenotype of these cells under both the lean and obese conditions. Based on the expression of the M1 marker CD11c and the M2 marker CD206 mannose receptor, it was suggested that a switch from an anti-inflammatory M2 phenotype to a pro-inflammatory M1 activation state was occurring during weight gain [16, 17]. Therefore, macrophages are the major source of innate inflammatory factors including IL-1β and TNF-α in the visceral adipose tissue during obesity [18]. Indeed, selective ablation of CD11c+ cells leads to a decrease in adipose and systemic inflammation during high-fat feeding [19]. Yet, more studies have highlighted the heterogeneity and plasticity of adipose tissue macrophage phenotypes, further demonstrating that the M1/M2 paradigm may not be applicable to in vivo tissues [20,21,22]. Instead, macrophages from mouse and human obese adipose tissue adopt a distinct metabolic activation state in response to metabolic stimuli, i.e., high glucose, high insulin, and the saturated fatty acid palmitate [20]. This metabolic phenotype is characterized by upregulation of both intracellular glycolysis and oxidative phosphorylation, increased expression of pro-inflammatory factors including IL-1β and TNF-α in association with markers of lipid transport and storage such as the fatty acid translocase CD36, the cholesterol transporter Abca1, and the lipid droplet-associated protein Plin2 [20, 21].
In the context of obesity, insulin resistance in hypertrophic adipocytes promotes a continuous and excessive exposure of macrophages to lipid species. Therefore, ATM store surplus lipid flux, leading to lipid droplet formation and foam cell-like lipid-laden macrophages [23, 24]. These cells greatly accumulate during obesity at sites known as crown-like structures around dead or dying adipocytes. In response to increased adiposity, ATM activates a program of lysosomal biogenesis, associated with lipid accumulation and catabolism [25]. This metabolic program was initially associated with M2-like macrophage activation and suggests that a major function of ATM is to clear dead adipocyte lipid reservoir [26]. In parallel, ATM also express high levels of the very low-density lipoprotein (VLDL) receptor, potentiating their pro-inflammatory profile via the uptake of ceramides during diet-induced obesity [27].
To refine the phenotypes of ATM, Hill and colleagues performed single-cell RNA sequencing and identified the transmembrane glycoprotein CD9 and the monocyte marker Ly6C as novel macrophage markers in the adipose tissue [28]. Reconciling previous publications with their novel findings, they conclude that three main populations of macrophages co-exist within the adipose tissue. The CD11b+Ly6c+ freshly recruited macrophages accumulate in the adipose tissue during obesity in interstitial spaces and are associated with potential adipogenic functions. The F4/80lowCD64+Ly6c−CD9− macrophage population, expressing CD206, predominates in the lean adipose tissue but are maintained during obesity, hence recapitulating the phenotype of resident macrophages. Finally, the F4/80highCD64+Ly6c−CD9+ cells are pro-inflammatory ATM with a high intracellular lipid content contained in lysosomes, resembling the initially described CD11chigh macrophage population [28]. The transcriptional regulation of adipose tissue macrophage phenotype is reviewed by Venteclef and colleagues in this issue. As an example, we have shown that the transcription factor interferon regulatory factor (IRF)5 controlled the pro-inflammatory polarization of ATM during diet-induced obesity. Therefore, preventing IRF5-dependent M1 macrophage polarization prevented adipose tissue inflammation and promoted a type 2 immune regulatory environment during obesity [29].
In humans, macrophages were also shown to accumulate and proliferate in adipose tissue during obesity [10, 28]. Several groups have examined their phenotype and came to the conclusions that ATM, including those localized into crown-like structures, express a mixed of M1 and M2 markers including CD206, CD163, CD11c, and pro-inflammatory cytokines [30,31,32]. Adipose tissue lipid-laden macrophages were also found to be CD9+ and accumulate proportionally to body mass [28].
The surprising phenotype of pancreatic islet macrophages
Distributed within the exocrine pancreas, the pancreatic islets are micro-organs that are essential for glucose homeostasis. β cells are the major cellular component of islets. In response to glucose, they produce within seconds the amount of insulin required for optimal energy supply to the insulin-sensitive tissues. Innate immune cells also reside inside pancreatic islets and are mainly composed of macrophages in mice and humans under steady state [5, 33,34,35]. Islet macrophages originate from definitive hematopoiesis, strongly depend on colony stimulatory factor (CSF)-1, and are self-maintained by in situ proliferation [34, 36]. More than 20 years after their discovery, the phenotype of these cells remains unclear. Contrary to adipose tissue, islet macrophages mitigate the M2 versus M1 immune paradigm associated with metabolic protection and dysfunction, respectively. Indeed, macrophages of healthy islets constitutively express M1-like markers including CD11c and class II histocompatibility molecules (MHCII) while expressing high levels of IL-1β, TNF-α, and the transcription factor IRF5 [33, 34, 36]. Plus, they do not express M2-like markers such as CD206, in comparison to the neighboring stromal macrophages from the exocrine pancreas [34]. Islet-resident macrophages thus adopt an M1-like activated phenotype at steady state.
During obesity, pancreatic islets also undergo inflammation with elevated production of inflammatory cytokines and chemokines, such as IL-1β, TNF-α, and CCL2, contributing to β cell dysfunction and progression towards type 2 diabetes [37,38,39,40,41,42]. Local inflammation is associated with an increased number of islet macrophages in diet-induced or genetically obese rodents and in type 2 diabetic patients [5, 38, 40, 43, 44]. Two studies suggest the existence of different subsets of islet macrophages, differentiating the resident from the pro-inflammatory ones. The islet-resident CD11b+Ly6C− or F4/80highCD11clow macrophages predominate at steady state and the CD11b+Ly6C+ or F4/80lowCD11chigh macrophages accumulate inside islets during the course of obesity [38, 44]. While the CD11b+Ly6C+ macrophages are recruited from circulating monocytes, F4/80lowCD11chigh macrophages were shown to proliferate in situ. Furthermore, despite an increase in macrophage number, diet-induced obesity does not seem to markedly alter islet macrophage phenotype in opposition to ATM [34, 44]. Endocrine cells themselves, including β cells, are a source of inflammatory factors and may orchestrate islet inflammation. Indeed, RNA sequencing analyses comparing islet cells from type 2 diabetic patients and healthy controls revealed an important inflammatory signature associated with β cell dysfunction and described that not only immune cells but also endocrine cells fuel local inflammation [45, 46]. These results, sometimes contradictory, suggest that macrophages may not be solely responsible for islet inflammation during obesity, and more studies are warranted to fully define their phenotype in physiology and in pathology.
A metabolic reservoir of innate lymphoid cells
ILCs have emerged as an important family of innate immune cells over the past decade [47]. Being lymphocytes that do not express adaptive antigen receptors, ILCs are largely tissue-resident cells and react promptly to stress signals, secreting large amounts of effector cytokines. As such, ILCs play important roles in early inflammatory responses during infections, but also contribute to tissue development, function, protection, and repair processes. The ILC family is divided into three groups: group 1 (ILC1 and NK cells), group 2 (ILC2), and group 3 (ILC3) based on their transcriptional and secretory characteristics as well as the resemblance with their adaptive T helper (Th) cell counterparts [47]. While group 1 and 2 ILCs have been largely involved in metabolic homeostasis, group 3 ILCs have not yet been described.
Group 1 ILCs
Group 1 ILCs have been divided into two main populations, conventional NK cells and ILC1 cells, expressing the receptors NK1.1 and NCR1 (also known as NKp46 in humans). They are early potent producers of IFN-γ and are capable of carrying out major cytotoxic actions. As such, group 1 ILCs are important sentinels of the body that survey tissues for infected or tumor cells. Besides, they were shown to accumulate inside the white adipose tissue of obese mice compared to controls [48,49,50]. On contrary, one study have shown a decrease in group 1 ILC number during long-term diet-induced obesity and in human obese patients compared to controls [51]. Group 1 ILCs represent the second biggest innate immune populations in adipose tissue after macrophages, through local proliferation in an IL-12-dependent manner [48,49,50,51,52].
Obesity promotes the expansion of a specific myeloid gene-expressing NK cell subpopulation characterized by high expression of the receptors for IL-6 and CSF-1 [52]. During the course of high-fat diet, abdominal adipocytes increased expression of ligands for NCR1, a potent activating receptor on group 1 ILCs, stimulating them to produce IFN-γ [48]. Based on the expression of the transcription factor Eomes and the integrin CD49b (also known as DX5), adipose tissue group 1 ILCs were further classified as circulating DX5+Eomes+ mature NK cells, tissue-resident DX5−Eomes+ immature NK cells, and DX5−Eomes− specific ILC1 subset [50]. All three cell populations are increased in the adipose tissue during obesity, ILC1s being the most poised to produce IFN-γ. Since cytokines are required to activate macrophages, group 1 ILC–derived IFN-γ appears to be fundamental for the polarization of M1-like macrophages in the visceral adipose tissue during obesity. Indeed, adoptive transfers or depletion of group 1 ILCs show that these cells are necessary and sufficient to regulate CD11c+ M1-like macrophage number and activation [48,49,50,51, 53]. In return, adipose tissue CD11c+ macrophages may produce IL-12, further activating group 1 ILCs.
Recently, Boulenouar and colleagues proposed that group 1 ILCs display cytotoxic activity and control macrophage death, primarily M2 macrophages, at steady state. After engulfing dying adipocytes or other potential hazardous extracellular material, ATM may get killed by group1 ILCs, preventing the development of inflammation. In contrast, this property may be lost during chronic obesity, contributing to the increase in pro-inflammatory ATM number during weight gain [51]. In humans, adipose tissue group 1 ILCs also show an activated phenotype in adipose tissue of obese subjects compared to lean controls, accumulating preferentially in the intra-abdominal omentum compared to other adipose depots [51, 53].
Group 2 ILCs
Group 2 ILCs (ILC2s) are rare yet potent resident cells that mediate tissue protection and repair processes. ILC2s respond to epithelial signals such as the IL-1 family alarmin IL-33 and coordinate type 2 immune responses by producing type 2 cytokines including IL-5 and IL-13. While IL-5 is required for eosinophil accumulation, IL-13 is a major upstream regulator of M2-like macrophage polarization. It is now acknowledged that ILC2 reside in the white adipose tissue of lean mice and nonobese individuals [54, 55]. Notably, IL-33 is locally expressed in adipose tissue in fibroblast-like adventitial stromal cells, endothelial cells, or adipocyte precursors depending on the studies [56,57,58] (Dahlgren et al., Immunity, 2019). In contrast, adipose ILC2 number drastically declines during diet-induced obesity and in obese patients [54, 55]. Mechanistically, the loss of ILC2s in the obese adipose tissue may result from the conversion of plastic ILC2s into pro-inflammatory counterparts via IL-1β [59] or from repression of ILC2 function. Indeed, group 1 ILC-derived IFN-γ may repress ILC2 activation in the adipose tissue [57]. ILC2 were also shown to up-regulate the expression of the inhibitory receptor programmed death (PD)-1 while M1-like macrophages express its ligand PD-Ligand 1 during diet-induced obesity. Therefore, the engagement of PD-1 on ILC2s with PD-L1 on macrophages may destabilize ILC2 function, decreasing their IL-13 production and subsequently M2-like macrophage number [58]. Conversely, type 2 immune pathologic conditions, such as helminth infections, or recombinant IL-33 injections were shown to restore the number of ILC2s and downstream immune cells in the adipose tissue of obese mice [55, 60, 61].
Beside macrophages, pancreatic islets also contain other cells from specific branches of innate immunity, including ILCs [33]. While very few NK cells were detected, ILC2s were found to reside inside islets isolated from C57BL/6 and BALB/c mice under steady state and associated with local IL-33 production from islet mesenchymal cells. In diet-induced obese mice, both islet ILC2 number and IL-33 gene expression were found to be decreased compared to lean controls. Upon IL-33 activation, the number of ILC2s is also increased, inducing eosinophil and dendritic cell accumulation and polarizing neighboring myeloid populations into regulatory retinoic acid-producing cells [33].
Other innate immune cells
Beside the initial description of ATM, a major breakthrough in immunometabolism was the discovery of resident eosinophils located between adipocytes under steady state [62]. This paved the way to the study of the implication of type 2 immunity in metabolism. As downstream target of IL-5-secreting ILC2s, eosinophil number is decreased in adipose tissue during obesity [62]. Surprisingly, eosinophils seem to accumulate back in the adipose tissue beyond 16 weeks of high-fat feeding [63]. Other granulocytes including neutrophils and mast cells were also described in adipose tissue contributing to adipose tissue inflammation [64,65,66].
Less explored than macrophages, dendritic cells also reside inside adipose tissue. Both ATM and dendritic cells express CD11c and MHC-II. It has been difficult to clearly differentiate the phenotypes and roles of dendritic cells in adipose tissue from those of macrophages. Selective ablation of CD11c+ cells therefore impacts both the number of M1-like macrophages and dendritic cells [19]. Appropriate study of adipose tissue dendritic cells requires the absence of expression of the macrophage markers MERKT and CD64 on their surface [67]. In mice, dendritic cells (as defined by CD11chigh and F4/80low or CD64− cells) were shown to accumulate in the adipose tissue during obesity [68, 69]. Similarly, the presence of dendritic cells in the adipose tissue of obese patients correlates with the body mass. Adipose tissue dendritic cells were originally associated with the polarization of pro-inflammatory Th17 cells [69]. More recently, Macdougall and colleagues identified two subsets of adipose tissue dendritic cells. These cells show an anti-inflammatory phenotype under steady state, following activation of the β catenin and PPARγ pathways, which was impaired during a high-fat feeding. Indeed, deletion of these pathways in dendritic cells accelerated obesity-induced inflammation in the adipose tissue [70]. In pancreatic islets, a subset of dendritic cells was also identified in mice under steady state [33]. While it is clear that dendritic cells populate metabolic tissues, further studies are required to fully evaluate their contribution to the maintenance of metabolic homeostasis and during obesity-induced inflammation.
Immunoregulation of metabolic functions
A protective type 2 immune axis in adipose tissue homeostasis
Type 2 immune cells including ILC2s and eosinophils are enriched in the white adipose tissue under steady state but are lost during obesity. As IL-5 producer, ILC2s regulate the accumulation of adipose tissue-resident eosinophils [54]. Together ILC2s and eosinophils produce IL-13 and IL-4 which are upstream signals promoting M2-like macrophage polarization. Indeed, experimental loss of ILC2s and eosinophils decreased the number of M2-like ATM and favors glucose intolerance in obese mice [54, 62]. Thus, ILC2s are a prerequisite for the maintenance of adipose tissue M2-like macrophages and subsequent insulin sensitivity in physiology (Fig. 1). Alteration of the M2 polarization in macrophages by targeting transcriptional programs including IRF4, Trib1, and Kruppel-like factor-4 (KLF4) also predispose to developing obesity-induced insulin resistance and adipocyte lipolysis, and the progression toward type 2 diabetes [71,72,73]. However, a recent study showed that adipose tissue CD206+ M2-like macrophages create a microenvironment that inhibits differentiation of adipocyte progenitors and, thereby, impairs systemic insulin sensitivity [74]. The complete mechanisms by which adipose tissue M2 macrophages not only limit excessive type 1 inflammation but also influence metabolic homeostasis in adipose tissue require further study.
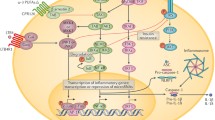
Interactions between adipose tissue-resident macrophages, innate lymphoid cells (ILCs), and adipocytes. At steady state, ILC2s and eosinophils coordinately promote M2-like macrophage polarization that predominate in the lean adipose tissue. These type 2 immune cells maintain adipocyte insulin sensitivity by the secretion of regulatory factors including TGF-β and IL-10 and yet unknown mechanisms. Upon sustained weight gain, adipocytes become hypertrophic, insulin resistant, hypoxic, and eventually apoptotic. In parallel, macrophages become metabolically activated (by high glucose, insulin, and palmitate concentrations) and stimulated by upstream group 1 ILCs (NK cells and ILC1s) secreting high amounts of IFN-γ. Macrophages produce pro-inflammatory factors such IL-1β and TNF-α and upregulate a lysosomal program to internalize and catabolize lipid species from moribund adipocytes. This is mediated by exocytosis in “lysosomal synapse” between the adipocyte and macrophages or the active release of triglyceride (TG)-filled vesicles by adipocytes. When required, group 1 ILCs may kill lipid-laden macrophages upon signs of inflammation. Group 1 ILC1s and metabolically activated macrophages both contribute to the loss of ILC2s during obesity, via IFN-γ secretion and upregulation of PDL1
In parallel, type 2 immunity has also been involved in cold-induced subcutaneous adipose tissue beiging. While the primary function of white adipose tissue is to store excess nutrients, beige adipose tissue aims at maintaining thermal homeostasis. Indeed, upon prolonged cold stress or adrenergic stimulation, de novo recruitment of beige adipocytes occurs mainly in the subcutaneous depot to support thermogenesis [75]. Cold-induced adipose tissue beiging was found to be dependent on the presence of ILC2s, eosinophils, and the IL-4/IL-13 pathway [55, 76]. Upon activation, ILC2s promote the proliferation of adipocyte precursors and their subsequent commitment to the beige fat linage [76]. ILC2s were also found to produce methionine-enkephalin peptides that can target adipocytes and favor beige fat formation [55]. Recombinant IL-33-driven activation of ILC2s is sufficient to promote adipose tissue beiging and whole-body glucose tolerance in lean and obese mice [33, 55]. Helminth parasitic infections that are also associated with high IL-33 production levels recapitulate the same metabolic phenotype [60,61,62]. Conversely, IL-33-deficient mice are prone to develop increased adiposity associated with decreased energy expenditure and impaired glucose tolerance [33, 55].
Thus, it is now acknowledged that type 2 immunity sustains metabolic homeostasis during physiology, and this protective environment is lost during weight gain [54, 55, 62]. Because beige adipocytes have the capacity to consume glucose to produce heat, it is likely that type 2 immunity-driven beiging mediates blood glucose-lowering effects, independently of insulin sensitivity. Furthermore, when type 2 immunity was promoted by myeloid deletion of IRF5, we observed that M2-like macrophages could orchestrate beneficial remodeling in the intra-abdominal adipose tissue in a TGF-β-dependent manner. This resulted in lipid partitioning in favor of healthy subcutaneous fat depots and an insulin-sensitizing effect despite obesity [29]. Altogether, these observations further confirm the capacity of type 2 immune cell subsets to actively regulate metabolism.
Macrophages as scavengers of adipocyte demise
White adipocytes store triglycerides in the form of a single large lipid droplet. During weight gain, adipocytes enlarge and eventually become insulin resistant and moribund. As a result, obesity promotes excessive release of triglycerides and nonesterified fatty acids in the extracellular milieu, through adipocyte lipolysis or death. ATM seem to play an active role in the clearance of lipids and adipocyte debris. As such, they preferentially encircle dead or dying adipocytes in crown-like structures [77, 78]. While CD11c+ M1-like macrophages accumulate in the adipose tissue from the first weeks of high-fat diet, the number of lipid-laden macrophages peaks when the frequencies of hypoxic and apoptotic adipocytes are the highest after 16 weeks of regimen [78,79,80]. In both human and mouse adipose tissue, CD11c+ macrophages were the main lipid-laden cells compared to CD11c− macrophages and dendritic cells [79]. Transgenic model of adipocyte apoptosis or caloric restriction-induced weight loss both led to increased lipid release and the concomitant influx of M2-like macrophages in the remnant adipose tissue [81, 82]. Conversely, alteration of macrophage capacities to recognize apoptotic cells impairs adipose tissue remodeling during diet-induced obesity and induces a massive M2-like macrophage accumulation [83]. Thus, the presence of ATM dynamically corresponds to adipocyte dysfunction and lipid surplus (Fig. 1).
Recent studies propose that ATM do not internalize lipids through standard endocytic mechanisms. Moreover, they seem to store lipid in vesicles and not typical lipid droplets like cholesterol-laden foam cells in atherosclerosis (Flaherty et al., Science, 2019). In response to increased adiposity, ATM activate a program of lysosome biogenesis associated with lipid uptake and catabolism but independently of their inflammatory status and autophagy [25, 84]. Such lysosomal pathway was shown to be essential for fatty acid mobilization and the M2 activation program in macrophages, questioning the in vivo phenotype of lipid-laden CD11c+ macrophages during obesity [26]. Inhibition of these lysosomal functions increased lipid storage in ATM and reduced the release of lipids from adipocytes. Indeed, ATM seem to actively participate in lipid liberation from the dying adipocytes. Macrophages located inside crown-like structures were shown to form an extracellular acidic hydrolytic compartment containing lysosomal enzymes delivered by exocytosis [80, 85]. In this “lysosomal synapse”, catabolism of the dead adipocyte begins allowing macrophages to internalize debris and lipid species from the adipocytes [85]. Indeed, loss of ATM increased the release of nonesterified fatty acids by adipocytes [25]. More specifically, inhibition of macrophage lysosomal exocytosis impaired macrophage lipid uptake and induced the accumulation of dead adipocytes in the adipose depot. This phenotype was observed after 16 weeks of high-fat feeding, when adipocytes undergo apoptosis, and not 8 weeks, amplifying local inflammation and metabolic dysfunction [80, 85]. Another study found that myeloid deletion of the neurolipin-1 (NRP1, the co-receptor for VEGF) blocked lipid internalization and β-oxidation in ATM. These macrophages shifted their metabolism towards a pro-inflammatory glycolitic profil, resulting in an exaggerated obese and glucose-intolerant mouse phenotype [86]. Thus, during adipose tissue expansion, macrophages upregulate a specific program of lysosomal exocytosis to internalize and catabolize adipocyte lipid surplus and extracellular material, preventing weight gain and ectopic lipid deposition.
Adipocytes also actively participate in supplying lipids to macrophages by releasing exosome-sized triglyceride-filled vesicles that are taken up intact by endocytosis (Flaherty et al., Science, 2019). These adipocyte-derived vesicles are increased during obesity and detectable in the circulation (Flaherty et al., Science, 2019). Thus, lipid internalization by ATM may represent a protective mechanism to prevent lipid species to target and activate adjacent immune cells, limiting adipose tissue inflammation during obesity. But it may also be an adipocyte-driven mechanism to locally control macrophage differentiation. Plus, if the intracellular lipid load triggers a maladaptive and inflammatory response in ATM, they might regulate “kill me” signals and program their own death with the help of cytotoxic group 1 ILCs [51].
Role of macrophages in adipose tissue angiogenesis
Increased adiposity requires a dynamic and sufficient adipose tissue vascularization, supporting adequate energy and oxygen supply to growing adipocytes or proliferating pre-adipocytes. Thus, healthy adipose tissue expansion relies on angiogenesis, i.e., when new blood vessels develop from those pre-existing within the tissue [87]. Conversely, inappropriate angiogenesis may lead to adipocyte dysfunction, local hypoxia, and inflammation, increasing the risks to develop insulin resistance and type 2 diabetes. ATM have been proposed to stimulate new capillary and vessel formation during the course of obesity. Indeed, they express numerous pro-angiogenic factors including matrix metalloproteases, platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF) [88,89,90]. Clodronate-derived depletion of macrophages or NRP1 deficiency in macrophages reduced the formation of adipose tissue blood vessels and inhibited local angiogenesis [86, 88]. This phenotype was associated with local hypoxia and inflammation and compromised insulin sensitivity. These studies further demonstrate that macrophage presence is required to license appropriate adipose tissue remodeling and expansion [86].
A possible link to obesity-induced insulin resistance
In animal models, a role for ATM in inducing insulin resistance has been demonstrated through diet-induced, genetic, or pharmacological manipulations of macrophage numbers in adipose tissue. In these studies, increased numbers of macrophages in adipose tissue have been associated with deteriorated glucose homeostasis [3, 4, 11,12,13]. Depletion of ATM using clodronate-loaded liposomes or the inducible CD11c knockout mice underlies that macrophages are mainly responsible for obesity-induced inflammation and impairment of insulin sensitivity and glucose tolerance [19, 63, 91, 92]. This association only holds true in the context of chronic diet-induced obesity since clodronate treatment did not have any effect after only 1 week of high-fat diet [92]. Most of pro-inflammatory factors secreted by M1-like ATM, including TNF-α and IL-1β, trigger insulin resistance in adipocytes by interfering with insulin signaling through activation of serine/threonine kinases [7]. Thus, preventing pro-inflammatory M1 polarization during obesity by targeting Irf5 in myeloid cells maintained glucose homeostasis in obese mice [29]. Similarly, selective Tnfa silencing in intra-abdominal ATM through administration of glucan shell-encapsulated siRNA significantly improved glucose tolerance in obese mice [93]. A study showed that ATM also secreted miRNA-containing exosomes that may target adjacent adipocytes and promote insulin resistance [94]. Thus, adipose tissue macrophage number, activation state, and secretory profile are key determinants of local inflammation and insulin sensitivity during increased adiposity (Fig. 1).
As upstream regulators of macrophage activation, ILCs orchestrate adipose tissue inflammation. On one side, decreased number and impaired function of ILC2s during obesity lead to diminished M2-polarizing IL-13 and eosinophil-derived IL-4 production in adipose tissue [54]. As already mentioned, in the absence of ILC2s or eosinophils, the number of M2-like macrophages is reduced in the adipose tissue while the glucose tolerance is markedly impaired in diet-induced obesity [54, 62]. On the other side, accumulation of NK cells and ILC1s is responsible for massive IFN-γ production, favoring an M1-like polarizing adipose milieu during weight gain. Ablation of NK cells or ILC1 in obese mice reduced the density of pro-inflammatory M1 macrophages in the adipose tissue and promoted metabolic homeostasis and inversely with adoptive transfer experiments [48,49,50, 52, 53]. Altogether, these results propose that adipose-resident group 1 ILCs are sufficient to contribute to obesity-associated insulin resistance through IFN-γ-mediated polarization of adipose tissue M1 macrophages. However, in humans, the pathological consequences of macrophage (or any other innate immune cell) infiltration into adipose tissue are more difficult to prove and are mainly based on correlative studies.
Innate immune priming of insulin secretion under steady state
Islet-resident macrophages harbor an M1-like activated phenotype at steady state. As such, their phenotype resembles barrier macrophages as characterized in the lung and the gastro-intestinal tract [95], questioning their physiological role in β cell function. In situ imaging of mouse islets showed that macrophages are in close contact with both β cells and blood vessels. Thanks to their long filopodia, resident macrophages can dynamically probe whole islet area [96]. They were shown to monitor β cell secretory activity by detecting endogenous levels of ATP via their purinergic receptors [97]. ATP is co-released with insulin in response to glucose and may trigger important processes in islet macrophages. It has long been recognized that acute but not chronic exposure of islets to IL-1β promotes insulin secretion in mouse and human islets [98, 99]. The underlying mechanisms are still unclear but may involve increased exocytosis subsequent to enhanced insulin granule docking at the plasma membrane [98]. Importantly, β cells have the highest expression of the signaling IL-1 receptor 1 (IL-1R1) of any other tissues, pointing to a physiological role of IL-1β in islet function [37, 100]. Using transgenic mouse models, two studies confirmed this hypothesis. Specific deletion of IL-1R1 on β cells impaired whole-body glucose tolerance via the reduction of glucose-stimulated insulin secretion [101]. Furthermore, feeding was found to induce a physiological rise in circulating IL-1β that potentiates postprandial insulin secretion [102]. In this context, macrophages from the peritoneal cavity produced IL-1β in response to bacterial products and glucose metabolism, acting back on the β cells [102]. As macrophages are likely to be the main source of IL-1β inside islets, it is not ruled out that islet-resident macrophages may also produce local IL-1β during this postprandial window. Thus, IL-1β at physiological doses appears to play a critical role in potentiating insulin secretion. Notably, islets express all members of the IL-1 regulatory system, highlighting the needs for subtle control of IL-1β signaling. As such, deletion of the IL-1R antagonist IL-1Ra in β cells disrupts β cell function and proliferation [103].
Pancreatic islets also produce IL-33 in mesenchymal cells. Both endogenous IL-33 expression and the presence of its target cell ILC2s inside islets were correlated with an optimal β cell insulin secretion in different mouse models [33]. Besides, IL-33 expression was increased in response to acute metabolic cues and streptozotocin-induced β cell death, proposing IL-33 as a new islet stress signal. Conversely, IL-33-deficient mice showed impaired glucose-induced insulin secretion. When activated by recombinant IL-33, islet-resident ILC2s license myeloid cells with retinoic acid-producing capacities and in turn, the retinoic acid signals to the β cells to promote insulin secretion. This islet IL-33/ILC2 axis may be a physiological pathway to preserve or restore islet secretory function in the context of acute metabolic stress. Notably, it is lost during chronic obesity, which may set the stage for type 2 diabetes development [33]. Overall, the IL-1 family appears to be an important physiological chaperone of β cell activities (Fig. 2). While macrophage-derived IL-1β potentiates insulin secretion, islet IL-33/ILC2-mediated type 2 immune responses promote β cell adaptation to acute metabolic stress and β cell injury.
Islet macrophage regulation of insulin secretion. Under steady state, islet macrophages harbor an M1-like phenotype with high IL-1β expression. Upon feeding, glucose and bacterial products trigger IL-1β release from macrophages. IL-1β then signals to the β cells via the IL-1R1 to potentiate insulin secretion. In turn, islet macrophage may get activated by β cell-derived insulin and ATP secretion. Upon acute metabolic stress, the IL-33/ILC2 circuit is activated and polarizes islet-resident macrophages to produce retinoic acid in an IL-13- and GM-CSF-dependent manner. In turn, retinoic acid signals to the β cells and stimulates insulin secretion. This loop is lost during chronic stress such as obesity. During obesity, macrophages accumulate inside islets and contribute to β cell dysfunction, eventually leading to type 2 diabetes. Macrophages impair glucose-stimulated insulin secretion by unclear cell-cell interactions and by internalizing β cell-derived insulin secretory granules. In parallel, islet macrophages also promote β cell proliferation to ensure adequate β cell mass
Dual role of islet macrophages during obesity
Chronic obesity-induced type 2 diabetes is associated with impaired and insufficient insulin secretion in response to glucose. This β cell failure is associated with local islet inflammation and macrophage accumulation [5, 37,38,39,40, 42,43,44]. Indeed, macrophage depletion obtained by in vivo clodronate liposome treatments rescued glucose-induced insulin secretion in transgenic mouse models of obesity and in palmitate-infused mice [38]. Similarly, ex vivo clodronate treatment rescued glucose-induced insulin secretion in islets isolated from diet-induced obese mice [44]. Conversely, co-culture of Min6 cells (a β cell line) with CD11c+ macrophages isolated from obese mouse islets impaired their function in a cell-cell contact-dependent manner [44]. In their article, Ying and colleagues propose that intra-islet macrophages phagocytize β cell insulin secretary granules, which may contribute to the impaired β cell insulin secretion in obese mice.
During weight gain and the development of insulin resistance, mouse pancreatic islets have to increase their insulin production mainly by β cell proliferation [104]. This proliferation was promoted by macrophages isolated from obese but not lean mice via the PDGFR signaling pathway [44]. Importantly, β cell abilities to proliferate and adapt to meet insulin needs are crucial. Failure to do so is associated with a high risk of developing type 2 diabetes [104]. Thus, macrophages residing inside islets of obese mice may support β cell adaptation to metabolic stress and maintain an appropriate β cell mass. This property was already demonstrated in different models of experimental β cell ablation, wherein M2-like macrophages eliciting a robust proliferative response in surviving β cells [105,106,107,108]. In humans, macrophages were also described to accumulate inside islets during type 2 diabetes [5, 43]. Notably, the physiological insulinotropic effect of IL-1β is lost in islets isolated from type 2 diabetic donors and sustained high levels of IL-1β are associated with β cell dysfunction and apoptosis [37, 42, 98]. Clinical studies targeting IL-1β pathway have shown beneficial effects on β cell secretory function [109]. Accordingly, anti-inflammatory drugs are in development for the treatment of type 2 diabetes [110].
Thus, the phenotype and role of islet macrophages are not completely unraveled. In the obese context, they may promote β cell proliferation while contributing to their functional decline, and yet their pro-inflammatory activation is not definite (Fig. 2). Further studies are required to fully explore their monitoring and detrimental properties. Beside, other immune compartment may also be involved including the pancreatic lymph nodes as recently reported in the context of autoimmune diabetes [111].
Concluding remarks and future perspectives
Mounting evidence demonstrates that components of the innate immune system play a crucial role in regulating metabolic homeostasis. The impact of each innate immune cell subsets is summarized in Table 1. Immunometabolism is supported by intense communication and interactions between metabolic cells, i.e., adipocytes and β cells, and resident innate immune cells. Under steady state, studies have shown that immune cells contribute to the maintenance of appropriate metabolic functions including β cell secretory function and adipose tissue angiogenesis or beiging when necessary. Yet, more studies are warranted to fully explore the physiological role of innate immune cells in everyday life and learn about intrinsic protective mechanisms when facing nutritional stress. In contrast, in the context of obesity and type 2 diabetes, innate immune cell frequencies are intensively altered in metabolic tissues and their impact of metabolic functions are still debated. Indeed, a lot of interrogations are still pending including the “chicken-and-egg” question: does inflammation cause metabolic dysfunction or vice versa?
With the high-fat diet model, scientists have the opportunity to study the different stages of obesity progression, from early weight gain to established insulin resistance. It appears that obvious glucose intolerance is already observed by 3 days of regimen, without obvious signs of inflammation [92, 113]. This phenotype was not improved by macrophage deletion following clodronate liposome treatment as it was observed in chronically obese mice [48, 63, 91, 92]. These results emphasize the importance of kinetics and suggest that diet-induced metabolic dysfunction may have different etiologies in the course of weight gain, with a negative impact of innate immunity in the long term only. Surprisingly, inflammation may remain elevated in the adipose tissue of formerly obese mice, despite fat mass and glucose tolerance normalization, further dissociating immune activation from metabolic function [9]. Other studies propose that insulin resistance would develop first and that the subsequent hyperinsulinemia may cause inflammation [114, 115]. In humans, in whom local and systemic inflammation, insulin resistance and perhaps type 2 diabetes are already established for a very long time, it is virtually impossible to decipher a possible causative relationship. Another concept that was recently introduced in immunometabolism is the notion of cell memory and “trained immunity.” Christ and colleagues have shown that Western diet feeding induced long-term epigenetic reprogramming in myeloid progenitors, which may result in an exaggerated response of macrophages to new nutritional stress [116]. Trained immunity may play a role in the pathophysiology of type 2 diabetes and should be considered in translational research considering the human tendency for weight variations [117].
Another question that remains is whether obesity-induced inflammation may only be considered as detrimental. As demonstrated earlier, innate immune cells are involved in maintaining metabolic functions by inducing β cell proliferation, buffering lipid surplus of moribund adipocytes, or killing potential stressed cells during obesity. Beside, metabolic cells actively participate in regulating local macrophage differentiation and activation by β cell release of ATP or adipocyte release of lipid-filled vesicles [96] (Flaherty et al., Science, 2019). Indeed, pro-inflammatory signaling may be a prerequisite for appropriate expansion of mouse adipose tissue in response to high-fat feeding. Reduced inflammation impaired local adipogenesis, leading to increased ectopic fat deposition, and worsened glucose intolerance [118]. Similarly, we have shown that promoting M2-like macrophages was sufficient to trigger a drastic intra-abdominal adipose tissue remodeling, redirecting lipid flux to healthy subcutaneous fat depot [29]. Therefore, innate immune activation can support the body adaption to nutritional stress and may be considered as endogenous protective mechanisms. Of note, publications in immunometabolism have highlighted the need for thorough study of the different cell subsets in each innate immune family with the use of specific markers. The heterogeneity of macrophage and ILC subpopulations might reflect the wide range of their immune cell functions, beneficial or detrimental.
Thus, these immunometabolic properties represent a growing field of interest with innovative therapeutic potential. Macrophages are still the predominant immune cells in metabolic tissues but ILCs are emerging as key sentinels to sense early cell distress. While inflammation has long been considered as a pathogenic trait of diabetes, it is relevant to wonder whether a fine-tuning of the immune responsiveness, rather than shutting it off with anti-inflammatory treatments, may be an interesting approach to preserve metabolic homeostasis. Exciting discoveries are yet to come, especially with the rise of neuroimmunology that may disturb our initial view of immunometabolism. For instance, a novel population of ATM was found located on adipose sympathetic nerve bundles, suggesting an additional role for these cells in the nerve regulation of adipose tissue function [119].
References
Kazankov K, Jorgensen SMD, Thomsen KL, Moller HJ, Vilstrup H, George J, Schuppan D, Gronbaek H (2019) The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol 16:145–159
Hotamisligil GS, Shargill NS, Spiegelman BM (1993) Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259:87–91
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112:1821–1830
Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, Gueripel X, Ellingsgaard H, Schneider MK, Biollaz G et al (2007) Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 56:2356–2370
Donath MY, Dalmas E, Sauter NS, Boni-Schnetzler M (2013) Inflammation in obesity and diabetes: islet dysfunction and therapeutic opportunity. Cell Metab 17:860–872
Gregor MF, Hotamisligil GS (2011) Inflammatory mechanisms in obesity. Annu Rev Immunol 29:415–445
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969
Zamarron BF, Mergian TA, Cho KW, Martinez-Santibanez G, Luan D, Singer K, DelProposto JL, Geletka LM, Muir LA, Lumeng CN (2017) Macrophage proliferation sustains adipose tissue inflammation in formerly obese mice. Diabetes 66:392–406
Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, Shen Y, Czech MP, Aouadi M (2014) Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab 19:162–171
Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW Jr (2006) CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 116:115–124
Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M (2006) MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116:1494–1505
Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T (2006) Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem 281:26602–26614
Inouye KE, Shi H, Howard JK, Daly CH, Lord GM, Rollins BJ, Flier JS (2007) Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes 56:2242–2250
Kirk EA, Sagawa ZK, McDonald TO, O'Brien KD, Heinecke JW (2008) Monocyte chemoattractant protein deficiency fails to restrain macrophage infiltration into adipose tissue [corrected]. Diabetes 57:1254–1261
Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M, Kobayashi M, Tobe K (2009) Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 58:2574–2582
Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR (2008) Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 57:3239–3246
Dalmas E, Venteclef N, Caer C, Poitou C, Cremer I, Aron-Wisnewsky J, Lacroix-Desmazes S, Bayry J, Kaveri SV, Clement K et al (2014) T cell-derived IL-22 amplifies IL-1beta-driven inflammation in human adipose tissue: relevance to obesity and type 2 diabetes. Diabetes 63:1966–1977
Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG (2008) Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab 8:301–309
Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, Schoenfelt KQ, Kuzma JN, Larson I, Billing PS, Landerholm RW, Crouthamel M, Gozal D, Hwang S, Singh PK, Becker L (2014) Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab 20:614–625
Boutens L, Hooiveld GJ, Dhingra S, Cramer RA, Netea MG, Stienstra R (2018) Unique metabolic activation of adipose tissue macrophages in obesity promotes inflammatory responses. Diabetologia 61:942–953
Li P, Lu M, Nguyen MT, Bae EJ, Chapman J, Feng D, Hawkins M, Pessin JE, Sears DD, Nguyen AK et al (2010) Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J Biol Chem 285:15333–15345
Aouadi M, Vangala P, Yawe JC, Tencerova M, Nicoloro SM, Cohen JL, Shen Y, Czech MP (2014) Lipid storage by adipose tissue macrophages regulates systemic glucose tolerance. Am J Physiol Endocrinol Metab 307:E374–E383
Prieur X, Mok CY, Velagapudi VR, Nunez V, Fuentes L, Montaner D, Ishikawa K, Camacho A, Barbarroja N, O'Rahilly S et al (2011) Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes 60:797–809
Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, Ferrante AW Jr (2013) Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab 18:816–830
Huang SC, Everts B, Ivanova Y, O'Sullivan D, Nascimento M, Smith AM, Beatty W, Love-Gregory L, Lam WY, O'Neill CM et al (2014) Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol 15:846–855
Shin KC, Hwang I, Choe SS, Park J, Ji Y, Kim JI, Lee GY, Choi SH, Ching J, Kovalik JP, Kim JB (2017) Macrophage VLDLR mediates obesity-induced insulin resistance with adipose tissue inflammation. Nat Commun 8:1087
Hill DA, Lim HW, Kim YH, Ho WY, Foong YH, Nelson VL, Nguyen HCB, Chegireddy K, Kim J, Habertheuer A, Vallabhajosyula P, Kambayashi T, Won KJ, Lazar MA (2018) Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc Natl Acad Sci U S A 115:E5096–E5105
Dalmas E, Toubal A, Alzaid F, Blazek K, Eames HL, Lebozec K, Pini M, Hainault I, Montastier E, Denis RG et al (2015) Irf5 deficiency in macrophages promotes beneficial adipose tissue expansion and insulin sensitivity during obesity. Nat Med 21:610–618
Bourlier V, Zakaroff-Girard A, Miranville A, De Barros S, Maumus M, Sengenes C, Galitzky J, Lafontan M, Karpe F, Frayn KN et al (2008) Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation 117:806–815
Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Gyori G, Zlabinger GJ, Stulnig TM (2007) Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes 31:1420–1428
Aron-Wisnewsky J, Tordjman J, Poitou C, Darakhshan F, Hugol D, Basdevant A, Aissat A, Guerre-Millo M, Clement K (2009) Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab 94:4619–4623
Dalmas E, Lehmann FM, Dror E, Wueest S, Thienel C, Borsigova M, Stawiski M, Traunecker E, Lucchini FC, Dapito DH, Kallert SM, Guigas B, Pattou F, Kerr-Conte J, Maechler P, Girard JP, Konrad D, Wolfrum C, Böni-Schnetzler M, Finke D, Donath MY (2017) Interleukin-33-activated islet-resident innate lymphoid cells promote insulin secretion through myeloid cell retinoic acid production. Immunity 47:928–942 e927
Calderon B, Carrero JA, Ferris ST, Sojka DK, Moore L, Epelman S, Murphy KM, Yokoyama WM, Randolph GJ, Unanue ER (2015) The pancreas anatomy conditions the origin and properties of resident macrophages. J Exp Med 212:1497–1512
Banaei-Bouchareb L, Gouon-Evans V, Samara-Boustani D, Castellotti MC, Czernichow P, Pollard JW, Polak M (2004) Insulin cell mass is altered in Csf1op/Csf1op macrophage-deficient mice. J Leukoc Biol 76:359–367
Carrero JA, McCarthy DP, Ferris ST, Wan X, Hu H, Zinselmeyer BH, Vomund AN, Unanue ER (2017) Resident macrophages of pancreatic islets have a seminal role in the initiation of autoimmune diabetes of NOD mice. Proc Natl Acad Sci U S A 114:E10418–E10427
Boni-Schnetzler M, Boller S, Debray S, Bouzakri K, Meier DT, Prazak R, Kerr-Conte J, Pattou F, Ehses JA, Schuit FC et al (2009) Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology 150:5218–5229
Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, Yagi N, Ohto U, Kimoto M, Miyake K, Tobe K, Arai H, Kadowaki T, Nagai R (2012) Saturated fatty acid and TLR signaling link beta cell dysfunction and islet inflammation. Cell Metab 15:518–533
Hasnain SZ, Borg DJ, Harcourt BE, Tong H, Sheng YH, Ng CP, Das I, Wang R, Chen AC, Loudovaris T et al (2014) Glycemic control in diabetes is restored by therapeutic manipulation of cytokines that regulate beta cell stress. Nat Med 20:1417–1426
Jourdan T, Godlewski G, Cinar R, Bertola A, Szanda G, Liu J, Tam J, Han T, Mukhopadhyay B, Skarulis MC, Ju C, Aouadi M, Czech MP, Kunos G (2013) Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat Med 19:1132–1140
Nackiewicz D, Dan M, He W, Kim R, Salmi A, Rutti S, Westwell-Roper C, Cunningham A, Speck M, Schuster-Klein C et al (2014) TLR2/6 and TLR4-activated macrophages contribute to islet inflammation and impair beta cell insulin gene expression via IL-1 and IL-6. Diabetologia 57:1645–1654
Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY (2002) Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest 110:851–860
Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG (2009) Islet-associated macrophages in type 2 diabetes. Diabetologia 52:1686–1688
Ying W, Lee YS, Dong Y, Seidman JS, Yang M, Isaac R, Seo JB, Yang BH, Wollam J, Riopel M, McNelis J, Glass CK, Olefsky JM, Fu W (2019) Expansion of islet-resident macrophages leads to inflammation affecting beta cell proliferation and function in obesity. Cell Metab 29:457–474.e455
Segerstolpe A, Palasantza A, Eliasson P, Andersson EM, Andreasson AC, Sun X, Picelli S, Sabirsh A, Clausen M, Bjursell MK et al (2016) Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab 24:593–607
Mahdi T, Hanzelmann S, Salehi A, Muhammed SJ, Reinbothe TM, Tang Y, Axelsson AS, Zhou Y, Jing X, Almgren P et al (2012) Secreted frizzled-related protein 4 reduces insulin secretion and is overexpressed in type 2 diabetes. Cell Metab 16:625–633
Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE et al (2018) Innate lymphoid cells: 10 years on. Cell 174:1054–1066
Wensveen FM, Jelencic V, Valentic S, Sestan M, Wensveen TT, Theurich S, Glasner A, Mendrila D, Stimac D, Wunderlich FT et al (2015) NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat Immunol 16:376–385
Lee BC, Kim MS, Pae M, Yamamoto Y, Eberle D, Shimada T, Kamei N, Park HS, Sasorith S, Woo JR et al (2016) Adipose natural killer cells regulate adipose tissue macrophages to promote insulin resistance in obesity. Cell Metab 23:685–698
O'Sullivan TE, Rapp M, Fan X, Weizman OE, Bhardwaj P, Adams NM, Walzer T, Dannenberg AJ, Sun JC (2016) Adipose-resident group 1 innate lymphoid cells promote obesity-associated insulin resistance. Immunity 45:428–441
Boulenouar S, Michelet X, Duquette D, Alvarez D, Hogan AE, Dold C, O'Connor D, Stutte S, Tavakkoli A, Winters D et al (2017) Adipose type one innate lymphoid cells regulate macrophage homeostasis through targeted cytotoxicity. Immunity 46:273–286
Theurich S, Tsaousidou E, Hanssen R, Lempradl AM, Mauer J, Timper K, Schilbach K, Folz-Donahue K, Heilinger C, Sexl V, Pospisilik JA, Wunderlich FT, Brüning JC (2017) IL-6/Stat3-dependent induction of a distinct, obesity-associated NK cell subpopulation deteriorates energy and glucose homeostasis. Cell Metab 26:171–184.e176
O'Rourke RW, Meyer KA, Neeley CK, Gaston GD, Sekhri P, Szumowski M, Zamarron B, Lumeng CN, Marks DL (2014) Systemic NK cell ablation attenuates intra-abdominal adipose tissue macrophage infiltration in murine obesity. Obesity (Silver Spring) 22:2109–2114
Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM (2013) Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med 210:535–549
Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, Artis D (2015) Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519:242–246
Cayrol C, Girard JP (2018) Interleukin-33 (IL-33): a nuclear cytokine from the IL-1 family. Immunol Rev 281:154–168
Molofsky AB, Van Gool F, Liang HE, Van Dyken SJ, Nussbaum JC, Lee J, Bluestone JA, Locksley RM (2015) Interleukin-33 and interferon-gamma counter-regulate group 2 innate lymphoid cell activation during immune perturbation. Immunity 43:161–174
Oldenhove G, Boucquey E, Taquin A, Acolty V, Bonetti L, Ryffel B, Le Bert M, Englebert K, Boon L, Moser M (2018) PD-1 is involved in the dysregulation of type 2 innate lymphoid cells in a murine model of obesity. Cell Rep 25:2053–2060.e4
Ohne Y, Silver JS, Thompson-Snipes L, Collet MA, Blanck JP, Cantarel BL, Copenhaver AM, Humbles AA, Liu YJ (2016) IL-1 is a critical regulator of group 2 innate lymphoid cell function and plasticity. Nat Immunol 17:646–655
Hams E, Bermingham R, Wurlod FA, Hogan AE, O'Shea D, Preston RJ, Rodewald HR, McKenzie AN, Fallon PG (2016) The helminth T2 RNase omega1 promotes metabolic homeostasis in an IL-33- and group 2 innate lymphoid cell-dependent mechanism. FASEB J 30:824–835
Hussaarts L, Garcia-Tardon N, van Beek L, Heemskerk MM, Haeberlein S, van der Zon GC, Ozir-Fazalalikhan A, Berbee JF (2015) Willems van Dijk K, van Harmelen V, et al.: Chronic helminth infection and helminth-derived egg antigens promote adipose tissue M2 macrophages and improve insulin sensitivity in obese mice. FASEB J 29:3027–3039
Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM (2011) Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332:243–247
Kumar D, Pandya SK, Varshney S, Shankar K, Rajan S, Srivastava A, Gupta A, Gupta S, Vishwakarma AL, Misra A, Gaikwad AN (2018) Temporal immmunometabolic profiling of adipose tissue in HFD-induced obesity: manifestations of mast cells in fibrosis and senescence. Int J Obes. https://doi.org/10.1038/s41366-018-0228-5
Talukdar S, Oh DY, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, Ofrecio J, Lin M, Brenner MB, Olefsky JM (2012) Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med 18:1407–1412
Elgazar-Carmon V, Rudich A, Hadad N, Levy R (2008) Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res 49:1894–1903
Zhang J, Shi GP (1822) Mast cells and metabolic syndrome. Biochim Biophys Acta 2012:14–20
Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S et al (2012) Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 13:1118–1128
Cho KW, Zamarron BF, Muir LA, Singer K, Porsche CE, DelProposto JB, Geletka L, Meyer KA, O'Rourke RW, Lumeng CN (2016) Adipose tissue dendritic cells are independent contributors to obesity-induced inflammation and insulin resistance. J Immunol 197:3650–3661
Bertola A, Ciucci T, Rousseau D, Bourlier V, Duffaut C, Bonnafous S, Blin-Wakkach C, Anty R, Iannelli A, Gugenheim J, Tran A, Bouloumie A, Gual P, Wakkach A (2012) Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes 61:2238–2247
Macdougall CE, Wood EG, Loschko J, Scagliotti V, Cassidy FC, Robinson ME, Feldhahn N, Castellano L, Voisin MB, Marelli-Berg F, Gaston-Massuet C, Charalambous M, Longhi MP (2018) Visceral adipose tissue immune homeostasis is regulated by the crosstalk between adipocytes and dendritic cell subsets. Cell Metab 27:588–601.e4
Eguchi J, Kong X, Tenta M, Wang X, Kang S, Rosen ED (2013) Interferon regulatory factor 4 regulates obesity-induced inflammation through regulation of adipose tissue macrophage polarization. Diabetes 62:3394–3403
Liao X, Sharma N, Kapadia F, Zhou G, Lu Y, Hong H, Paruchuri K, Mahabeleshwar GH, Dalmas E, Venteclef N, Flask CA, Kim J, Doreian BW, Lu KQ, Kaestner KH, Hamik A, Clément K, Jain MK (2011) Kruppel-like factor 4 regulates macrophage polarization. J Clin Invest 121:2736–2749
Satoh T, Kidoya H, Naito H, Yamamoto M, Takemura N, Nakagawa K, Yoshioka Y, Morii E, Takakura N, Takeuchi O, Akira S (2013) Critical role of Trib1 in differentiation of tissue-resident M2-like macrophages. Nature 495:524–528
Nawaz A, Aminuddin A, Kado T, Takikawa A, Yamamoto S, Tsuneyama K, Igarashi Y, Ikutani M, Nishida Y, Nagai Y, Takatsu K, Imura J, Sasahara M, Okazaki Y, Ueki K, Okamura T, Tokuyama K, Ando A, Matsumoto M, Mori H, Nakagawa T, Kobayashi N, Saeki K, Usui I, Fujisaka S, Tobe K (2017) CD206(+) M2-like macrophages regulate systemic glucose metabolism by inhibiting proliferation of adipocyte progenitors. Nat Commun 8:286
Harms M, Seale P (2013) Brown and beige fat: development, function and therapeutic potential. Nat Med 19:1252–1263
Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A (2015) Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 160:74–87
Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS (2005) Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 46:2347–2355
Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS (2007) Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 56:2910–2918
Muir LA, Kiridena S, Griffin C, DelProposto JB, Geletka L, Martinez-Santibanez G, Zamarron BF, Lucas H, Singer K, O'Rourke RW et al (2018) Frontline science: rapid adipose tissue expansion triggers unique proliferation and lipid accumulation profiles in adipose tissue macrophages. J Leukoc Biol 103:615–628
Coats BR, Schoenfelt KQ, Barbosa-Lorenzi VC, Peris E, Cui C, Hoffman A, Zhou G, Fernandez S, Zhai L, Hall BA, Haka AS, Shah AM, Reardon CA, Brady MJ, Rhodes CJ, Maxfield FR, Becker L (2017) Metabolically activated adipose tissue macrophages perform detrimental and beneficial functions during diet-induced obesity. Cell Rep 20:3149–3161
Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW Jr (2010) Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest 120:3466–3479
Fischer-Posovszky P, Wang QA, Asterholm IW, Rutkowski JM, Scherer PE (2011) Targeted deletion of adipocytes by apoptosis leads to adipose tissue recruitment of alternatively activated M2 macrophages. Endocrinology 152:3074–3081
Stienstra R, Dijk W, van Beek L, Jansen H, Heemskerk M, Houtkooper RH, Denis S, van Harmelen V, Willems van Dijk K, Tack CJ, Kersten S (2014) Mannose-binding lectin is required for the effective clearance of apoptotic cells by adipose tissue macrophages during obesity. Diabetes 63:4143–4153
Grijalva A, Xu X, Ferrante AW Jr (2016) Autophagy is dispensable for macrophage-mediated lipid homeostasis in adipose tissue. Diabetes 65:967–980
Haka AS, Barbosa-Lorenzi VC, Lee HJ, Falcone DJ, Hudis CA, Dannenberg AJ, Maxfield FR (2016) Exocytosis of macrophage lysosomes leads to digestion of apoptotic adipocytes and foam cell formation. J Lipid Res 57:980–992
Wilson AM, Shao Z, Grenier V, Mawambo G, Daudelin JF, Dejda A, Pilon F, Popovic N, Boulet S, Parinot C, Oubaha M, Labrecque N, de Guire V, Laplante M, Lettre G, Sennlaub F, Joyal JS, Meunier M, Sapieha P (2018) Neuropilin-1 expression in adipose tissue macrophages protects against obesity and metabolic syndrome. Sci Immunol 3:eaan4626
Corvera S, Gealekman O (1842) Adipose tissue angiogenesis: impact on obesity and type-2 diabetes. Biochim Biophys Acta 2014:463–472
Xu F, Burk D, Gao Z, Yin J, Zhang X, Weng J, Ye J (2012) Angiogenic deficiency and adipose tissue dysfunction are associated with macrophage malfunction in SIRT1−/− mice. Endocrinology 153:1706–1716
Cho CH, Koh YJ, Han J, Sung HK, Jong Lee H, Morisada T, Schwendener RA, Brekken RA, Kang G, Oike Y, Choi TS, Suda T, Yoo OJ, Koh GY (2007) Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circ Res 100:e47–e57
Pang C, Gao Z, Yin J, Zhang J, Jia W, Ye J (2008) Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. Am J Physiol Endocrinol Metab 295:E313–E322
Feng B, Jiao P, Nie Y, Kim T, Jun D, van Rooijen N, Yang Z, Xu H (2011) Clodronate liposomes improve metabolic profile and reduce visceral adipose macrophage content in diet-induced obese mice. PLoS One 6:e24358
Lee YS, Li P, Huh JY, Hwang IJ, Lu M, Kim JI, Ham M, Talukdar S, Chen A, Lu WJ, Bandyopadhyay GK, Schwendener R, Olefsky J, Kim JB (2011) Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 60:2474–2483
Aouadi M, Tencerova M, Vangala P, Yawe JC, Nicoloro SM, Amano SU, Cohen JL, Czech MP (2013) Gene silencing in adipose tissue macrophages regulates whole-body metabolism in obese mice. Proc Natl Acad Sci U S A 110:8278–8283
Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, Ofrecio JM, Wollam J, Hernandez-Carretero A, Fu W, Li P, Olefsky JM (2017) Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell 171:372–384.e12
Ferris ST, Zakharov PN, Wan X, Calderon B, Artyomov MN, Unanue ER, Carrero JA (2017) The islet-resident macrophage is in an inflammatory state and senses microbial products in blood. J Exp Med 214:2369–2385
Zinselmeyer BH, Vomund AN, Saunders BT, Johnson MW, Carrero JA, Unanue ER (2018) The resident macrophages in murine pancreatic islets are constantly probing their local environment, capturing beta cell granules and blood particles. Diabetologia 61:1374–1383
Weitz JR, Makhmutova M, Almaca J, Stertmann J, Aamodt K, Brissova M, Speier S, Rodriguez-Diaz R, Caicedo A (2018) Mouse pancreatic islet macrophages use locally released ATP to monitor beta cell activity. Diabetologia 61:182–192
Hajmrle C, Smith N, Spigelman AF, Dai X, Senior L, Bautista A, Ferdaoussi M, MacDonald PE (2016) Interleukin-1 signaling contributes to acute islet compensation. JCI Insight 1:e86055
Zawalich WS, Zawalich KC (1989) Interleukin 1 is a potent stimulator of islet insulin secretion and phosphoinositide hydrolysis. Am J Phys 256:E19–E24
Benner C, van der Meulen T, Caceres E, Tigyi K, Donaldson CJ, Huising MO (2014) The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics 15:620
Burke SJ, Batdorf HM, Burk DH, Martin TM, Mendoza T, Stadler K, Alami W, Karlstad MD, Robson MJ, Blakely RD, Mynatt RL, Collier JJ (2018) Pancreatic deletion of the interleukin-1 receptor disrupts whole body glucose homeostasis and promotes islet beta-cell de-differentiation. Mol Metab 14:95–107
Dror E, Dalmas E, Meier DT, Wueest S, Thevenet J, Thienel C, Timper K, Nordmann TM, Traub S, Schulze F et al (2017) Postprandial macrophage-derived IL-1beta stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat Immunol 18:283–292
Boni-Schnetzler M, Hauselmann SP, Dalmas E, Meier DT, Thienel C, Traub S, Schulze F, Steiger L, Dror E, Martin P et al (2018) Beta cell-specific deletion of the IL-1 receptor antagonist impairs beta cell proliferation and insulin secretion. Cell Rep 22:1774–1786
Aguayo-Mazzucato C, Bonner-Weir S (2018) Pancreatic beta cell regeneration as a possible therapy for diabetes. Cell Metab 27:57–67
Brissova M, Aamodt K, Brahmachary P, Prasad N, Hong JY, Dai C, Mellati M, Shostak A, Poffenberger G, Aramandla R, Levy SE, Powers AC (2014) Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes beta cell regeneration. Cell Metab 19:498–511
Criscimanna A, Coudriet GM, Gittes GK, Piganelli JD, Esni F (2014) Activated macrophages create lineage-specific microenvironments for pancreatic acinar- and beta-cell regeneration in mice. Gastroenterology 147:1106–1118.e11
Riley KG, Pasek RC, Maulis MF, Dunn JC, Bolus WR, Kendall PL, Hasty AH, Gannon M (2015) Macrophages are essential for CTGF-mediated adult beta-cell proliferation after injury. Mol Metab 4:584–591
Xiao X, Gaffar I, Guo P, Wiersch J, Fischbach S, Peirish L, Song Z, El-Gohary Y, Prasadan K, Shiota C et al (2014) M2 macrophages promote beta-cell proliferation by up-regulation of SMAD7. Proc Natl Acad Sci U S A 111:E1211–E1220
Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY (2007) Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 356:1517–1526
Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, Group CT (2018) Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet 391:319–328
Wan X, Zinselmeyer BH, Zakharov PN, Vomund AN, Taniguchi R, Santambrogio L, Anderson MS, Lichti CF, Unanue ER (2018) Pancreatic islets communicate with lymphoid tissues via exocytosis of insulin peptides. Nature 560:107–111
Hams E, Locksley RM, McKenzie AN, Fallon PG (2013) Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J Immunol 191:5349–5353
Ji Y, Sun S, Xia S, Yang L, Li X, Qi L (2012) Short term high fat diet challenge promotes alternative macrophage polarization in adipose tissue via natural killer T cells and interleukin-4. J Biol Chem 287:24378–24386
Shimobayashi M, Albert V, Woelnerhanssen B, Frei IC, Weissenberger D, Meyer-Gerspach AC, Clement N, Moes S, Colombi M, Meier JA, Swierczynska MM, Jenö P, Beglinger C, Peterli R, Hall MN (2018) Insulin resistance causes inflammation in adipose tissue. J Clin Invest 128:1538–1550
Pedersen DJ, Guilherme A, Danai LV, Heyda L, Matevossian A, Cohen J, Nicoloro SM, Straubhaar J, Noh HL, Jung D, Kim JK, Czech MP (2015) A major role of insulin in promoting obesity-associated adipose tissue inflammation. Mol Metab 4:507–518
Christ A, Gunther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, Scholz CJ, Oosting M, Haendler K, Bassler K et al (2018) Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 172:162–175.e14
Mourits VP, Wijkmans JC, Joosten LA, Netea MG (2018) Trained immunity as a novel therapeutic strategy. Curr Opin Pharmacol 41:52–58
Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, Scherer PE (2014) Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab 20:103–118
Pirzgalska RM, Seixas E, Seidman JS, Link VM, Sanchez NM, Mahu I, Mendes R, Gres V, Kubasova N, Morris I et al (2017) Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat Med 23:1309–1318
Acknowledgements
This work was supported by the European Foundation for the Study of Diabetes (EFSD) Research Programme, the French Society for Diabetes (SFD), and the ATIP-Avenir Programme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that she has no conflict of interest.
Additional information
This article is a contribution to the special issue on Inflammation and Type 2 Diabetes - Guest Editor: Marc Y. Donath
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dalmas, E. Role of innate immune cells in metabolism: from physiology to type 2 diabetes. Semin Immunopathol 41, 531–545 (2019). https://doi.org/10.1007/s00281-019-00736-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-019-00736-5