Abstract
This review is not intended to cover in detail all aspects of the discovery and evolution of our understanding of the “alternative pathway” of complement activation, there are many excellent reviews that do this (see Fearon (CRC Crit Rev Immunol 1:1–32, 1979), Pangburn and Müller-Eberhard (Springer Semin Immunopathol 7:163–192, 1984)), but instead to give sufficient background for current concepts to be put in context. The prevailing textbook view, of components having a primary role as an alternative “pathway” for C3 activation, is challenged, with an argument developed for the primary role of the system being that of providing a surface-dependent amplification loop for both C3 and C5 activation. Whatever the mechanism by which the initial C3b molecule is generated, deposition onto a surface has the potential to target that surface for elimination. Elimination or escape from initial targeting is determined by a sophisticated and highly regulated amplification loop for C3 activation. This viewpoint of the system is then briefly developed to provide a context for therapeutic treatment of disease caused, at least in part, by dysregulated amplification of C3 activation, and to highlight some of the challenges that such therapies will face and need to address.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The discovery of properdin and of its role in complement activation
Complement, as a heat-labile effector system of blood involved in pathogen elimination, was first studied by Bordet in the late nineteenth century. In 1945, Landsteiner noted that substances, “similar to in some respects, but not identical to, antibodies, and including complement”, are produced in some animals. These substances, without prior exposure to antigen, provided a natural immunity for the host, protecting it against infection. It was the analysis of these substances that led to the discovery of properdin. Early work had identified four “components” of complement, C’1, C’2, C’3, and C’4. Then, in the mid-1950s, Pillemer and coworkers, while attempting to isolate C’3 through study of its selective inactivation by yeast cell walls (C’1, C’4 and C’2 were largely unaffected), discovered a new protein that they named properdin. The dependence of properdin on the C’3 component of complement for elicitation of complement effector functions is summarized in their seminal 1954 publication [1]. A dependence of properdin on Mg2+ ions, and on heat labile and hydrazine-sensitive serum factors, was also shown by Pillemer, Lepow, and their co-workers, with recognition that the hydrazine-sensitive factor resembled C4. Crucially, properdin was identified as being distinct from antibodies, lacking an antibody-like specificity in its initial recognition function. Subsequent work showed that C’3 itself was compromised of multiple proteins and included not just what is now known as C3 but also C5, C6, C7, C8, and C9. (Throughout this review, in order to remove unnecessary confusion, the current recommendations for complement nomenclature [2] have been used. See Table 1 for earlier nomenclatures.) Pillemer and coworkers interpreted their work as evidence for properdin having a direct role in the recognition of pathogens, recruiting C3 and triggering complement-dependent effector functions without the participation of antibody. This viewpoint was later challenged by Nelson [3], who argued that natural antibody (nAb) recognition of polysaccharide epitopes contained in yeast cell walls was sufficient for low level activation of C1, C4, and C2, and for C3 (as C3b) deposition, and that deposited C3b was then responsible for the recruitment of properdin. Whatever the sequence, the work of Pillemer and colleagues had demonstrated unequivocally a new complexity to the complement system, and had identified a new component, properdin, that was capable of playing a critical role in C3 activation. This system was initially termed the “properdin system”.
Identification and characterization of components of the properdin system
The 1960s and early 1970s experienced rapid progress in the identification of essential components of the properdin system. Originally identified as factors A, B, C, and D, progress in understanding of their individual contributions and mechanisms of action came from multiple parallel lines of investigation. These included immunohistochemical and biochemical characterization of the isolated serum proteins, as well as detailed analysis of three distinct phenomena that resulted in both C3 and C5 activation. These phenomena, depletion of complement activity in serum by complex polysaccharides such as yeast cell walls and inulin, depletion of complement activity by a factor present in cobra venom (CVF), and depletion of complement activity by C3 nephritic factor (C3NeF), a factor present in the sera of certain patients with progressive kidney disease, led to a robust understanding of the essentials of the system. Analysis of a rare complement component deficiency, now known as a factor I (FI) deficiency, also provided first insights into the sophisticated regulation of the system.
Biochemical and immunohistochemical analysis of the activating components of the properdin system
Properdin
Properdin, while stimulating interest in an alternative (to the classical pathway) mechanism of C3 activation, and of initiation of multiple effector systems of complement, was not the first component of the system to be isolated and characterized. This was C3. Partial purification of properdin, in 1968, enabled unequivocal distinction from immunoglobulin and other known serum proteins, including the identified complement proteins. Of particular note, highly purified properdin did not combine with zymosan, but was able to restore activity to properdin-depleted serum in the zymosan assay [4]. Later procedures yielded highly purified properdin and also gave rise to the concept of “native” and “activated” properdin as the isolated protein did not behave in an identical manner to properdin in serum [5, 6]. It was preparations such as these that were used in reassembling the properdin system from isolated components. They also gave first insight into the difficulties of working with the isolated protein.
C3
C3 can be identified with the heat-stable component of complement removed by yeast, and also the hydrazine-sensitive Factor A of the properdin system partially isolated in Pillemer’s pioneering investigations. It was first purified to homogeneity in 1960, with decay of activity of both the protein in serum and the isolated protein over time, together with generation of an inactive antigenically related molecule, noted [7]. Hydrazine or ammonia treatment of serum also resulted in a C3dg molecule with altered electrophoretic mobility when compared to C3dg generated in serum with aging, the first hint that these treatments subtly altered the C3dg component of C3.
FB
FB is the heat-labile serum factor identified in early investigations of the properdin system [8]. Isolated independently by many groups, its relationship to the complement system was not always immediately recognized. This was defined in experiments with activators of the properdin system that showed that it could be cleaved by an unidentified serum protein to generate a complex with C3-cleaving activity [9]. Rapid confirmation of the identity of FB with the proteins isolated by other groups was key to developing understanding of its role in complement activation [10]. While these studies of FB had established that that it possessed enzymatic activity, its identity as a serine protease came later. The binding of FB to C3b is Mg2+ −dependent [11].
FD
FD was the last “activating” component of the properdin system to be identified. Its proteolytic nature was surmised from the mechanism by which FB was activated [9,10,11,12] and, in 1974, it too was characterized as a serine protease [13]. One unresolved aspect of this initial characterization was that of the nature of FD in serum, as an inactive precursor molecule or as the active enzyme. While initially presumed to circulate as a precursor or zymogen, in part by analogy with classical serine proteases such as trypsin and chymotrypsin, and in part because of its resistance to inactivation by diisopropylfluorophosphate (DFP) [14], this interpretation could have been influenced by the cryptic nature of the FD active site [15, 16]. Others reported that FD circulated in serum as the active enzyme [16].
Dissection of the mechanism of cobra venom factor (CVF) in complement depletion
While it was shown as early as 1902 that cobra venom contained a complement-depleting factor capable of removing from serum, from multiple species, factor(s) required for bactericidal activity, further progress on understanding the mechanism by which this occurred came much later. In 1969, Ballow and Cochrane identified and partially characterized a factor in cobra venom that combined with an unknown factor in serum and, together with post-C3 components, was capable of lysing erythrocytes through a passive mechanism (a mechanism now known as reactive or bystander lysis) [17]. This occurred without the involvement of antibody and the early-acting (antibody-dependent) components, C1, C4, and C2. The unknown serum factor, which was heat-labile, formed a stable complex with CVF, the complex acquiring C3- and, in some species, C5-inactivating properties [18]. Isolation of CVF in 1971 led rapidly to detailed characterization of its requirements for C3 activation. The reaction required Mg2+ ions and, for C3 activation, the complement proteins now known as Factor B (FB) and Factor D (FD). C5 activation required, in addition to Mg2+, FB and FD, C3. Initially CVF binds FB to form a loose Mg2+-dependent CVF.B complex. This is activated, by FD, to generate the C3 cleaving complex CVFBb and release the fragment Ba [9, 12]. These data, largely obtained in human serum, were paralleled by investigations in other species, notably the guinea pig, highlighting the overall similarities in the CVF-dependent C3 activation and depletion mechanism across mammalian phylogeny. The studies also led to the hypothesis that a similar mechanism might operate in antibody-antigen-independent endotoxin-, immune aggregate-, and zymosan-activation of C3 and C5 [9].
Although these investigations had shown that FB and FD were the same serum proteins as those required for other, often properdin-dependent, mechanisms of C3 activation, it should be noted that CVF formed a stable C3 convertase, with no requirement for properdin for its complement-depleting activity.
Dissection of the mechanism of C3 nephritic factor (C3NeF) in complement depletion
In the 1960s, many studies of patients with renal disease had identified increased levels of activation products of C3 and, in 1969, West and coworkers identified a factor in the serum of some patients with glomerulonephritis that, in the presence of Mg2+ and an unidentified serum protein, catalyzed specifically cleavage of C3 [19]. Further characterization of this factor, named C3 nephritic factor (C3NeF), showed that it acted independently of C4 and C2, and of immune complexes [20]. Subsequently, the C3NeF serum cofactor was identified as FB. While these features of C3NeF were strikingly analogous to those of CVF, differences in their mechanisms of action were also identified. C3NeF did not bind FB in the absence of C3 or FD and, in some cases, properdin. In the presence of these proteins, an active C3-cleaving enzyme was generated [21]. Further characterization of C3NeF showed it to be an immunoglobulin, but with considerable heterogeneity in C3NeFs from different patients. Despite their heterogeneity, C3NeFs had one common feature, that of stabilizing, through their Fab domains, both the fluid-phase and surface-bound C3bBb- and/or C3bBbP C3-cleaving enzymes [22]. In many cases, C3NeFs recognized epitopes on FB or Bb when bound to the C3bB or C3bBb complexes, indicating that complexed FB or Bb is locked in a metastable conformation [23]. Both these studies and those with CVF led to a functional analogy between the C3 and FB activation mechanism, dependent on FD, and that of C4 and C2 activation, dependent on C1, being made; with hindsight, this may have adversely biased subsequent investigations and hypotheses relating to the properdin system.
The amplification convertase
Together, the above studies enabled the mechanism of assembly of the amplification loop C3 convertase to be elucidated. C3b, however generated, acquires a Mg2+-dependent binding site for FB. The resulting C3bB complex is then activated by FD to generate the active C3bBb enzyme.
The amplification loop for C3 activation and its fluid-phase regulation
The concept of positive feedback of C3 activation by components of the properdin system came from Müller-Eberhard and Götze’s early studies with partially purified components and serum [24]. A positive feedback or amplification loop for C3 activation requires regulation if components of the system are not to be depleted to exhaustion [25]. Significant insight into regulation came from studies with a unique patient with accelerated C3 consumption and circulating C3b. This patient, who had no active factor I (FI) in his circulation, was unable to break down C3 beyond C3b and also lacked intact FB. This resulted in a functional deficiency of amplification. Infusion of plasma from a healthy individual or of purified FI resulted in destruction of circulating C3b and transient restoration of normal C3 and FB levels [26]. Conclusions drawn from studies with this first patient have been confirmed with identification of further unrelated FI-deficient individuals.
Biochemical and immunohistochemical analysis of fluid-phase regulatory components of the properdin system
FI
FI was first identified as a natural inhibitor of C3 in both rabbit and guinea pig serum and later identified as identical to the “conglutinogen-activating factor” described by Lachmann and Müller-Eberhard [27]. Present at relatively low concentrations in serum, it acted specifically, presumed as an enzyme, on surface-bound C3b, exposing a site that was recognized by bovine conglutinin, and rendering C3b sensitive to further enzymatic attack. Ruddy and Austen [28] confirmed the enzymatic nature of FI and demonstrated that its action resulted in the loss of hemolytic activity associated with cell-bound C3b. It was later reported that phosphorylation of the alpha chain of C3 inhibits FI activity; the physiological significance of this remains unclear [29].
FH
FH was first identified as both an accelerator of the FI-mediated inactivation of C3b, and a C3b-binding protein that inhibited the activity of the C3bBb amplification convertase by accelerating its decay [30, 31]. Further analysis of its mode of action showed it to be an essential cofactor for FI-mediated inactivation of C3b and confirmed FI as a protease, cleaving C3 at two closely adjacent sites to generate iC3b and releasing a small peptide, C3f [32, 33]. The second mode of action of FH, acceleration of C3bBb (and C3bB) decay, was the consequence of competition between FB/Bb and FH for binding to C3b.
The initial step in recruitment of components of the properdin system
Possibly because of the strong similarities between C4 and C3, and between C2 and FB (now known to be consequent on a common evolutionary origin), attempts have been made to identify an activation pathway for the properdin system, analogous to antibody-antigen-dependent classical pathway (CP) activation. In the CP, recruitment of C1q (the recognition factor) by aggregated Ig results in first activation of C1r and C1s, and then sequential activation of C4 (generating C4b) and C2, when complexed with C4b, by C1s. These attempts included identification of an “initiating factor” [34], and of a “properdin convertase” [35] (neither of which survived further scrutiny), and the concept of activated properdin (see later). There are however also striking differences between the systems. Unlike C1r and C1s, FD circulates, in man, at least in part (see later), as an active enzyme waiting for assembly of its complex substrate, C3bB, generating C3bBb. Unlike C3bBb, which is a C3-activating enzyme, amplifying C3b and C3bBb generation, the analogous enzyme of the classical pathway, C4b2a cannot amplify its own production, but only activate C3.
The C3 tickover hypothesis.
Whether or not nAbs (or other factors) are involved in the recruitment of properdin onto zymosan, identification of amplification and regulatory mechanisms within the properdin system, together with analysis of complement activation in systems such as the C4-deficient guinea pig, or with complement activators such as bacteria, endotoxin, or non-C1-activating immune aggregates [9], had allowed the principle of antibody/classical pathway-independent C3 activation to be established beyond doubt. While the mechanism by which initial C3 activation occurred was not addressed, Lachmann and coworkers proposed that, in serum, continual low level C3 activation generated C3b and coined the term “C3 tickover” for the hypothesis [36]. The fate of C3b thus generated would be determined by the balance of amplification or down-regulatory factors, the balance itself determined by the local environment in which the C3b was generated. In the fluid-phase, down-regulation is heavily favored, with generation of fluid-phase iC3b (often mistakenly identified as C3c, see [26]) as an inactive end product. The hypothesis explained the observations with CVF, with C3NeF, and with FI deficiency; all conditions where down-regulation was compromised; and emphasized the critical role that down-regulation plays in controlling C3 activation in the fluid-phase.
Proteolytic activation of C3
While the tickover hypothesis did not address directly the mechanism of initial C3b generation, it was already well-known that C3b could be formed not just by the action of the classical pathway C3 convertase, C4b2a, but also by many different less-specific proteases [37,38,39], and that the C3b thus generated was highly resistant to further proteolytic attack, only becoming protease-sensitive after the generation of iC3b by the combined actions of FI and FH [27]. Lectin pathway proteases, acting independently of C4 and C2, can now be added to that list [40]. Indeed, trypsin was routinely used as a tool protease to generate C3b, both in the fluid phase and on surfaces [41]. Sites of inflammation, coagulation, and infection, where C3 activation is required, are likely to be sites in which non-specific protease activity, from inflammatory cells and/or pathogens, is abundant, and this the predominant source of early focused C3b generation. (While the LP was not recognized at the time of these early investigations, it is probable that it too was often activated and contributed to initial C3 activation.)
Summary of the fluid-phase amplification loop for C3 activation
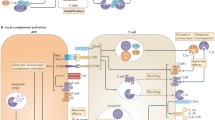
The system, as outlined above, appears sufficient and contains all that is necessary, for controlled fluid-phase activation of C3 activation, and generation of fully functional C3b. In the fluid-phase, properdin plays no part. [42], down-regulation predominates, and amplification of C3b generation is not seen (see Fig. 1b). Nevertheless, if activation occurs close to surfaces, allowing a high probability of surface-binding of C3b, there is potential for surface-dependent amplification.
a The generation of nascent C3b (*C3b). Nascent C3b has the transient ability to bind to any surface with suitable acceptor hydroxyl- or amino- groups. b If nascent C3b does not bind covalently to a surface, the activated acyl group of the internal thioester is rapidly hydrolysed and C3b efficiently inactivated by the action of FI and FH (or FHL-1). c If nascent C3b binds to a surface the consequence of binding is determined by the nature of the surface. If the surface is non-protective (NPS), any FB that binds is actively displaced, primarily by CD55 and/or FH, and the C3b rapidly inactivated by the combined actions of FI and, primarily, FH and/or CD46. d If nascent C3b binds to a protective surface, dissociation of C3bB or C3bBb is inhibited, the bimolecular complex may be stabilized by recruitment of properdin, and amplification of C3b (and C5b) generation is seen, with recruitment of C3- and C5-dependent effector functions
The surface dependence of recruitment of effector functions of the amplification loop
A different picture emerges with surface-bound C3b. On activation of C3, C3b acquires a transient ability to bind to a surface (a property shared with nascent C4b). Surface binding requires generation in close proximity to the surface [43]. This provides a comprehensive innate immune surveillance mechanism, C3b “identifying” all surfaces with which it is in contact as potential targets for elimination. However, it is non-discriminatory. The host requires additional mechanisms to differentiate between beneficial and non-beneficial targeting.
Focused targeting
C3b is deposited in clusters around the activation site, emphasizing both the transient nature of surface-binding and the impact of amplified generation [44]. While clustering of C4b around activation sites has also been claimed, this may be less pronounced, a reflection of an inability of C4b to amplify its own production. Focused targeting of C5-dependent effector functions is propagated by the requirement for a second C3b molecule to present C5 to C3bBb in its activation [45], and by the ability of C3b to “dock” C5b in the initial step of MAC assembly, C5b6 generation. MAC-dependent lysis of cells is up to 100-fold more efficient on surfaces containing bound C3b than on surfaces devoid of C3b [46].
The nature of the C3b surface attachment
In their initial investigations of the properdin system, Pillemer and coworkers had noted that, using conventional techniques, C3 could not be eluted from zymosan. In 1963, noting that hydrazine inactivation of C3 and C4 resembled nuclephilic attack at an ester, Mueller-Eberhard and Biro speculated that the interaction of C4b (and, by analogy, C3b) with membranes might be covalent. Confirmation of a covalent interaction between C3b and activating surfaces, through a bond susceptible to nucleophilic attack, came much later [47], and it was not until 1979 that evidence for ester bond formation between C3b and receptive surfaces was produced [48]. First insight into the mechanism of covalent bond formation came with the observation that chemical or enzymatic inactivation of C3 resulted in the release of a single thiol group, with speculation that, on activation, this was released from an internal thioester. Further evidence came from studies on the inactivation of C3 with small nitrogen nucleophiles such as methylamine, amino acid sequence analysis of the affected region in C3 [49], and investigation of the autolytic cleavage reaction of native C3. Evidence for an identical reaction mechanism in C4 followed rapidly. Investigations of the interaction of nascent C3b with small molecules indicated that covalent attachment to a receptive surfaces was the result of a transacylation reaction from the newly exposed reactive acyl group of the internal thioester, either to surface hydroxyl (the preferred acceptor for C3b) or to surface amino groups (forming oxyester or amide bonds respectively). The extremely short half-life of the exposed thioester, estimated at around 60 μs, limits surface attachment to the immediate vicinity of C3 activation [50], and transacylation was believed to be the direct consequence of a conformational change following proteolytic activation of C3 to C3a and C3b. Final confirmation of these deductions came with the crystal structures of the native protein and its activation and inactivation products [51]. Transacylation to a surface hydroxyl group, while not irreversible, provides a long-lived C3b, iC3b, or C3dg tag to the surface.
Surface-bound C3 convertase stability
Surface-bound C3bB and C3bBb complexes, similarly to the fluid-phase complexes, are intrinsically unstable [31], with a short half-life. However, and in contrast to the fluid-phase complexes, surface-bound C3bB and C3bBb convertases can be stabilized by properdin [52, 53]. The strength of the interaction of properdin with surface-bound C3b is weak, but is enhanced on C3bB, and enhanced further when C3bBb is generated. The avidity of properdin for surface-bound iC3b is even less than that for bound C3b. Properdin also inhibits inactivation of bound C3b by FI (but not the inactivation of fluid-phase C3b) [54]. The products of FI action on surface-bound C3b remain the same as those generated in the fluid phase.
Membrane regulators of amplification loop activity
Clearly, as uptake of C3b onto a surface is essentially agnostic, the host requires mechanisms to protect homologous surfaces, particularly those normally exposed to complement such as blood cells and the vascular endothelium, from potentially damaging complement attack.
CD55
The first indication of a membrane-bound factor that blocked the activity of membrane-bound C3 convertases came from the experiments of Hoffmann in 1969, who identified a factor in the erythrocyte membrane, a factor that accelerated the decay of the surface-bound C4b2a classical pathway C3 and C5 convertases. Subsequently isolated, initially from the guinea pig then subsequently from other species including man, the protein, now known as CD55, was shown to accelerate the decay of not just surface-bound C4b2a but also the surface-bound C3bBb enzyme [55]. Its major role in regulating the amplification loop convertase on homologous cells was emphasized by retention of decay-accelerating activity even on neuraminidase-treated cells (see later) [56]. Understanding of the role of CD55 was greatly enhanced by analysis of the defect in a rare blood disorder, paroxysmal nocturnal hemoglobinuria (PNH). PNH is now known to be the consequence of a defect in the synthesis of the glycosylphosphatidylinositol (GPI) membrane anchor, and CD55 is one of many proteins attached to the cell membrane through a GPI anchor. PNH red blood cells, which are highly susceptible to complement-mediated lysis, were shown to lack CD55 and to be heavily coated with C3 [57]. Insertion of human CD55 into the cell membrane of sheep erythrocytes profoundly affected their ability to support amplification of C3 deposition [58] and, when inserted into the membrane of PNH cells, restored their ability to regulate amplification of C3 deposition onto their surface [59]. As might be expected for such a critical regulatory protein, CD55 is found on most cell types normally exposed to blood.
CR1 (CD35)
Though CR1 was the first regulator to be identified, it was not the first isolated. This was CR1, identified initially as an inhibitor of the amplification loop C3 convertase [60]. Unlike CD55, which has only decay-accelerating activity, CR1 has both decay-accelerating and FI cofactor activities. Not only could it replace FH in the generation of iC3b, irreversibly removing the ability of C3b to reform a C3 convertase, but expressed cofactor activity for FI in the generation of C3c (released from the C3b acceptor) and C3dg, which remained attached [61]. When solubilized and isolated from red blood cell membranes, inhibitory activity was manifested in the accelerated decay of properdin-stabilized C3bBb enzymes, an accelerated decay that was unaffected by sialic acid removal from the erythrocyte (see later) [60]. Further characterization showed it to be the C3b or immune adherence receptor. CR1 showed subtle differences from FH in both its decay-accelerating and FI cofactor activities. It was more effective in dissociating some (but not all) C3NeF-stabilized C3bBb convertases [62], and in its cofactor activity on C3b bound to IgG [63]. These subtle differences are supported by overlapping, but not identical, binding sites for CR1 and FH on C3b. A differential effect of CR1 on C3 and C5 convertases, with CR1 more effective against C5 convertases, may be attributable to competition between C3b and C5 rather than C3b and FB or Bb. An understanding of the role of CR1 in amplification loop regulation was complicated by the existence of genetic variants carrying different numbers of C3b-binding domains. However, as PNH cells carried normal levels of CR1 [64], their susceptibility to enhanced C3b deposition and lysis gave unequivocal demonstration of the primary role of CD55, and not CR1, as the major intrinsic regulator of amplification convertase activity on homologous cells. This conclusion was supported by normal decay-accelerating activity on CR1-deficient erythrocytes [57]. CD55 acts as an intrinsic (same cell) regulator of the amplification loop C3 convertase, whereas CR1 acts primarily as an extrinsic regulator.
CD46
CD46 was the last membrane-associated regulator of the amplification loop convertase to be identified. Isolated as a C3b-binding protein from leukocytes and platelets, it was subsequently shown to have cofactor activity for FI in the inactivation of both C3b (to iC3b) and C4b, but no decay acceleration activity [65]. Cofactor activity was preferentially expressed by the membrane-bound rather than solubilized protein [66], to be primarily directed against the amplification loop convertase, not the classical pathway convertase [65, 67], and, possibly, to have greater efficacy in regulation of C5 rather than C3 activation [68]. As with other cofactors, this preferential effect on C5 vs. C3 activation may reflect a greater accessibility of the C5-binding vs. FB-binding C3b molecule.
Together, these three proteins form an integral protection for cells against homologous attack, possibly acting synergistically. Not all cells exposed to complement express all three proteins—indeed the late discovery of CD46 may be a reflection of its absence, in man, from the red blood cell. It should also be noted that these homologous restriction factors (HRFs), as they came to be known, are, as with most protein-protein interactions, conserved across species. They are generally active against heterologous complement and do not form the molecular basis for species-specific amplification of C3 activation.
The influence of the surface on amplification loop activity
Parallel to investigations into HRFs were investigations of the influence of the surface on its ability to support amplification. These uncovered yet another mechanism, possibly the most critical, by which the host protects itself from homologous complement attack. In 1974, the ability of rabbit erythrocytes to support C3 activation and cell lysis, in the absence of C1 activation, was reported. This was shown to be a consequence of protection of deposited C3b from the action of FI and FH [69]. Similar protection against inactivation by FI and FH was argued for C3b deposited on zymosan [42]. Further analysis showed that protection of C3b against FI-mediated inactivation correlated with sialic acid content of cell surfaces. Removal of sialic acid from sheep erythrocytes resulted in amplified C3 activation by components of the properdin system [70]; this effect could be reversed by resialylation [71]. Mouse strains with genetically determined low sialic acid content supported amplification, whereas those with high sialic acid content did not [72]. A low sialic acid content of the cell membrane correlated with a decreased affinity for FH binding, and hence favored formation of the C3bB, C3bBb, and C3bBbP enzymes [73]. Further evidence pointing to the relevance of sialic acid in protection against amplified C3 activation came from a study with group B streptococci possessing capsular sialic acid that failed to amplify C3b deposition unless the sialic acid was masked or removed [74], and from the incorporation of sialoglycolipid GM3 into liposomes [75]. Investigations of the properties of C3b bound to different ligands demonstrated that, additionally, properties of the acceptor also influence the rate at which C3b can be inactivated by FI and FH, again primarily because of surface influence on the C3b-FH interaction [76]. Even enhanced C3b deposition can, with time, change a surface from activating to non-activating [77], possibly a consequence of an increased avidity of FH binding, as a dimer or higher multimer, at regions of high C3b density.
It is less clear why antibody may enhance amplification of C3 activation under conditions where classical pathway activation is inhibited. One possibility is that antibody binding alters the cell surface, masking FH binding. Alternatively, antibody binding might provide protected C3b acceptor sites, on which amplified C3 activation occurs, close to the cell surface. C3b bound to antibody is protected from the action of FI and FH [78]. Similarly, C3b covalently bound to C4b in a classical pathway C3 convertase is protected from the action of FI, enhancing both amplification potential and C5 activation.
Detailed analysis of the molecular basis of FH augmentation of host defense against homologous attack has given considerable insight into the complexities surrounding this key aspect of regulation. Incorporation of polyanions such as heparin onto the surface of an activator such as zymosan resulted in decreased amplification [79], with high concentrations of polyanions even in the fluid phase limiting FH availability for effective regulation of fluid-phase amplification. Interpretation of early investigations into the nature of the heparin effect on amplification was complicated by the observations that heparin not only potentiated the C3b-FH interaction but also inhibited the C3b-FB interaction. Nevertheless, it became clear that FH possessed one or more polyanion binding sites, and that engagement of these enhanced the affinity of FH for C3b [80], thus favoring FI-mediated inactivation of C3b to iC3b. The site in the complement control protein repeats CCPs 19-20 appears critical for cell recognition and protection [81]. Not all polyanions enhanced FH binding to C3b, indicating subtle structural requirements in the spatial arrangement of anions [82], and pointing to a “pattern recognition” role for FH [83]. FH was also demonstrated to have at least two sites of interaction with C3b [84]. In a series of experiments, the heparin interaction sites on FH were finally identified as primarily being in CCP7 and CCP20, with some contribution from adjacent CCP repeats [85]. Binding sites of FH for C3 fragments were identified in CCPs 1-5 (C3b only), CCPs 19-20 (C3b and C3d) and, less well defined, CCPs 12-14 [86]. Subsequent studies have failed to confirm a specific role for CCPs 12-14, though disruption of the unusual constrained structure of these domains might have a distal effect on heparin-binding. The ability of the heparin-binding site of CCP7 to recognize a site in CRP might also play a role in enhanced amplification-dependent clearance of dead or dying cells expressing phosphocholine on their surface [87]. These data from domain deletion, site-directed mutagenesis, and monoclonal antibody probes have subsequently been validated by structural studies [88, 89] and by characterization of disease-related mutations in FH or by epitope definition of anti-FH autoantibodies [90], where the disease is consequent on dysregulated amplification of C3 activation. The picture that has emerged is that weak interactions between FH and C3b, and FH and polyanions such as surface heparin sulfate and glycosaminoglycans, act synergistically in protecting host cells against inappropriate C3b-initiated amplification of the complement response [91]. Tissue specificity of FH-surface interactions may play a role in determining the importance of specific interactions. Oligomerization of FH might also be important in increasing the avidity of FH-C3b interactions at areas of high C3b density.
Summary of the surface-bound amplification loop for C3 activation
It is clear from the sophisticated and yet simple mechanisms described above that evolution has provided, in the complement system, a highly effective surveillance mechanism for potentially damaging “contamination.” The initial step in this is non-discriminatory. Continual low-level proteolytic activation of C3 generates C3b (C3 “tick-over”), confers on C3b a transient ability to bind to a surface (see Fig. 1a). Generation of C3b in close proximity to a surface is enhanced by the innate pattern-recognition-dependent focused activation of the lectin pathway), by a link to the focused adaptive antibody response through the classical pathway, and by the local protease-rich environment at sites of infection, inflammation, and coagulation. In this sense, the classical activation pathway is primarily an effector arm of the antibody response and is not a truly innate response. It should also be noted that the existence of the LP was established long after the molecular mechanisms of the Properdin System were defined, and it is possible that the LP, with its multiple pattern recognition molecules, plays a more significant role in initial C3 activation than is currently believed. The consequence of C3b deposition is determined by host recognition of the nature of the surface, not simply self or non-self, but malignant (potentially damaging) or benign (non-threatening). FH plays a key part in this, as a pattern recognition molecule, but protective. The completeness of the system was amply demonstrated by reconstitution from its isolated components on natural and unnatural substrates [92, 93]. Interestingly, the impact of properdin in the lysis of rabbit and desialylated sheep erythrocytes was minimal, giving only a doubling in their rate of lysis [92], an early indication that not all amplification is properdin-dependent, and later substantiated in the mouse by properdin knock-out [93].
Recognition of the surface as potentially damaging results in amplification of C3 activation by components of the properdin system, and recruitment of the major effector functions of complement to the targeted surface. With the exception of some pathogenic C3NeFs, C5 activation is essentially a surface-dependent phenomenon [94]. Some reports suggest that the efficacy of the amplification convertase is enhanced by the formation of covalently linked C3b dimers. Surface-dependent recruitment of effector arms is also evident in the effects of soluble products of C3 activation, ineffective except at unphysiologically high concentrations. Soluble monomeric C3b does not engage CR1, soluble iC3b does not engage CR3, and soluble monomeric C3d or C3dg does not engage CR2. The argument against soluble C3 activation products having effector roles possibly extends to C3a, where responses might be expected to be concentration-dependent, only synergistic with C5a at the higher concentrations that would be found close to sites of amplified generation (see [95]). The importance of amplified C3 activation at a surface is reflected in the different early responses to infection in amplification-sufficient and amplification-deficient animals, and the system has apparently evolved to be highly responsive to this need. It is a systemic surveillance system providing a local focused effector response.
Effective functioning of the system appears dependent on highly specific enzymes, either circulating in their active form ready to act on their substrates as they are assembled (FD, FI), or as a zymogen waiting to be activated at a C3b-tagged surface (FB). Site-directed mutagenesis and structural studies of expressed recombinant protease domains, of the intact enzymes, and of enzymes in complexes, have supported this view. In free FD, the active site is poorly configured, only adopting a productive configuration in the C3bBD complex [15, 96]. Kinetic experiments also support an induced fit mechanism of action on binding its complex substrate, C3bB. This accounts for its poor esterolytic activity on low molecular weight substrates and its high degree of substrate specificity, FB in C3bB being its only substrate. It can however be inactivated by DFP [13]. While a conformational change in the active site of FI is induced on binding to C3b, it requires the presence of a cofactor, FH, MCP, or CR1 to be positioned correctly to attack scissile bonds in C3b [97]. The active site of FB appears less disorganized in the zymogen as it is accessible to DFP. This might account for early reports of C3-cleaving activity by uncleaved FB [98]. Dissociation of Bb from the C3bBb complex results in irreversible loss of Bb activity; this might reflect a requirement for the Ba domain in its initial docking on C3b or in the initial C3bBP complex. Structures of FB either free or complexed with C3b or CVF highlight the considerable conformational changes associated with its binding to C3b [99]. Together with structures of C3b complexed with the FI cofactor, FH [100], and an understanding of the structural basis of the large conformational changes that occur in C3 on activation and subsequent inactivation [101], a detailed picture of the molecular structures of C3-containing amplification and down-regulatory complexes has emerged.
These data support the mechanistic concept that surface-binding of C3b, however generated, serves as an early warning alert of host exposure to potentially damaging events. Focused C3b generation is provided by the CP or LP, or by the amplification convertase C3bBb. Despite the similarity between the C4b2a and C3bBb enzymes, there is no “alternative” C3 activation pathway, simply a multiplicity of ways in which C3b can be generated and initiate amplification of its own generation. Surface binding of C3b triggers the system, binding FB, and, activated by FD, generating C3bBb. Bb can be displaced by FH or membrane-bound CD55 (or CR1). FI can act on C3bH or C3b complexed with CD46 (or CR1) to generate iC3b. FD is believed to be the rate-limiting enzymes in the activation of C3 (by C3bBb), and FI rate-limiting for the inactivation of C3b. Efficacy of the soluble regulators of the amplification convertase is surface-determined. Properdin has a low affinity for C3b, greater affinity for C3bB, and greatest affinity for C3bBb, enhancing the half-life of the surface-bound amplification convertase several-fold. As properdin is a variable multimer, mostly comprised of three or four identical subunits, it is possible that focused C3b deposition leads to enhanced properdin binding. FH has a relatively low affinity for C3b, sufficient for fluid-phase regulation, but the affinity of the C3b-FH interaction, favoring down-regulation of the convertase, is greatly enhanced by concomitant surface recognition through specific polyanion binding. Again, the ability of FH to form oligomers might enhance directly binding at sites of focused C3b deposition, but the ability of FH to down-regulate the amplification, and compete with properdin stabilization, and is heavily dependent on engagement of its C-terminal domains with suitably orientated self-protective polyanions. The fate of surface-bound C3b is determined by competition between FH and properdin. On HRF-protected surfaces, or surfaces offering enhanced interaction between C3b and FH, the balance is heavily in favor of down-regulation (Fig. 1c). Only where these regulatory processes are absent or negated does amplification of C3 activation occur (Fig. 1d).
There have however been specific challenges to this view:
The role of thioester-hydrolyzed C3 (C3(H2O)) in initiation of the amplification loop
Concomitantly with the identification of the metastable thioester in C3 came the realization that C3 in which the thioester had been broken, as a result of nucleophilic attack at the activated acyl group, either by water hydroxyl (greatly accentuated by disruption of the structure by chaotrophic agents) or by a small nitrogen nucleophile, acquired C3b-like properties without cleavage and release of the C3a peptide [102]. Nucleophilic attack was accompanied by the same conformational changes as those seen in the C3 to C3b transformation [103]. Realization that C3(H2O), as thioester hydrolyzed C3 is now known, acquired C3b-like properties led to the proposal that C3(H2O), on recruiting FB, formed the initiating C3 convertase of the amplification loop [104] and was the initial event in C3 tickover-dependent generation of C3b, a hypothesis that has acquired textbook status. Early comparison of the kinetics of the C3(H2O) and C3b complexes with FB and FH showed that the C3(H2O) complex with Bb was both less stable and less active than the equivalent complex with C3b [11], and the association constant of FB with C3(H2O) was fivefold lower than that with C3b [105]. Later studies, using different methodology, have given different results, favoring slightly enhanced C3(H2O)-FB association and a slightly more active C3(H2O)Bb enzyme when compared to C3b [106]. The same study suggested that FH-dependent FI inactivation of C3(H2O) was marginally slower than C3b inactivation. Enhanced generation of C3(H2O) has been claimed in specific situations such as forced blood oxygenation, and deposition of C3(H2O) onto protein adsorbed to biomaterials has been argued as the preliminary step in C3 amplification on the polymer surface. Biomaterials, long known to support amplification of C3 activation, are however believed to induce significant protease/protease imbalance, so non-complement proteolytic activation of C3 is hard to exclude. Nevertheless, these more recent data led to a refined proposal, that accelerated generation of C3(H2O), “contact activation”, be regarded as the initiating step in amplified C3b generation [107].
While there is no doubting the properties of C3(H2O), there are difficulties with a C3(H2O)-dependent tickover hypothesis. C3(H2O) cannot attach covalently to a surface as the activated acyl group has been hydrolyzed by water. There are significant spatial and temporal constraints to the covalent attachment of C3b to a surface; generation of C3b by a C3(H2O)Bb enzyme is a random event, with a high likelihood of decay of the activated acyl group prior to a productive surface encounter. Whatever the precise kinetics of C3(H2O)- and C3b-dependent convertase formation and activity, regulation of fluid-phase convertases remains heavily in favor of inactivation. Incubation of all components of the amplification loop (and lytic pathway proteins) at physiological concentrations at 37 °C gave no C3 or FB (or C5) consumption; the mixture was stable [92]. This correlates well with the experiences of component isolation from serum or plasma. Finally, the plasma half-lives of the highly structurally homologous proteins C3, C4, and C5 are similar, of the order of 60 h in healthy individuals [108, 109]. C4, while it has an internal thioester and is subject to water hydrolysis, has no self-catalyzed amplified activation, and C5 has no internal thioester. This argues in favor of minimal thioester-hydrolysis-dependent C3 consumption. It is therefore the view of the author that C3(H2O) generation is unlikely to be the initial step in generating surface-bound amplification convertases. This is far more likely to be a consequence of focused activation consequent on CP or LP activation, or of non-specific protease activation at sites of inflammation, infection, or coagulation. There may be specific instances where C3(H2O) plays a role, but these are likely to be the exception, not the rule.
The role of properdin
Controversies have surrounded the role of properdin since its discovery by Pillemer in 1954 [1]: initiator of amplification, stabilization of amplification convertases once they were formed, or a mixture of the two? Pillemer himself believed that properdin, in its interaction with zymosan, had a recognition function, but this view was challenged by Nelson in 1958, who believed that properdin binding required the presence of surface-bound C3b [3]. A second controversy has centered on the existence or not of an activated form of properdin. Much of this controversy is likely to be a consequence of the acknowledged difficulty of working with isolated properdin, and of its molecular heterogeneity.
Properdin is a multimer of identical subunits, circulating in plasma as a mixture of cyclic oligomers, mostly dimers, trimers, and tetramers [6, 110]. It is not known what influences the degree of oligomerization: isolated oligomers are stable and, unless first subjected to denaturation and renaturation [111], do not re-equilibrate. Higher oligomers have a higher affinity for C3b and its complexes with FB and Bb [111], possibly influencing isolation procedures that utilize C3b-binding. Each properdin monomer is comprised of six non-identical thrombospondin type-1 repeats (TSR1s). Domain deletion analysis has ascribed specific association functions to individual domains and suggests that each monomer has a C3b and C3bBb binding site [112]. This view is supported by analysis of a recently described dysfunctional properdin that does not form oligomers [113], the analysis confirming also the importance of oligomerization for function. Electron microscopic analysis hints at a properdin-induced conformational change in C3b in the C3bBbP complex; this may account for protection against down-regulation. One intriguing aspect of properdin that has had very little attention is that it is heavily C-mannosylated. C-mannosylation is a rare post-synthetic modification of tryptophan residues, and its purpose is not understood. A recent study has highlighted considerable heterogeneity in C-mannosylation in properdin [114], and possible roles in properdin could be in determining TSR flexibility and preferred oligomerization structure, or in ligand affinity.
Possibly in an attempt to force parallels with activation of the classical pathway, much early work with properdin centered on attempts to define “activated” properdin, and the mechanism by which it was generated. Several reports of an altered electrophoretic mobility of properdin under conditions in which the amplification convertase was active were interpreted as evidence for activation. With hindsight it seems probable that the change in mobility was caused by association of properdin, either as a tight non-covalent complex, or even covalently, with the amplification convertase or its products, or it possibly reflects a selective depletion of higher oligomers or otherwise functionally differentiated species. Reports of a properdin converting enzyme as an initiator of amplified C3 activation [35] were not substantiated, and in some investigations of amplification, it was simply assumed that if properdin was functional, it must be in the activated state [5, 53]. Some ascribed generation of “activated” properdin to a conformational change [34] or to proteolytic cleavage. The latter was not compatible with the reported reversible transition between “activated” and “precursor” states [115]. Finally, in the late 1980s, two groups independently reported that properties associated with “activated” properdin were associated with artefactual aggregates associated with purification and/or freeze-thaw of the isolated protein. Properdin dissociated from C3bBb complexes retained the discrete cyclic oligomer structure [110, 111].
The question of whether or not properdin can act as an initiator of amplification has proven much harder to resolve. In 1968, Pensky and coworkers found that highly purified properdin did not bind zymosan [4], a finding in agreement with the Nelson and not the Pillemer view of the system. The finding that in C2-deficient serum, zymosan, but not other initiators of amplification, bound properdin may well hint at non-CP activation of C3. In a series of publications, the La Jolla group developed the concept of C3b, optimally “dimeric,” as a properdin receptor for further recruitment of amplification convertases, with properdin the terminal, not initial, step in their formation [34, 52]. Later work by Lutz and coworkers supported this concept of preferential recruitment of properdin to C3b dimers (see [116]). Only artefactual “activated” properdin appeared to be able to bind surfaces and initiate amplification. Binding of properdin to solid-phase immune complexes was strictly C3-dependent [117]. The concept of initial C3b-dependent capture of properdin a requirement for further amplification convertase recruitment, at that site, is in keeping with the observation that cells expressing transmembrane recombinant properdin can initiate amplification [118]. The later SPR findings of Hourcade [119] and the data obtained with fungal recruitment of the amplification convertase [120] also support this view. Despite this, a number of more recent publications have again argued for a recognition role for properdin, on pathogens [117, 121, 122], on tubular epithelial cells, and on apoptotic and other cell populations [123]. With these data, the concept of properdin having a dual role, both as a stabilizer of the C3bBb amplification convertase and as an initiating component, in a properdin “pathway”, with properdin defined as a pattern recognition molecule, has re-emerged [124]. The re-emergent “properdin as a recognition molecule” hypothesis is strongly contested. Reinvestigation of some of the above systems used in recent support of a pattern recognition role for properdin, but in the presence of compstatin, which totally blocks C3 activation, demonstrates an absolute requirement for C3 activation prior to properdin-binding, negating the pattern recognition concept [125, 126].
In defining a role for surface binding of properdin as an initiator of the amplification convertase, it is clearly necessary to rule out any possible contribution from C3b, generated either by CP or LP C3 convertase activity (e.g.by using a compstatin derivative) or by other proteolytic activity, which may not be inhibited by compstatin. The presence of “activated” properdin must also be rigorously excluded. The 1976 comment by Chapitis and Lepow, “caution (is required) in interpretation of immunopathological studies in which properdin deposits are found in the presence of C3”, [6] seems as pertinent now as it was then.
The nature of circulating factor D
FD is an unusual serine protease in that the zymogen possesses an unique short 5-residue propeptide. When first isolated, FD was thought to be in its zymogen form and, by analogy with the classical pathway, requiring activation prior to acting on the C3bB complex [13]. Activation by trypsin or by preparations of properdin was claimed, with the zymogen DFP-resistant, and trypsin-cleaved FD DFP-sensitive [14]. Interestingly, citrated plasma was used as the source for FD in this isolation. Others, using different isolation procedures and/or a different source for isolation (EDTA-plasma or serum), purified FD only in its active form. It was also claimed that purified FD had a different electrophoretic mobility to FD in serum. Nevertheless, throughout the late twentieth and early twenty-first centuries, the consensus was that FD circulated, in plasma, as an active enzyme. This was fully in accord with a role in immune surveillance through amplification of C3 activation. FD has exquisitely high specificity and is only active on its sole substrate FB when FB is in a productive complex with C3b. This is the consequence of its poorly organized active site, which is only expressed when bound to C3bB [96]. In 2010, this view was challenged by new data that showed that in mice deficient in MASP-1/3, FD circulated in its zymogen form, and that the zymogen could be activated by MASP-1 or MASP-3 to the active enzyme [127]. This data was substantiated by the finding that MASP-1/3 deficiency had the same protective effect in a mouse model of disease, collagen antibody-induced arthritis, previously shown to be FD-dependent [128]. Against this was the finding that MASP-1/3 deficiency did not overcome the amplification-driven pathology seen in FH-deficient mice [129]. This was followed by the claim that the generation of active FD from pro-FD in man was similarly MASP-1- or MASP-3-dependent [130]. (MASP-1 and MASP-3 come from alternative transcripts of the MASP-1/3 gene, and differ only in their protease domains.) Others have argued against this, showing that patients with a MASP-1/3 deficiency disease called Malpuech–Michels–Mingarelli–Carnevale (3MC) syndrome had a functional amplification loop [131], and that both pro-FD and active FD could be detected in their serum [132]. While MASP-3 made a major contribution to FD activation, it was not the only enzyme, in man, capable of effecting this change. Additionally, while FD deficiency in man is associated with an increased infection risk, 3MC syndrome does not appear to carry the same risk; in none of the reports of 3MC syndrome, with some patients in adulthood, are there reports of increased infection rates.
It is possible that with respect to FD processing, man and mice are not the same. This would not be the first difference between mouse and man noted within the complement system. For example, while the functions ascribed to the membrane bound molecules of the Regulators of Complement Activation (RCA) family are conserved between mouse and man, the molecules that affect these functions are often structurally different, or show different cell distribution. Might there be reasons for differences in FD processing?
-
MASP-3 circulates as an inactive zymogen; activation requires surface-binding. In man, FD is a small non-glycosylated protein, comprising only the serine protease domain, and with a short half-life (ca. 1 h) [133]. Murine FD in contrast is glycosylated, and this will be reflected in a considerably longer half-life. Residual active FD from a MASP-dependent activation event, sufficient to provide surveillance, might persist longer.
-
Recent studies on the regulation of FD biosynthesis by mouse adipocytes have suggested that, in the mouse, FD might not be the rate-limiting enzyme that it is believed to be in man [134]. In this context, patients with end-stage renal disease and consequent increase in circulating FD levels have only a modest increase in activation products of the amplification loop and lytic pathway.
-
FD (adipsin) deficiency is associated with an obese phenotype in the mouse, a phenotype that is not seen in FD-deficient humans. Adipocytes are believed to be the major site of FD synthesis, yet FD mRNA expression is significantly reduced by insulin (tenfold), with potentially large variation in the amount of circulating FD, something not compatible with a rate-limiting role in amplification convertase generation.
-
Might there be differences in processing dependent on the site of synthesis? In man the adipocyte, with processing of FD claimed to occur in intracellular vesicles, is believed to be a major site of synthesis. MASP-3 is not synthesized in the adipocyte. In the mouse, adipocyte FD activity presumably proceeds in the absence of any recognized “immune surveillance” activity.
Clearly, this is a controversy waiting to be resolved. However, pro-FD activation requiring a surface-mediated event to activate the enzyme responsible for pro-FD activation and therefore to initiate amplification of C3 activation is counter-intuitive to the immediate response required in an effective surveillance model. While augmented pro-FD activation at sites of MASP-3 activation might be beneficial, this is something which is yet to be demonstrated.
The role of factor H-like protein 1 (FHL-1)
In 1987, an alternative transcript of the FH gene was identified [135] and subsequently shown to code for a protein, FHL-1, that possessed cofactor activity for FI in the inactivation of C3b to iC3b. FHL-1 comprises the N-terminal 7 CCP domains of FH [136], and therefore, in addition to the FH cofactor domains, contains the CCP7 heparin-binding domain. FHL-1 is relatively unstudied. FH and FHL-1 are differently expressed in some inflammatory diseases [137], but the consequences of this are unclear. FHL-1 does not possess the C-terminal heparin-binding domain of FH, so cannot be recruited to a surface to augment host defense. It has recently been suggested that FHL-1 might play a role in protection against amplified C3 activation in extravascular spaces such as Bruch’s membrane of the eye where FH, because of its highly asymmetric structure, cannot freely diffuse [138]. With equivalent fluid-phase regulatory properties to FH, it is possible that the role of FHL-1 in regulation of C3 activation has been underestimated.
The role of factor H-related proteins
At the same time as FHL-1 was identified as the product of an alternative transcript of the FH gene, encompassing the amino-terminal seven CCPs, additional mRNA species encoding FH-related proteins, containing two CCPs with high homology, but not identity, to the carboxy-terminal CCPs of FH [139, 140] were identified. These mRNAs encoded proteins now known as factor H-related proteins (FHRs). Although high structural similarity, particularly in the C-terminal domains, led to suggestions that they might have properties in common with FH, they did not appear to have any FH-like regulatory function in down-regulation of the amplification loop [140, 141]. A lack of FH-like regulatory function is not unexpected as they lack CCP repeats with high homology to the four N-terminal CCPs of FH required for decay-accelerating and FI-cofactor activity. Despite one claim to the contrary, this appears largely to be born out experimentally. A lack of down-regulatory activity is also apparent from FH-deficient individuals, who lack FH and FHL-1, but not FHRs, yet are unable to regulate amplification of C3 activation [142]. FHRs show glycosylation heterogeneity and, in one case (FHR-4A and FHR-4B), alternative splicing variation. The extremely high sequence homology between the C-terminal CCP domains of FHRs and FH suggested that heparin/glycosaminoglycan and C3d/C3b binding might be conserved, and this has been born out for all FHRs identified to date. Specific properties for individual FHRs have also been reported, for example the surface-binding of FHR-4, or the FHR-5-dependent recruitment of properdin, to form platforms for amplification convertase assembly. Against this are the seemingly contradictory claims of FHR-1 as an inhibitor of C5 activation [143], and FHR-2 as an inhibitor of C3 activation by the C3bBb C3 convertase [144].
It seems possible that different assay procedures used in different laboratories might have contributed to apparently contradictory roles of FHRs. Two recent discoveries have however given significant insight into the possible role of FHRs: The first is the observation that FHRs can form either homo- or hetero-dimers [145]. Thus, while the affinity of specific interactions, whether to C3b/C3d or to the cell surface, might be low, the avidity of these interactions is greatly enhanced by dimer formation. The second insight came from analysis of the molecular defects in diseases associated with dysregulated complement activation, particularly atypical hemolytic uremic syndrome (aHUS). aHUS has long been known to be associated with complement activation, with increased generation of amplification convertase activation products. In a number of cases, the causative agent appears to be hybrid proteins combining FHRs in non-natural combinations. Together, these data have led to the hypothesis that FHRs, through their surface and C3b/C3d binding, act as antagonists to FH, adding a further layer of sophistication to host/non-host discrimination. Where pathologic hybrid molecules are formed the balance of regulation, or possibly specific host/non-host recognition, is lost, and the critical balance of regulation of the amplification convertase is compromised [146]. Analysis of two hybrid proteins, an FH-FHR-1 hybrid and an FH-FHR3 hybrid, has also emphasized the critical nature of spatial organization of the complement control protein repeat domains in FH for optimal function [147]. The hypothesis of FHRs as antagonists to FH is reviewed in more depth in [148].
It is also possible that FHRs have evolved to combat pathogen complement evasion. Many pathogens have evolved strategies to evade complement attack, and many of these subvert the complement response by recruiting FH to their surface, in order to down-regulate the amplification convertase. One host response could be to generate molecules such as FHRs that compete with FH for binding to the pathogen FH-binding protein, blocking the ability of the pathogen to subvert amplification.
This is clearly a developing story, and it will be intriguing to see how it develops in the next few years.
Therapeutic implications
With increased understanding of the complement system, and particularly activation and regulation of the amplification convertase, has come recognition that inappropriate regulation is a component of many diseases. While aHUS is one of the most studied and understood of these, dysregulation is suspected to be a causative agent in many other diseases, for example age-related macular degeneration (AMD) (see [149]). Now more than ever, there is a need for amplification-directed agents to test these hypotheses in man and, if proven, to provide effective therapy. One possible approach to inhibition of amplification is to target the amplification proteases, FD and FB. Are there reasons to prefer one over the other?
The choice of target, FD, or FB?
Two low molecular weight (LMW) orally bioavailable highly specific FD inhibitors have been described in the literature [150, 151], but, as yet, no FB inhibitor. While at first sight the two targets might seem equivalent in terms of efficacy in inhibiting amplification of C3b generation, older literature contains many reports of non-FD activation of FB in the C3bB complex. While some are non-physiological, they do indicate accessibility of the scissile bond to proteases other than FD, and activation by kallikrein [152] might have relevance under specific circumstances. There are also reports of C3-cleaving activity by intact FB in the C3bB complex [98, 153]. While considerably less efficient than FD activation of C3bB to C3bBb, in conditions where, once formed, the C3bBb enzyme is stabilized, as in C3NeF complexes, non-FD activation could negate any beneficial effect of FD inhibition. There are pros and cons too to the targets as suitable therapeutic targets. In plasma, FD is a far less abundant protein than FB, making it in some ways more appealing; with similar potency against the target, lower drug levels would be needed to effect equivalent inhibition. Against this is the far higher turnover of FD when compared to FB. While FB, with its higher plasma concentration, might seem a less attractive target, the existence of different conformations of the protease domain in free FB, complexed as C3bB or C3bBb, or released as inactive Bb, suggests that there might be an opportunity for selectively targeting the active form of the enzyme. There is also an open question over the configuration of the protease active site that is recognized by an inhibitor. If it is only that expressed in the enzyme-substrate complex, greater FD inhibition for equivalent effect might be required. FD activates a single FB only in any one C3bB complex, before diffusing to the next proenzyme complex, whereas each C3bBb, particularly if stabilized, has the potential to activate many C3 molecules. Amplification of C3b generation occurs when the rate of C3b generation exceeds that of its FI-mediated inactivation, and it may be easier to achieve this therapeutic goal with a FB inhibitor. On the plus side, the fact that both FD and FB are highly unusual serine proteases with cryptic active sites increases the probability that effective inhibitors will be highly selective. It will require clinical testing in different diseases before answers to these theoretical considerations become apparent.
The goal of amplification convertase inhibition
Amplification convertase-driven diseases are highly sensitive both to the levels of individual components [154] and to their genetically determined polymorphism. Factor I levels in particular might have a physiologically meaningful effect on the balance between amplification and down-regulation [36, 155]. The plasma levels of individual complement proteins are under both genetic and environmental control. For example, FH levels can vary up to fivefold between individuals [156]. The term complotype has been coined to describe the polymorphic make-up of individuals [157], and an individual’s complotype argued to influence significantly their susceptibility to disease risk. In many diseases, such as AMD or aHUS, the goal of therapeutic inhibition would be to convert an individual from a high risk to a low risk phenotype (see Fig. 2). This would not require complete shut-down of amplification. In practice, given the variability between individuals, it could be challenging to achieve this with a LMW inhibitor and might require refinements such as controlled release formulation (feasible) and/or individualized dosing (more challenging). Other diseases such as C3 glomerulopathy, driven by C3NeF-stabilized convertases, might require a more complete shutdown of amplification. Additionally, the degree of inhibition require to control disease, or disease-related pathology, may well be disease-specific.
a In amplification loop-driven disease down-regulation of the amplification convertase is lost, C3 and C5 activation is enhanced, with concomitant and damaging recruitment of C3- and C5-dependent effector mechanisms (e.g. opsonization, neutrophil chemotaxis, MAC assembly). b The optimal goal of an amplification loop-directed therapeutic (e.g., a FD or FB inhibitor) is to restore the normal regulatory balance such that disease-specific pathologic activation is controlled, without affecting the response to pathogens (e.g., encapsulated bacteria)
The challenge of infection—is there a therapeutic window?
The biggest challenge to acceptable inhibition of the C3 amplification convertase, certainly perceptually, and probably in practice, will be generating an appropriate balance between disease control and infection risk. It has long been known that genetic deficiency of circulating amplification pathway components carries a greatly increased risk of infection, particularly bacterial infections. Heterozygous deficiency appears not to be associated with infection, nor does a low-risk complotype for disease (e.g., AMD) carry any discernable increased infection risk. In 1983, Pangburn and coworkers described amplification as having three stages, a lag phase, an amplification phase, and a plateau phase [77]. The delay between the lag phase and amplification varied dependent on the trigger. Might there be a therapeutic window for a systemic inhibitor, particularly in chronic disease, in which the disease trigger can be down-regulated without adversely affecting control of infection? (This seems less likely for targeted inhibition, where both beneficial and deleterious C3b deposition would be impacted.) It is also possible that in diseases for which vaccination is possible (e.g., Meningococcus, Pneumococcus, Haemophilus influenza), an effective antibody response would give sufficiently strong classical pathway-dependent C3 activation for effector functions to be recruited without the participation of the amplification convertase. Such “drive-through” elicitation of opsonization, neutrophil and macrophage recruitment, and lytic functions could ease concerns over specific life-threatening infections (see Fig. 3).
a Under normal circumstances, and particularly in the early complement response to infection, much of the effector arm response (C3-dependent opsonization, C5a-dependent cell recruitment and activation, MAC-dependent membrane disruption and lysis) is driven by the amplification loop. b: In an effectively-immunized individual, Classical Pathway driven events predominate (the “Drive-Through” hypothesis), and effective recruitment of C3-dependent opsonization, C5a-dependent cell recruitment and activation and MAC-dependent membrane disruption and cell lysis occurs without any significant contribution from the amplification loop. (Note: Blockade of C5 would still disrupt C5-driven events, particularly Neisseria meningitidis killing, which is heavily MAC-dependent, whereas Amplification blockade would leave the Lytic Pathway intact)
Summary
Despite many decades of research into the amplification of C3 activation, and of its regulation, giving us a solid understanding of its basic mechanisms, some levels of intricacy remain to be fully understood, some are possibly as yet undiscovered. Nevertheless, the basic understanding that we have, that C3b, however generated, has the potential to bind to a surface, to amplify its own production and to recruit effector functions to the targeted surface, seems solid. These effector functions include C3-dependent opsonization, C5a-dependent cell recruitment, and membrane attack complex-dependent functions. C3b deposition is agnostic: any surface with exposed acceptor sites can be targeted; it is up to the host to discriminate between appropriate and non-appropriate targeting. The prospect of imminent therapeutic intervention studies gives a renewed impetus to refining both basic concepts and our understanding of complement and disease. The next few years promise to be both exciting and, particularly in the clinic, highly challenging.
References
Pillemer L, Blum L, Lepow IH, Ross OA, Todd EW, Wardlaw AC (1954) The properdin system and immunity. I. Demonstration and isolation of a new serum protein, properdin, and its role in immune phenomena. Science 120:279–285
Kemper C1, Pangburn MK2, Fishelson Z (2014) Complement nomenclature 2014. Mol Immunol 61:56–58. https://doi.org/10.1016/j.molimm.2014.07.004
Nelson RA Jr (1958) An alternative mechanism for the properdin system. J Exp Med 108:515–535
Pensky J, Hinz CF Jr, Todd EW, Wedgwood RJ, Boyer JT, Lepow IH (1968) Properties of highly purified human properdin. J Immunol 100:142–158
Götze O, Müller-Eberhard HJ (1974) The role of properdin in the alternate pathway of complement activation. J Exp Med 139:44–57
Chapitis J, Lepow IH (1976) Multiple sedimenting species of properdin in human serum and interaction of purified properdin with the third component of complement. J Exp Med 143:241–257
Müller-Eberhard HJ, Nilsson U, Aronsson T (1960) Isolation and characterization of two beta1-glycoproteins of human serum. J Exp Med 111:201–215
Blum L, Pillemer L, Lepow IH (1959) The properdin system and immunity. XIII. Assay and properties of a heat-labile serum factor (factor B) in the properdin system. Z Immun exp ther 118:349–357
Götze O1, Müller-Eberhard HJ (1971) The c3-activator system: an alternate pathway of complement activation. J Exp Med 134:90–108
Alper CA, Goodkofsky I, Lepow IH (1973) The relationship of glycine-rich glycoprotein to factor B in the properdin system and to the cobra factor-binding protein of human serum. J Exp Med 137:424–437
Pangburn MK, Müller-Eberhard HJ (1986) The C3 convertase of the alternative pathway of human complement. Enzymic properties of the bimolecular proteinase. Biochem J 235:723–730
Cooper NR (1973) Formation and function of a complex of the C3 proactivator with a protein from cobra venom. J Exp Med 137:451–460
Fearon DT, Austen KF, Ruddy S (1974) Properdin factor D: characterization of its active site and isolation of the precursor form. J Exp Med 139:355–366
Fearon DT, Austen KF, Ruddy S (1974) Properdin factor D. II. Activation to D by properdin. J Exp Med 140:426–436
SV1 N, Carson M, el-Kabbani O, Kilpatrick JM, Moore D, Chen X, Bugg CE, Volanakis JE, DeLucas LJ (1994) Structure of human factor D. A complement system protein at 2.0 A resolution. J Mol Biol 235:695–708
Lesavre PH, Müller-Eberhard HJ (1978) Mechanism of action of factor D of the alternative complement pathway. J Exp Med 148:1498–1509
Ballow M, Cochrane CG (1969) Two anticomplementary factors in cobra venom: hemolysis of guinea pig erythrocytes by one of them. J Immunol 103:944–952
Cochrane CG, Müller-Eberhard HJ, Aikin BS (1970) Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol 105:55–69
Vallota EH, Forristal J, Spitzer RE, Davis NC, West CD (1970) Characteristics of a non-complement-dependent C3-reactive complex formed form factors in nephritic and normal serum. J Exp Med 131:1306–1324
Vallota EH, Götze O, Spiegelberg HL, Forristal J, West CD, Müller-Eberhard HJ (1974) A serum factor in chronic hypocomplementemic hephritis distinct from immunoglobulins and activating the alternate pathway of complement. J Exp Med 139:1249–1261
Daha MR, Fearon DT, Austen KF (1976) C3 nephritic factor (C3NeF): stabilization of fluid phase and cell-bound alternative pathway convertase. J Immunol 116:1–7
Davis AE 3rd, Ziegler JB, Gelfand EW, Rosen FS, Alper CA (1977) Heterogeneity of nephritic factor and its identification as an immunoglobulin. Proc Natl Acad Sci U S A 74:3980–3983
Daha MR, Van Es LA (1981) Stabilization of homologous and heterologous cell-bound amplification convertases, C3bBb, by C3 nephritic factor. Immunology 43:33–38
Müller-Eberhard HJ, Götze O (1972) C3 proactivator convertase and its mode of action. J Exp Med 135:1003–1008
Lachmann PJ, Nicol P (1973) Reaction mechanism of the alternative pathway of complement fixation. Lancet 1(7801):465–467
Abramson N, Alper CA, Lachmann PJ, Rosen FS, Jandl JH (1971) Deficiency of C3 inactivator in man. J Immunol 107:19–27
Lachmann PJ, Müller-Eberhard HJ (1968) The demonstration in human serum of “conglutinogen-activating factor” and its effect on the third component of complement. J Immunol 100:691–698
Ruddy S, Austen KF (1969) C3 inactivator of man. I. Hemolytic measurement by the inactivation of cell-bound C3. J Immunol 102:533–543
Ekdahl KN1, Nilsson B (1995) Phosphorylation of complement component C3 and C3 fragments by a human platelet protein kinase. Inhibition of factor I-mediated cleavage of C3b. J Immunol 154:6502–6510
Whaley K, Ruddy S (1976) Modulation of C3b hemolytic activity by a plasma protein distinct from C3b inactivator. Science 193:1011–1013
Weiler JM, Daha MR, Austen KF, Fearon DT (1976) Control of the amplification convertase of complement by the plasma protein beta1H. Proc Natl Acad Sci U S A 73:3268–3272
Pangburn MK, Schreiber RD, Müller-Eberhard HJ (1977) Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med 146:257–270
Harrison RA, Lachmann PJ (1980) The physiological breakdown of the third component of human complement. Mol Immunol 17:9–20
Medicus RG, Schreiber RD, Götze O, Müller-Eberhard HJ (1976) A molecular concept of the properdin pathway. Proc Natl Acad Sci U S A 73:612–616
Spitzer RE, Stitzel AE, Urmson J (1976) Interaction of properdin convertase and properdin in the alternative pathway of complement activation. Immunochemistry 13:15–20
Lachmann PJ, Halbwachs L (1975) The influence of C3b inactivator (KAF) concentration on the ability of serum to support complement activation. Clin Exp Immunol 21:109–114
Bokisch VA, Müller-Eberhard HJ, Cochrane CG (1969) Isolation of a fragment (C3a) of the third component of human complement containing anaphylatoxin and chemotactic activity and description of an anaphylatoxin inactivator of human serum. J Exp Med 129:1109–1130
Johnson U, Ohlsson K, Olsson I (1976) Effects of granulocyte neutral proteases on complement components. Scand J Immunol 5:421–426
Kirschfink M, Borsos T (1988) Binding and activation of C4 and C3 on the red cell surface by non-complement enzymes. Mol Immunol 25:505–512
Matsushita M1, Fujita T (1995) Cleavage of the third component of complement (C3) by mannose-binding protein-associated serine protease (MASP) with subsequent complement activation. Immunobiology 194:443–448
Law SK, Minich TM, Levine RP (1984) Covalent binding efficiency of the third and fourth complement proteins in relation to pH, nucleophilicity, and availability of hydroxyl groups. Biochemistry 23:3267–3272
Fearon DT, Austen KF (1977) Activation of the alternative complement pathway due to resistance of zymosan-bound amplification convertase to endogenous regulatory mechanisms. Proc Natl Acad Sci U S A 74:1683–1687
Bokisch VA, Dierich MP, Müller-Eberhard HJ (1975) Third component of complement (C3): structural properties in relation to functions. Proc Natl Acad Sci U S A 72:1989–1993
Mardiney MR Jr, Müller-Eberhard HJ, Feldman JD. (1968) Ultrastructural localization of the third and fourth components of complement on complement-cell complexes. Am J Pathol 53:253–261.
Vogt W, Schmidt G, Von Buttlar B, Dieminger L (1978) A new function of the activated third component of complement: binding to C5, an essential step for C5 activation. Immunology 34:29–40
Hammer CH, Abramovitz AS, Mayer MM (1976) A new activity of complement component C3: cell-bound C3b potentiates lysis of erythrocytes by C5b,6 and terminal components. J Immunol 117:830–834
Law SK, Levine RP (1977) Interaction between the third complement protein and cell surface macromolecules. Proc Natl Acad Sci U S A 74:2701–2705
Law SK, Lichtenberg NA, Levine RP (1979) Evidence for an ester linkage between the labile binding site of C3b and receptive surfaces. J Immunol 123:1388–1394
Tack BF, Harrison RA, Janatova J, Thomas ML, Prahl JW (1980) Evidence for presence of an internal thiolester bond in third component of human complement. Proc Natl Acad Sci U S A 77:5764–5768
Sim RB, Twose TM, Paterson DS, Sim E (1981) The covalent-binding reaction of complement component C3. Biochem J 193:115–127
P1 G, Milder FJ, Janssen BJ (2008) Complement driven by conformational changes. Nat Rev Immunol 8:48–58
Medicus RG, Götze O, Müller-Eberhard HJ (1976) Alternative pathway of complement: recruitment of precursor properdin by the labile C3/C5 convertase and the potentiation of the pathway. J Exp Med 144:1076–1093
Fearon DT, Austen KF (1975) Properdin: initiation of alternative complement pathway. Proc Natl Acad Sci U S A 72:3220–3224
Farries TC, Lachmann PJ, Harrison RA (1988) Analysis of the interactions between properdin, the third component of complement (C3), and its physiological activation products. Biochem J 252:47–54
Nicholson-Weller A, Burge J, Fearon DT, Weller PF, Austen KF (1982) Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol 129:184–189
Okada H, Tanaka H, Okada N (1983) Prevention of complement activation on the homologous cell membrane of nucleated cells as well as erythrocytes. Eur J Immunol 13:340–344
Pangburn MK, Schreiber RD, Müller-Eberhard HJ (1983) Deficiency of an erythrocyte membrane protein with complement regulatory activity in paroxysmal nocturnal hemoglobinuria. Proc Natl Acad Sci U S A 80:5430–5434
Medof ME, Kinoshita T, Nussenzweig V (1984) Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med 160:1558–1578
Medof ME, Kinoshita T, Silber R, Nussenzweig V (1985) Amelioration of lytic abnormalities of paroxysmal nocturnal hemoglobinuria with decay-accelerating factor. Proc Natl Acad Sci U S A 82:2980–2984
Fearon DT (1979) Regulation of the amplification C3 convertase of human complement by an inhibitory protein isolated from human erythrocyte membrane. Proc Natl Acad Sci U S A 76:5867–5871
Medicus RG, Melamed J, Arnaout MA (1983) Role of human factor I and C3b receptor in the cleavage of surface-bound C3bi molecules. Eur J Immunol 13:465–470
Daha MR, Kok DJ, Van Es LA (1982) Regulation of the C3 nephritic factor stabilized C3/C5 convertase of complement by purified human erythrocyte C3b receptor. Clin Exp Immunol 50:209–214
Fries LF, Prince GM, Gaither TA, Frank MM (1985) Factor I co-factor activity of CR1 overcomes the protective effect of IgG on covalently bound C3b residues. J Immunol 135:2673–2679
Roberts WN, Wilson JG, Wong W, Jenkins DE Jr, Fearon DT, Austen KF, Nicholson-Weller A (1985) Normal function of CR1 on affected erythrocytes of patients with paroxysmal nocturnal hemoglobinuria. J Immunol 134:512–517
Seya T, Turner JR, Atkinson JP (1986) Purification and characterization of a membrane protein (gp45-70) that is a cofactor for cleavage of C3b and C4b. J Exp Med 163:837–855
Seya T1, Atkinson JP (1989) Functional properties of membrane cofactor protein of complement. Biochem J 264:581–588
Kojima A1, Iwata K, Seya T, Matsumoto M, Ariga H, Atkinson JP, Nagasawa S (1993) Membrane cofactor protein (CD46) protects cells predominantly from alternative complement pathway-mediated C3-fragment deposition and cytolysis. J Immunol 151:1519–1527
T1 S, Okada M, Matsumoto M, Hong KS, Kinoshita T, Atkinson JP (1991) Preferential inactivation of the C5 convertase of the alternative complement pathway by factor I and membrane cofactor protein (MCP). Mol Immunol 28:1137–1147
Fearon DT, Austen KF (1977) Activation of the alternative complement pathway with rabbit erythrocytes by circumvention of the regulatory action of endogenous control proteins. J Exp Med 146:22–33
Fearon DT (1978) Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A 75:1971–1975
Tomlinson S, Pontes de Carvalho L, Vandekerckhove F, Nussenzweig V (1992) Resialylation of sialidase-treated sheep and human erythrocytes by Trypanosoma cruzi trans-sialidase: restoration of complement resistance of desialylated sheep erythrocytes. Glycobiology 2:549–551
Nydegger UE, Fearon DT, Austen KF (1978) Autosomal locus regulates inverse relationship between sialic acid content and capacity of mouse erythrocytes to activate human alternative complement pathway. Proc Natl Acad Sci U S A 75:6078–6082
Kazatchkine MD, Fearon DT, Austen KF (1979) Human alternative complement pathway: membrane-associated sialic acid regulates the competition between B and beta1 H for cell-bound C3b. J Immunol 122:75–81
Edwards MS, Kasper DL, Jennings HJ, Baker CJ, Nicholson-Weller A (1982) Capsular sialic acid prevents activation of the alternative complement pathway by type III, group B streptococci. J Immunol 128:1278–1283
Okada N, Yasuda T, Tsumita T, Okada H (1983) Membrane sialoglycolipids regulate the activation of alternative complement pathway by liposomes containing trinitrophenylaminocaproyldipalmitoylphosphatidylethaolamine. Immunology 48:129–140
Pangburn MK, Morrison DC, Schreiber RD, Müller-Eberhard HJ (1980) Activation of the alternative complement pathway: recognition of surface structures on activators by bound C3b. J Immunol 124:977–982
Pangburn MK, Schreiber RD, Müller-Eberhard HJ (1983) C3b deposition during activation of the alternative complement pathway and the effect of deposition on the activating surface. J Immunol 131:1930–1935
Fries LF, Gaither TA, Hammer CH, Frank MM (1984) C3b covalently bound to IgG demonstrates a reduced rate of inactivation by factors H and I. J Exp Med 160:1640–1655
Kazatchkine MD, Fearon DT, Silbert JE, Austen KF (1979) Surface-associated heparin inhibits zymosan-induced activation of the human alternative complement pathway by augmenting the regulatory action of the control proteins on particle-bound C3b. J Exp Med 150:1202–1215
Meri S, Pangburn MK (1990) Discrimination between activators and nonactivators of the alternative pathway of complement: regulation via a sialic acid/polyanion binding site on factor H. Proc Natl Acad Sci U S A 87:3982–3986
Ferreira VP, Herbert AP, Hocking HG, Barlow PN, Pangburn MK (2006) Critical role of the C-terminal domains of factor H in regulating complement activation at cell surfaces. J Immunol 177:6308–6316
Meri S, Pangburn MK (1994) Regulation of alternative pathway complement activation by glycosaminoglycans: specificity of the polyanion binding site on factor H. Biochem Biophys Res Commun 198:52–59
Blaum BS (2017) The lectin self of complement factor H. Curr Opin Struct Biol 44:111–118. https://doi.org/10.1016/j.sbi.2017.01.005
Sharma AK, Pangburn MK (1996) Identification of three physically and functionally distinct binding sites for C3b in human complement factor H by deletion mutagenesis. Proc Natl Acad Sci U S A 93:10996–11001
Blackmore TK, Hellwage J, Sadlon TA, Higgs N, Zipfel PF, Ward HM, Gordon DL (1998) Identification of the second heparin-binding domain in human complement factor H. J Immunol 160:3342–3348
Jokiranta TS, Hellwage J, Koistinen V, Zipfel PF, Meri S (2000) Each of the three binding sites on complement factor H interacts with a distinct site on C3b. J Biol Chem 275:27657–27662
Giannakis E, Male DA, Ormsby RJ, Mold C, Jokiranta TS, Ranganathan S, Gordon DL (2001) Multiple ligand binding sites on domain seven of human complement factor H. Int Immunopharmacol 1:433–443
Schmidt CQ, Herbert AP, Hocking HG, Uhrín D, Barlow PN (2008) Translational mini-review series on complement factor H: structural and functional correlations for factor H. Clin Exp Immunol 151:14–24
Kajander T, Lehtinen MJ, Hyvärinen S, Bhattacharjee A, Leung E, Isenman DE, Meri S, Goldman A, Jokiranta TS (2011) Dual interaction of factor H with C3d and glycosaminoglycans in host-nonhost discrimination by complement. Proc Natl Acad Sci U S A 108:2897–2902. https://doi.org/10.1073/pnas.1017087108
de Córdoba SR, de Jorge EG (2008) Translational mini-review series on complement factor H: genetics and disease associations of human complement factor H. Clin Exp Immunol 151:1–13
Blaum BS, Hannan JP, Herbert AP, Kavanagh D, Uhrín D, Stehle T (2015) Structural basis for sialic acid-mediated self-recognition by complement factor H. Nat Chem Biol 11:77–82. https://doi.org/10.1038/nchembio.1696.
Schreiber RD, Müller-Eberhard HJ (1978) Assembly of the cytolytic alternative pathway of complement from 11 isolated plasma proteins. J Exp Med 148:1722–1727
Kimura Y, Miwa T, Zhou L, Song WC (2008) Activator-specific requirement of properdin in the initiation and amplification of the alternative pathway complement. Blood 111:732–740
Rawal N, Pangburn MK (1998) C5 convertase of the alternative pathway of complement. Kinetic analysis of the free and surface-bound forms of the enzyme. J Biol Chem 273:16828–16835
Coulthard LG, Woodruff TM (2015) Is the complement activation product C3a a proinflammatory molecule? Re-evaluating the evidence and the myth. J Immunol 194(8):3542. https://doi.org/10.4049/jimmunol.1403068
Jing H, Macon KJ, Moore D, DeLucas LJ, Volanakis JE, Narayana SV (1999) Structural basis of profactor D activation: from a highly flexible zymogen to a novel self-inhibited serine protease, complement factor D. EMBO J 18:804–814
Xue X, Wu J, Ricklin D, Forneris F, Di Crescenzio P, Schmidt CQ, Granneman J, Sharp TH, Lambris JD, Gros P (2017) Regulator-dependent mechanisms of C3b processing by factor I allow differentiation of immune responses. Nat Struct Mol Biol 24:643–651. https://doi.org/10.1038/nsmb.3427
Daha MR, Fearon DT, Austen KF (1976) Formation in the presence of C3 nephritic factor (C3NeF) of an alternative pathway C3 convertase containing uncleaved B. Immunology 31:789–796
Milder FJ, Gomes L, Schouten A, Janssen BJ, Huizinga EG, Romijn RA, Hemrika W, Roos A, Daha MR, Gros P (2007) Factor B structure provides insights into activation of the central protease of the complement system. Nat Struct Mol Biol 14:224–228
Pechtl IC, Neely RK, Dryden DT, Jones AC, Barlow PN (2011) Use of time-resolved FRET to validate crystal structure of complement regulatory complex between C3b and factor H (N terminus). Protein Sci 20(12):2102. https://doi.org/10.1002/pro.738
Berends ET, Gorham RD Jr, Ruyken M, Soppe JA, Orhan H, Aerts PC, de Haas CJ, Gros P, Rooijakkers SH (2015) Molecular insights into the surface-specific arrangement of complement C5 convertase enzymes. BMC Biol 13:93. doi: https://doi.org/10.1186/s12915-015-0203-8.
Pangburn MK, Müller-Eberhard HJ (1980) Relation of putative thioester bond in C3 to activation of the alternative pathway and the binding of C3b to biological targets of complement. J Exp Med 152:1102–1114
Isenman DE, Kells DI, Cooper NR, Müller-Eberhard HJ, Pangburn MK (1981) Nucleophilic modification of human complement protein C3: correlation of conformational changes with acquisition of C3b-like functional properties. Biochemistry 20:4458–4467
Pangburn MK, Schreiber RD, Müller-Eberhard HJ (1981) Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b-like activities by spontaneous hydrolysis of the putative thioester in native C3. J Exp Med 154:856–867
Pryzdial EL, Isenman DE (1988) A thermodynamic study of the interaction between human complement components C3b or C3(H2O) and factor B in solution. J Biol Chem 263:1733–1738
Bexborn F, Andersson PO, Chen H, Nilsson B, Ekdahl KN (2008) The tick-over theory revisited: formation and regulation of the soluble alternative complement C3 convertase (C3(H2O)Bb). Mol Immunol 45:2370–2379
Nilsson B, Nilsson Ekdahl K (2012) The tick-over theory revisited: is C3 a contact-activated protein? Immunobiology 217:1106–1110. https://doi.org/10.1016/j.imbio.2012.07.008
Carpenter CB, Ruddy S, Shehadeh IH, Müller-Eberhard HJ, Merrill JP, Austen KF (1969) Complement metabolism in man: hypercatabolism of the fourth (C4) and third (C3) components in patients with renal allograft rejection and hereditary, angioedema (HAE). J Clin Invest 48:1495–1505
Sissons JG, Liebowitch J, Amos N, Peters DK (1977) Metabolism of the fifth component of complement, and its relation to metabolism of the third component, in patients with complement activation. J Clin Invest 59:704–715
Farries TC, Finch JT, Lachmann PJ, Harrison RA (1987) Resolution and analysis of “native” and “activated” properdin. Biochem J 243:507–517
Pangburn MK (1989) Analysis of the natural polymeric forms of human properdin and their functions in complement activation. J Immunol 142:202–207
Higgins JM, Wiedemann H, Timpl R, Reid KB (1995) Characterization of mutant forms of recombinant human properdin lacking single thrombospondin type I repeats. Identification of modules important for function. J Immunol 155:5777–5785
Pedersen D1, Roumenina L, Jensen RK, Gadeberg TA, Marinozzi C, Picard C, Rybkine T2, Thiel S, Sørensen UB, Stover C, Fremeaux-Bacchi V, Andersen GR (2017) Functional and structural insight into properdin control of complement alternative pathway amplification. EMBO J 36:1084–1099. 10.15252/embj.201696173
Yang Y, Liu F, Franc V, Halim LA, Schellekens H, Heck AJ (2016) Hybrid mass spectrometry approaches in glycoprotein analysis and their usage in scoring biosimilarity. Nat Commun 7:13397. https://doi.org/10.1038/ncomms13397
Medicus RG, Esser AF, Fernandez HN, Müller-Eberhard HJ (1980) Native and activated properdin: interconvertibility and identity of amino- and carboxy-terminal sequences. J Immunol 124:602–606
Lutz HU, Fumia S, Schurtenberger C, Alaia V (2007) Opinion paper: stimulation of complement amplification or activation of the alternative pathway of complement? Mol Immunol 44:3862–3865
Junker A, Baatrup G, Svehag SE, Wang P, Holmström E, Sturfelt G, Sjöholm AG (1998) Binding of properdin to solid-phase immune complexes: critical role of the classical activation pathway of complement. Scand J Immunol 47:481–486
Vuagnat BB, Mach J, Le Doussal JM (2000) Activation of the alternative pathway of human complement by autologous cells expressing transmembrane recombinant properdin. Mol Immunol 37:467–478
Hourcade DE (2006) The role of properdin in the assembly of the alternative pathway C3 convertases of complement. J Biol Chem 281:2128–2132
Agarwal S, Specht CA, Haibin H, Ostroff GR, Ram S, Rice PA, Levitz SM (2011) Linkage specificity and role of properdin in activation of the alternative complement pathway by fungal glycans. MBio. 2. pii: e00178–11. doi: https://doi.org/10.1128/mBio.00178-11.
Cortes C, Ferreira VP, Pangburn MK (2011) Native properdin binds to Chlamydia pneumoniae and promotes complement activation. Infect Immun 79:724–731. https://doi.org/10.1128/IAI.00980-10
Spitzer D, Mitchell LM, Atkinson JP, Hourcade DE (2007) Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J Immunol 179:2600–2608
Xu W, Berger SP, Trouw LA, de Boer HC, Schlagwein N, Mutsaers C, Daha MR, van Kooten C (2008) Properdin binds to late apoptotic and necrotic cells independently of C3b and regulates alternative pathway complement activation. J Immunol 180:7613–7621
Kemper C, Atkinson JP, Hourcade DE (2010) Properdin: emerging roles of a pattern-recognition molecule. Annu Rev Immunol 28:131–155. https://doi.org/10.1146/annurev-immunol-030409-101250
Harboe M, Garred P, Lindstad JK, Pharo A, Müller F, Stahl GL, Lambris JD, Mollnes TE (2012) The role of properdin in zymosan- and Escherichia coli-induced complement activation. J Immunol 189:2606–2613. https://doi.org/10.4049/jimmunol.1200269
Harboe M, Johnson C, Nymo S, Ekholt K, Schjalm C, Lindstad JK, Pharo A, Hellerud BC, Nilsson Ekdahl K, Mollnes TE, Nilsson PH (2017) Properdin binding to complement activating surfaces depends on initial C3b deposition. Proc Natl Acad Sci U S A 114:E534–E539. https://doi.org/10.1073/pnas.1612385114
Takahashi M, Ishida Y, Iwaki D, Kanno K, Suzuki T, Endo Y, Homma Y, Fujita T (2010) Essential role of mannose-binding lectin-associated serine protease-1 in activation of the complement factor D. J Exp Med 207:29–37. https://doi.org/10.1084/jem.20090633
Banda NK, Takahashi M, Takahashi K, Stahl GL, Hyatt S, Glogowska M, Wiles TA, Endo Y, Fujita T, Holers VM, Arend WP (2011) Mechanisms of mannose-binding lectin-associated serine proteases-1/3 activation of the alternative pathway of complement. Mol Immunol 49:281–289. https://doi.org/10.1016/j.molimm.2011.08.021
Ruseva MM, Takahashi M, Fujita T, Pickering MC (2014) C3 dysregulation due to factor H deficiency is mannan-binding lectin-associated serine proteases (MASP)-1 and MASP-3 independent in vivo. Clin Exp Immunol 176:84–92. https://doi.org/10.1111/cei.12244
Dobó J, Szakács D, Oroszlán G, Kortvely E, Kiss B, Boros E, Szász R, Závodszky P, Gál P, Pál G (2016) MASP-3 is the exclusive pro-factor D activator in resting blood: the lectin and the alternative complement pathways are fundamentally linked. Sci Rep 6:31877. https://doi.org/10.1038/srep31877
Degn SE, Jensen L, Hansen AG, Duman D, Tekin M, Jensenius JC, Thiel S (2012) Mannan-binding lectin-associated serine protease (MASP)-1 is crucial for lectin pathway activation in human serum, whereas neither MASP-1 nor MASP-3 is required for alternative pathway function. J Immunol 189:3957–3969. https://doi.org/10.4049/jimmunol.1201736
Pihl R, Jensen L, Hansen AG, Thøgersen IB, Andres S, Dagnæs-Hansen F, Oexle K, Enghild JJ, Thiel S (2017) Analysis of factor D isoforms in Malpuech-Michels-Mingarelli-Carnevale patients highlights the role of MASP-3 as a maturase in the alternative pathway of complement. J Immunol. pii: ji1700518. doi: https://doi.org/10.4049/jimmunol.1700518.
Pascual M, Steiger G, Estreicher J, Macon K, Volanakis JE, Schifferli JA (1988) Metabolism of complement factor D in renal failure. Kidney Int 34:529–536
Wu X, Hutson I, Hourcade D, Harris C, Atkinson JP (2016) Regulation of the mouse adipose factor D biosynthesis by the endocrine hypothalamic-pituatory-adrenal axis. Immunobiology 221:1171
Misasi R, Huemer HP, Schwaeble W, Sölder E, Larcher C, Dierich MP (1989) Human complement factor H: an additional gene product of 43 kDa isolated from human plasma shows cofactor activity for the cleavage of the third component of complement. Eur J Immunol 19:1765–1768
Estaller C, Schwaeble W, Dierich M, Weiss EH (1991) Human complement factor H: two factor H proteins are derived from alternatively spliced transcripts. Eur J Immunol 21:799–802
Friese MA, Hellwage J, Jokiranta TS, Meri S, Müller-Quernheim HJ, Peter HH, Eibel H, Zipfel PF (2000) Different regulation of factor H and FHL-1/reconectin by inflammatory mediators and expression of the two proteins in rheumatoid arthritis (RA). Clin Exp Immunol 121:406–415
Clark SJ, Schmidt CQ, White AM, Hakobyan S, Morgan BP, Bishop PN (2014) Identification of factor H-liken 1 as the predominant complement regulator in Bruch’s membrane: implications for age-related macular degeneration. J Immunol 193:4962–4970. https://doi.org/10.4049/jimmunol.1401613
Estaller C, Koistinen V, Schwaeble W, Dierich MP, Weiss EH (1991) Cloning of the 1.4-kb mRNA species of human complement factor H reveals a novel member of the short consensus repeat family related to the carboxy terminal of the classical 150-kDa molecule. J Immunol 146:3190–3196
Timmann C, Leippe M, Horstmann RD (1991) Two major serum components antigenically related to complement factor H are different glycosylation forms of a single protein with no factor H-like complement regulatory functions. J Immunol 146:1265–1270
Zipfel PF, Skerka C (1994) Complement factor H and related proteins: an expanding family of complement-regulatory proteins? Immunol Today 15:121–126
Fijen CA, Kuijper EJ, Te Bulte M, van de Heuvel MM, Holdrinet AC, Sim RB, Daha MR, Dankert J (1996) Heterozygous and homozygous factor H deficiency states in a Dutch family. Clin Exp Immunol 105:511–516
Heinen S, Hartmann A, Lauer N, Wiehl U, Dahse HM, Schirmer S, Gropp K, Enghardt T, Wallich R, Hälbich S, Mihlan M, Schlötzer-Schrehardt U, Zipfel PF, Skerka C (2009) Factor H-related protein 1 (CFHR-1) inhibits complement C5 convertase activity and terminal complex formation. Blood 114:2439–2447. https://doi.org/10.1182/blood-2009-02-205641
Eberhardt HU, Buhlmann D, Hortschansky P, Chen Q, Böhm S, Kemper MJ, Wallich R, Hartmann A, Hallström T, Zipfel PF, Skerka C (2013) Human factor H-related protein 2 (CFHR2) regulates complement activation. PLoS One 8:e78617. https://doi.org/10.1371/journal.pone.0078617
Goicoechea de Jorge E, Caesar JJ, Malik TH, Patel M, Colledge M, Johnson S, Hakobyan S, Morgan BP, Harris CL, Pickering MC, Lea SM (2013) Dimerization of complement factor H-related proteins modulates complement activation in vivo. Proc Natl Acad Sci U S A 110:4685–4690. https://doi.org/10.1073/pnas.1219260110
Józsi M, Tortajada A, Uzonyi B, Goicoechea de Jorge E, Rodríguez de Córdoba S (2015) Factor H-related proteins determine complement-activating surfaces. Trends Immunol 36:374–384. https://doi.org/10.1016/j.it.2015.04.008
Valoti E, Alberti M, Tortajada A, Garcia-Fernandez J, Gastoldi S, Besso L, Bresin E, Remuzzi G, Rodriguez de Cordoba S, Noris M (2015) A novel atypical hemolytic uremic syndrome-associated hybrid CFHR1/CFH gene encoding a fusion protein that antagonizes factor H-dependent complement regulation. J Am Soc Nephrol 26:209–219. https://doi.org/10.1681/ASN.2013121339
Medjeral-Thomas N, Pickering MC (2016) The complement factor H-related proteins. Immunol Rev 274:191–201. https://doi.org/10.1111/imr.12477
Holers VM (2008) The spectrum of complement alternative pathway-mediated diseases. Immunol Rev 223:300–316. https://doi.org/10.1111/j.1600-065X.2008.00641.x
Maibaum J, Liao SM, Vulpetti A, Ostermann N, Randl S, Rüdisser S, Lorthiois E, Erbel P, Kinzel B, Kolb FA, Barbieri S, Wagner J, Durand C, Fettis K, Dussauge S, Hughes N, Delgado O, Hommel U, Gould T, Mac Sweeney A, Gerhartz B, Cumin F, Flohr S, Schubart A, Jaffee B, Harrison R, Risitano AM, Eder J, Anderson K (2016) Small-molecule factor D inhibitors targeting the alternative complement pathway. Nat Chem Biol 12:1105–1110. https://doi.org/10.1038/nchembio.2208
Yuan X, Gavriilaki E, Thanassi JA, Yang G, Baines AC, Podos SD, Huang Y, Huang M, Brodsky RA (2017) Small-molecule factor D inhibitors selectively block the alternative pathway of complement in paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. Haematologica 102:466–475. https://doi.org/10.3324/haematol.2016.153312
DiScipio RG (1982) The activation of the alternative pathway C3 convertase by human plasma kallikrein. Immunology 45:587–595
Day NK, Schreiber RD, Götze O, Müller-Eberhard HJ (1976) Reversible activation of proactivator (factor B) of the alternative pathway without cleavage of the molecule. Scand J Immunol 5:715–720
Nydegger UE, Fearon DT, Austen KF (1978) The modulation of the alternative pathway of complement in C2-deficient human serum by changes in concentration of the component and control proteins. J Immunol 120:1404–1408
Forristal J, Iitaka K, Vallota EH, West CD (1977) Correlations between serum factor B and C3b inactivator levels in normal subjects and in patients with infections, nephrosis and hypocomplementaemic glomerulonephritis. Clin Exp Immunol 28:61–71
Esparza-Gordillo J, Soria JM, Buil A, Almasy L, Blangero J, Fontcuberta J, Rodríguez de Córdoba S (2004) Genetic and environmental factors influencing the human factor H plasma levels. Immunogenetics 56:77–82
Heurich M, Martínez-Barricarte R, Francis NJ, Roberts DL, Rodríguez de Córdoba S, Morgan BP, Harris CL (2011) Common polymorphisms in C3, factor B, and factor H collaborate to determine systemic complement activity and disease risk. Proc Natl Acad Sci U S A 108:8761–8766. https://doi.org/10.1073/pnas.1019338108
Fearon DT (1979) Activation of the alternative complement pathway. CRC Crit Rev Immunol 1:1–32
Pangburn MK, Müller-Eberhard HJ (1984) The alternative pathway of complement. Springer Semin Immunopathol 7:163–192
Acknowledgements
The author would like to acknowledge, with gratitude, the contributions that Professor Peter Lachmann and the late Professor Fred Rosen have made to his understanding and thinking about the complement system.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a contribution to the special issue on Complement in Health and Disease: Novel Aspects and Insights - Guest Editors: Paul Morgan and David Kavanagh
Rights and permissions
About this article
Cite this article
Harrison, R.A. The properdin pathway: an “alternative activation pathway” or a “critical amplification loop” for C3 and C5 activation?. Semin Immunopathol 40, 15–35 (2018). https://doi.org/10.1007/s00281-017-0661-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-017-0661-x