Abstract
Recent studies demonstrated that basophils play crucial and non-redundant roles in the immune system, in spite of the fact that they are the rarest granulocytes and represent less than 1 % of peripheral blood leukocytes. In response to various stimuli, basophils release effector molecules stored in their cytoplasmic granules, including chemical mediators and proteases, and also secrete cytokines and chemokines. In this review, we will focus on the physiological and pathological roles of basophil-derived IL-4. Basophils can readily produce large quantities of IL-4 and are therefore the important source of IL-4. Basophil-derived IL-4 has been shown to regulate other immune cells, including T cells, B cells, group 2 innate lymphoid cells, monocytes, and macrophages. It also acts on non-hematopoietic cells such as fibroblasts and endothelial cells. Those cells stimulated with basophil-derived IL-4 contribute to the positive or negative regulation of a variety of immune responses in health and disease, including protection against parasitic and bacterial infections, allergy, and autoimmune diseases. Thus, basophil-derived IL-4 plays versatile roles in immunity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Basophils share common features with tissue-resident mast cells, including the cell surface expression of the high-affinity IgE receptor (FcεRI) and the ability to release chemical mediators such as histamine in response to IgE plus antigen stimulation [1]. Despite their phenotypic similarities with mast cells, the study on basophils remains behind because of their rarity and short life-span that make their functional analysis difficult. Therefore, the physiological significance of basophils had long been unknown, until the studies in the early 1990s have highlighted the importance of basophils by providing evidence that both human and murine basophils can rapidly secrete a large amount of IL-4 in response to FcεRI cross-linking [2, 3]. This finding puts basophils on the main stage of immunology, allowing researchers to appreciate previously neglected roles of basophils [4, 5]. In addition, the recent advantages of analytical tools for basophils, including genetically engineered mice in which only basophils are constitutively or inducibly ablated [6–9], have accelerated in vivo research on basophils. It is now clear that basophils function as an important source of IL-4 in vivo and that basophil-derived IL-4 has a significant effect on a variety of cells under both physiological and pathological conditions. In this review, we focus on versatile roles of basophil-derived IL-4 in the immune system.
Stimuli and signaling that induce IL-4 production by basophils
Basophils can release IL-4 within 5–10 min after stimulation even under pharmacological inhibition of transcriptional factors, indicating the existence of preformed IL-4 in steady-state basophils [10]. Indeed, basophils from IL-4/green fluorescent protein (GFP) reporter mice, in which IL-4 expression in basophils can be visualized, show constitutive GFP expression [11]. However, basophil-derived IL-4 mostly results from de novo synthesis of protein after basophil activation.
Basophils release IL-4 in IgE-dependent and IgE-independent manners [2, 3, 12–14] in response to a variety of stimuli such as IgE plus antigens; cytokines; pathogen-derived molecules including Toll-like receptor (TLR) ligands; and allergenic proteases, suggesting the involvement of basophils in both acquired and innate immunities (Fig. 1). The IgE-dependent mechanisms of basophil activation have been extensively examined and characterized, in which the Fc receptor γ-chain (FcRγ), spleen tyrosine kinase (Syk), and nuclear factor of activated T cells (NFAT) play pivotal roles in FcεRI-mediated signal transduction [15, 16], leading to a robust IL-4 production of basophils in response to IgE plus antigens.
Stimuli and their sensors for basophil IL-4 production. Basophils produce a large amount of IL-4 in response to a variety of stimuli, including IgE plus antigens, cytokines, TLR ligands, papain, and house dust mite protease Der f 1. Basophils release IL-4 in both IgE-dependent and IgE-independent manners, suggesting that basophil-derived IL-4 plays roles in both acquired and innate immunities, respectively
On the other hand, IgE-independent mechanisms underlying basophil IL-4 production remain incompletely defined. IL-3 (an important cytokine for basophil expansion [17]) can directly induce basophil IL-4 production and also enhance IL-4 production elicited by other types of stimuli [18, 19]. Intriguingly, it is reported that IL-3-induced IL-4 production of basophils requires FcRγ that is constitutively associated with the common β-chain of IL-3 receptor on basophils [20]. IL-18 and IL-33, which belong to the IL-1 family cytokines and have recently been shown to contribute to Th2 responses [21–24], can also induce basophil IL-4 production via a MyD88- and p38α-dependent pathway in an FcεRI-independent manner [25, 26]. Interestingly, basophils appear to show functional heterogeneity depending on cytokine milieu. Thymic stromal lymphopoietin (TSLP), as well as IL-3, has recently been identified as a key regulator of basophil expansion [27]. TSLP-elicited basophils exhibit higher expression of receptors for IL-18 and IL-33, along with lower responsiveness to IgE plus antigens, thereby producing more IL-4 in response to these cytokines than do IL-3-elicited conventional basophils, suggesting that the former may preferentially play roles in innate immunity rather than IgE-mediated acquired immunity [27, 28].
As basophils are thought to be involved in innate immunity, the roles of TLRs and their ligands in basophil activation have been investigated. Murine bone marrow-derived basophils (BMBAs) express TLR1, TLR2, TLR4, and TLR6, so that they can produce IL-4 in response to bacterial components including peptidoglycan (PGN) or lipopolysaccharide (LPS) in the presence of IL-3, even without FcεRI cross-linking [19, 22]. Human basophils are found to express high levels of TLR2 and TLR4 [29] and can secrete IL-4 in response to PGN but not to LPS via activation of nuclear factor-κB (NF-κB) [30]. The absence of CD14, a coreceptor for detection of LPS by TLR4, may account for the lack of LPS responsiveness in human basophils [29]. Notably, the TLR-mediated signaling has more impact on IL-4 production of basophils than that of mast cells [22]. These results suggest that basophils and basophil-derived IL-4 may play an important role in initiation of allergic diseases triggered by bacterial infections.
Basophils can also produce IL-4 in response to allergenic proteases such as papain and house dust mite proteases Der f 1 and Der p 1 [14, 31]. Interestingly, Rosenstein et al. have recently demonstrated that papain-induced IL-4 production is dependent on FcRγ, even though its immunoreceptor tyrosine-based activation motif (ITAM) and canonical signaling molecule Syk are not phosphorylated by papain stimulation [32]. More recently, it has been reported that FcRγ is indispensable for basophil IL-4 production not only by papain but also by other innate stimuli including Der f 1, IL-18, IL-33, and LPS, suggesting that FcRγ broadly controls basophil IL-4 production even in the case of IgE-independent fashion [19].
Effects of basophil IL-4 on T cells
It is widely accepted that IL-4 has a principal role in promoting differentiation of naïve CD4+ T cells to Th2 cells that in turn produce more IL-4, leading to allergic responses and protection against parasites [33, 34]. Although the initial source of IL-4 remains unclear, basophils are proposed as providers of initial IL-4 that primarily induces Th2 differentiation [35, 36]. Indeed, basophils can rapidly produce larger amounts of IL-4 with lower threshold for activation than do T cells [37]. Coculture of naïve CD4+ T cells with wild-type (WT) but not Il4 −/− basophils can promote a robust Th2 differentiation in the presence of conventional antigen-presenting cells (APCs) and antigens, suggesting the importance of basophil-derived IL-4 in promoting Th2 differentiation in vitro [38, 39]. The in vivo relevance of these findings is supported by analysis of mice with genetically or artificially induced basophilia in which Th2 differentiation is markedly accelerated [38–40]. Importantly, Sokol et al. have shown that subcutaneous injection of papain, a potent inducer of Th2 response, elicits a transient basophil migration into the draining lymph nodes where basophils produce IL-4 and encounter CD4 T cells [14]. Indeed, antibody-mediated depletion of basophils leads to a lack of basophil migration into the lymph nodes, resulting in a failure of Th2 differentiation [14]. These results suggest that basophil-derived IL-4 critically contributes to papain-induced Th2 differentiation in vivo, presumably in the lymph nodes.
In 2009, it has been reported by three independent groups that basophils act as not only providers of IL-4 but also critical APCs for driving a robust Th2 differentiation [41–43]. They have demonstrated that basophils express MHC class II and costimulatory molecules, such as CD80, CD86, and CD40, and can present antigens to naïve CD4+ T cells with providing IL-4, leading to Th2 differentiation even in the absence of dendritic cells (DCs). This is an exciting discovery as it is generally assumed that basophils and DCs have distinct roles in Th2 differentiation, a source of IL-4 and antigen presentation [44, 45], respectively. However, the full extent of capacity of basophils for antigen presentation and Th2 differentiation remains uncertain because conflicting results have appeared, probably depending on different experimental settings [7, 8, 46–55]. To address this issue, further investigation with more sophisticated tools, such as basophil-specific MHC class II-deficient mice, will be necessary.
Basophil-derived IL-4 also regulates other T cell subsets. Basophils appear to limit disease activity of experimental murine colitis, in which basophil-derived IL-4 suppresses harmful Th1 responses such as IFN-γ, IL-2, and TNF-α production of CD4+ T cells [56]. Unlike CD4+ T cells, naïve CD8+ T cells stimulated in the presence of basophils can produce IL-10, which requires basophil-derived IL-4 [57].
Effects of basophil IL-4 on B cells
It is well known that antigen-specific antibodies, including IgE, are produced by B cells in response to the first antigen exposure during the primary responses and that antigen-specific IgE-armed basophils produce robust IL-4 in response to corresponding antigens in the secondary memory responses [58]. However, physiological relevance of basophil-derived IL-4 in the memory responses had largely been unknown. Recent studies have revealed that basophil-derived IL-4 plays roles in protective immunity [59, 60] and in autoimmunity [61] through enhanced production of pathogen-specific and self-reactive antibodies, respectively. Thus, basophils have the potential to activate B cells through production of IL-4 that amplifies humoral memory responses.
Denzel and colleagues have demonstrated that basophils act together with CD4+ T cells to enhance B cell proliferation and immunoglobulin production by providing IL-4 and IL-6 in vitro [59]. Notably, they have also delineated the physiological significance of basophils in humoral memory responses in vivo through analysis of murine vaccination-infection model. When mice vaccinated with pneumococcal surface protein A (PspA) are subjected to sepsis induced by Streptococcus pneumoniae, depletion of basophils before second vaccination results in increased mortality with much lower titers of plasma PspA-specific IgG1 and IgG2a [59]. Another group has reported that circulating IgD constitutively binds to human basophils through an unknown calcium-fluxing receptor [60]. IgD cross-linking on basophils triggers sustained intracellular calcium flux that elicits basophils’ production of IL-4, IL-13, B cell-activating factor (BAFF), and a proliferation-inducing ligand (APRIL), leading to IgM secretion as well as IgG and IgA class switching in B cells. Since IgD+IgM− B cells derived from the upper respiratory mucosa are specialized to produce IgD that recognizes respiratory bacteria and their components, IgD-armed basophils may be involved in amplification of humoral memory responses for protection against bacterial infections in humans [60]. Although the full extent of its capacity remains to be determined, these results suggest that basophil-derived IL-4 may play roles in enhancement of production of pathogen-specific antibodies in B cells and thus contribute to humoral protective immunity against pathogens.
Basophil-derived IL-4 also has the potential to amplify autoantibody production in B cells. It has been reported that Lyn −/− mice exhibit a significant increase of basophil proliferation and IL-4 production because of the transcriptional factor GATA-3 upregulation [40]. Interestingly, basophils contribute to the production of autoantibodies including self-reactive IgE from B cells by providing IL-4, which causes lupus-like nephritis in aged Lyn −/− mice [61]. The self-reactive IgE in turn leads to basophil activation with IL-4 production, accelerating B cell autoantibody production and disease progression. Notably, either the depletion of basophils or the absence of IL-4 attenuates autoantibody production and thus protects mice from severe lupus nephritis [61], implicating the contribution of basophil-derived IL-4 to the pathogenesis of humoral autoimmunity.
Effects of basophil IL-4 on group 2 innate lymphoid cells
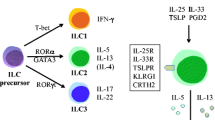
Innate lymphoid cells (ILCs) are the most recently identified cell population in the innate immune system [62]. Among them, group 2 ILCs (ILC2s) have been shown to play crucial roles in allergic inflammation and protective immunity against parasites through a robust production of Th2 cytokine in response to epithelial cell-derived factors such as IL-25, IL-33, and TSLP [62, 63]. Although ILC2s are a primary source of innate IL-5 and IL-13, there is little evidence that they produce IL-4 in mice [64], suggesting their unique role is distinct from that of IL-4-producing basophils in Th2 responses. In addition, recent studies have revealed that ILC2s express IL-4Rα and can proliferate in response to IL-4 [65, 66]. Collectively, these results suggest that basophil-derived IL-4 acts on ILC2s and that basophils and ILC2s have cooperative roles by providing different types of Th2 cytokines in controlling Th2 responses.
Motomura et al. have demonstrated that basophil-derived IL-4 contributes to the activation of ILC2s in cysteine protease-induced allergic asthma (Fig. 2) [65]. In mice administered intranasally with papain, basophils promote a robust production of IL-5 and IL-13 from ILC2s by providing IL-4 in collaboration with epithelial cell-derived IL-33, resulting in the exacerbation of lung eosinophilia and goblet cell hyperplasia [65]. Another group has also reported the importance of basophil-derived IL-4 in promoting ILC2 activation during allergic skin inflammation [66]. They have shown that basophils and ILC2s accumulate in close proximity in the skin lesions from both atopic dermatitis (AD) patients and AD-like mice induced by topical application of a vitamin D analog MC903. Analysis of mice with diphtheria toxin-based genetic depletion of basophils has revealed that basophils are required for substantial accumulation of ILC2s in the skin lesions. Notably, supernatants from in vitro activated WT basophils promote the proliferation of ILC2s while those from Il4 −/− basophils fail, suggesting that basophil-derived IL-4 is crucial for ILC2 proliferation [66].
Effects of basophil IL-4 on macrophages
Macrophages have a phenotypic heterogeneity and exhibit distinct features for activation under different conditions of immunological status [67]. Alternatively activated macrophages (AAMs), also called M2-type macrophages, are elicited by canonical Th2 cytokines IL-4 and IL-13 and are involved in Th2 responses during parasitic infection and allergic inflammation [68]. Recent studies have expanded their roles beyond conventional type 2 immunity to include maintenance of physiological homeostasis [69–71] and tissue repair [72, 73]. Although various cell types, including T cells, natural killer T (NKT) cells, mast cells, eosinophils, and basophils, show the potential to release IL-4 that may impact on macrophages [68], we and other groups have recently highlighted the importance of basophil-derived IL-4 in alternative activation of macrophages (Fig. 3) [74–76].
Basophil-derived IL-4 contributes to AAM generation from inflammatory monocytes. Basophil-derived IL-4 acts on inflammatory monocytes recruited into the skin, leading to the generation of AAMs. The AAMs contribute to larval trapping through arginase-1 production during the second helminth infestation or to attenuation of allergic inflammation (upper panel). During L. monocytogenes infection, bacteria-induced Kupffer cell death stimulates hepatocytes that release IL-33, triggering basophil IL-4 production in the liver. Basophil-derived IL-4 subsequently induces the generation of monocyte-derived AAMs that replace Kupffer cells ablated by bacterial infection, restoring liver homeostasis (lower panel)
Helminth infections provoke highly polarized Th2 responses, characterized by elevated levels of serum IgE, eosinophilia, mucus overproduction, and smooth muscle and fibroblast alternations along with AAM generation. Th2 cytokines IL-4 and IL-13 play a pivotal role at both the initiation and execution phases of the protective immunity against helminths [77]. Although the roles of both basophils and AAMs in Th2 responses during helminth infection remained controversial [7, 8, 68], our recent report has proposed an important role of basophil-derived IL-4 in AAM generation and protection against the intestinal helminth Nippostrongylus brasiliensis (Fig. 3) [75]. During the second infestation with N. brasiliensis, IgE-armed basophils infiltrate into the larva-infected skin and are activated in response to helminth antigens. Skin-infiltrating basophils produce IL-4 that subsequently promotes the generation of AAMs, leading to the larval trapping of the skin through arginase-1 production [75].
Th2 responses are also closely associated with allergic inflammation, in which basophil-derived IL-4 is implicated in AAM generation (Fig. 3). We have previously established a mouse model of basophil-dependent chronic skin allergic inflammation (designated IgE-CAI) [78], in which mice passively sensitized with antigen-specific IgE are challenged intradermally with corresponding antigens. The IgE-armed basophils produce IL-4 in response to antigens and initiate allergic inflammation in the skin lesions. Basophil-derived IL-4 acts on inflammatory monocytes recruited to the skin lesions and promotes their differentiation to AAMs, which in turn dampen inflammation [74]. Thus, basophil-derived IL-4 contributes to the termination of allergic skin inflammation.
Recent report has delineated a role of basophil-derived IL-4 in AAM generation to maintain liver homeostasis (Fig. 3) [76]. Bleriot and colleagues have demonstrated that, upon Listeria monocytogenes infection, bacteria-induced Kupffer cell death triggers IL-33 release from hepatocytes that induce IL-4 production by infiltrating basophils in the liver. Basophil-derived IL-4 elicits differentiation of recruited inflammatory monocytes into AAMs that in turn replace dead Kupffer cells, restoring liver homeostasis [76].
Basophil-derived IL-4 has been shown to promote increased expression of the inhibitory receptor FcγRIIB on macrophages that may in part account for the immunosuppressive effect of high-dose intravenous immunoglobulin (IVIG) [79]. Sialylated IgG, an effector component of IVIG, binds to DC-SIGN+ myeloid cells, resulting in the production of IL-33, which induces IL-4 production of basophils. Intriguingly, basophil-derived IL-4 promotes upregulation of FcγRIIB on the cell surface of macrophages, leading to immunosuppression in an arthritis model of mice when treated with IVIG [79]. Although a conflicting result has appeared recently [80], this observation suggests that basophils can regulate inflammation by providing IL-4 through enhanced expression of FcγRIIB on macrophages.
Effects of basophil IL-4 on non-hematopoietic cells
Beyond the hematopoietic lineages, recent studies have illuminated the roles of basophil-derived IL-4 in the regulation of other cell types including fibroblasts [81] and endothelial cells [82]. Basophils promote eosinophil migration especially in the presence of fibroblasts. In coculture with fibroblasts, basophils produce IL-4 and TNF-α, which elicit CCL11 expression in fibroblasts, leading to the enhanced eosinophil migration in vitro [81]. In allergic skin lesions, IgE/allergen-stimulated basophils alter their migratory kinetics during the transmigration through blood vessels and stay in the endothelium [82]. Sustained interaction of basophils with endothelium promotes delivery of basophil-derived IL-4 to the endothelium and subsequent induction of endothelial vascular cell adhesion molecule-1 (VCAM-1), leading to eosinophil accumulation in the skin.
Concluding remarks
Basophil research has been strongly accelerated over the past few years by generation of sophisticated analytical tools such as constitutive or inducible basophil-ablated mice. In addition to initiation of Th2 differentiation, recent advances have revealed the crucial roles of basophil-derived IL-4 in the activation of B cells, ILC2s, macrophages, and non-hematopoietic cells. Such advances have led to greater appreciation for functions of basophil-derived IL-4 beyond the conventional Th2 responses, including humoral memory responses and maintenance of physiological homeostasis. Thus, basophils now come into the forefront of immunological field. Given that the absolute number of basophils is relatively small, it is possible that basophils and their products including IL-4 become promising targets to treat immunological disorders, although we need to define the clinical relevance of findings in mice to human diseases.
References
Galli SJ (2000) Mast cells and basophils. Curr Opin Hematol 7:32–39
Seder RA, Paul WE, Dvorak AM, Sharkis SJ, Kagey-Sobotka A, Niv Y, Finkelman FD, Barbieri SA, Galli SJ, Plaut M (1991) Mouse splenic and bone marrow cell populations that express high-affinity Fc epsilon receptors and produce interleukin 4 are highly enriched in basophils. Proc Natl Acad Sci U S A 88:2835–2839
Piccinni MP, Macchia D, Parronchi P, Giudizi MG, Bani D, Alterini R, Grossi A, Ricci M, Maggi E, Romagnani S (1991) Human bone marrow non-B, non-T cells produce interleukin 4 in response to cross-linkage of Fc epsilon and Fc gamma receptors. Proc Natl Acad Sci U S A 88:8656–8660
Karasuyama H, Mukai K, Obata K, Tsujimura Y, Wada T (2011) Nonredundant roles of basophils in immunity. Annu Rev Immunol 29:45–69
Karasuyama H, Yamanishi Y (2014) Basophils have emerged as a key player in immunity. Curr Opin Immunol 31:1–7
Wada T, Ishiwata K, Koseki H, Ishikura T, Ugajin T, Ohnuma N, Obata K, Ishikawa R, Yoshikawa S, Mukai K et al (2010) Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest 120:2867–2875
Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D (2010) Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity 33:364–374
Sullivan BM, Liang HE, Bando JK, Wu D, Cheng LE, McKerrow JK, Allen CD, Locksley RM (2011) Genetic analysis of basophil function in vivo. Nat Immunol 12:527–535
Sawaguchi M, Tanaka S, Nakatani Y, Harada Y, Mukai K, Matsunaga Y, Ishiwata K, Oboki K, Kambayashi T, Watanabe N et al (2012) Role of mast cells and basophils in IgE responses and in allergic airway hyperresponsiveness. J Immunol 188:1809–1818
Gibbs BF, Haas H, Falcone FH, Albrecht C, Vollrath IB, Noll T, Wolff HH, Amon U (1996) Purified human peripheral blood basophils release interleukin-13 and preformed interleukin-4 following immunological activation. Eur J Immunol 26:2493–2498
Gessner A, Mohrs K, Mohrs M (2005) Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol 174:1063–1072
Schroeder JT, MacGlashan DW Jr, Kagey-Sobotka A, White JM, Lichtenstein LM (1994) IgE-dependent IL-4 secretion by human basophils. The relationship between cytokine production and histamine release in mixed leukocyte cultures. J Immunol 153:1808–1817
Schroeder JT (2011) Basophils: emerging roles in the pathogenesis of allergic disease. Immunol Rev 242:144–160
Sokol CL, Barton GM, Farr AG, Medzhitov R (2008) A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol 9:310–318
Siraganian RP, de Castro RO, Barbu EA, Zhang J (2010) Mast cell signaling: the role of protein tyrosine kinase Syk, its activation and screening methods for new pathway participants. FEBS Lett 584:4933–4940
Law M, Morales JL, Mottram LF, Iyer A, Peterson BR, August A (2011) Structural requirements for the inhibition of calcium mobilization and mast cell activation by the pyrazole derivative BTP2. Int J Biochem Cell Biol 43:1228–1239
Lantz CS, Boesiger J, Song CH, Mach N, Kobayashi T, Mulligan RC, Nawa Y, Dranoff G, Galli SJ (1998) Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature 392:90–93
Le Gros G, Ben-Sasson SZ, Conrad DH, Clark-Lewis I, Finkelman FD, Plaut M, Paul WE (1990) IL-3 promotes production of IL-4 by splenic non-B, non-T cells in response to Fc receptor cross-linkage. J Immunol 145:2500–2506
Kamijo S, Nunomura S, Ra C, Kanaguchi Y, Suzuki Y, Ogawa H, Okumura K, Takai T. (2015). Innate basophil IL-4 responses against allergens, endotoxin, and cytokines require the Fc receptor gamma-chain. J Allergy Clin Immunol 137:1613–1615.e2
Hida S, Yamasaki S, Sakamoto Y, Takamoto M, Obata K, Takai T, Karasuyama H, Sugane K, Saito T, Taki S (2009) Fc receptor gamma-chain, a constitutive component of the IL-3 receptor, is required for IL-3-induced IL-4 production in basophils. Nat Immunol 10:214–222
Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H (2001) Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol 19:423–474
Yoshimoto T, Nakanishi K (2006) Roles of IL-18 in basophils and mast cells. Allergol Int 55:105–113
Liew FY, Pitman NI, McInnes IB (2010) Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol 10:103–110
Oboki K, Ohno T, Kajiwara N, Saito H, Nakae S (2010) IL-33 and IL-33 receptors in host defense and diseases. Allergol Int 59:143–160
Yoshimoto T, Tsutsui H, Tominaga K, Hoshino K, Okamura H, Akira S, Paul WE, Nakanishi K (1999) IL-18, although antiallergic when administered with IL-12, stimulates IL-4 and histamine release by basophils. Proc Natl Acad Sci U S A 96:13962–13966
Kroeger KM, Sullivan BM, Locksley RM (2009) IL-18 and IL-33 elicit Th2 cytokines from basophils via a MyD88- and p38alpha-dependent pathway. J Leukoc Biol 86:769–778
Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, Wherry EJ, Jessup HK, Siegel LA, Kambayashi T et al (2011) TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature 477:229–233
Siracusa MC, Kim BS, Spergel JM, Artis D (2013) Basophils and allergic inflammation. J Allergy Clin Immunol 132:789–801, quiz 788
Sabroe I, Jones EC, Usher LR, Whyte MK, Dower SK (2002) Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J Immunol 168:4701–4710
Bieneman AP, Chichester KL, Chen YH, Schroeder JT (2005) Toll-like receptor 2 ligands activate human basophils for both IgE-dependent and IgE-independent secretion. J Allergy Clin Immunol 115:295–301
Kamijo S, Takeda H, Tokura T, Suzuki M, Inui K, Hara M, Matsuda H, Matsuda A, Oboki K, Ohno T et al (2013) IL-33-mediated innate response and adaptive immune cells contribute to maximum responses of protease allergen-induced allergic airway inflammation. J Immunol 190:4489–4499
Rosenstein RK, Bezbradica JS, Yu S, Medzhitov R (2014) Signaling pathways activated by a protease allergen in basophils. Proc Natl Acad Sci U S A 111:E4963–4971
Zhu J, Yamane H, Paul WE (2010) Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 28:445–489
Paul WE, Zhu J (2010) How are T(H)2-type immune responses initiated and amplified? Nature Reviews Immunology 10:225–235
Mitre E, Nutman TB (2006) Basophils, basophilia and helminth infections. Chem Immunol Allergy 90:141–156
Falcone FH, Zillikens D, Gibbs BF (2006) The 21st century renaissance of the basophil? Current insights into its role in allergic responses and innate immunity. Exp Dermatol 15:855–864
Mitre E, Taylor RT, Kubofcik J, Nutman TB (2004) Parasite antigen-driven basophils are a major source of IL-4 in human filarial infections. J Immunol 172:2439–2445
Hida S, Tadachi M, Saito T, Taki S (2005) Negative control of basophil expansion by IRF-2 critical for the regulation of Th1/Th2 balance. Blood 106:2011–2017
Oh K, Shen T, Le Gros G, Min B (2007) Induction of Th2 type immunity in a mouse system reveals a novel immunoregulatory role of basophils. Blood 109:2921–2927
Charles N, Watford WT, Ramos HL, Hellman L, Oettgen HC, Gomez G, Ryan JJ, O’Shea JJ, Rivera J (2009) Lyn kinase controls basophil GATA-3 transcription factor expression and induction of Th2 cell differentiation. Immunity 30:533–543
Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R (2009) Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol 10:713–720
Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, Nakanishi K (2009) Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol 10:706–712
Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, van Rooijen N et al (2009) MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol 10:697–705
Mellman I, Steinman RM (2001) Dendritic cells: specialized and regulated antigen processing machines. Cell 106:255–258
Kapsenberg ML (2003) Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol 3:984–993
Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, Muskens F, Lambrecht BN (2010) Inflammatory dendritic cells—not basophils—are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med 207:2097–2111
Kim S, Prout M, Ramshaw H, Lopez AF, LeGros G, Min B (2010) Cutting edge: basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J Immunol 184:1143–1147
Phythian-Adams AT, Cook PC, Lundie RJ, Jones LH, Smith KA, Barr TA, Hochweller K, Anderton SM, Hammerling GJ, Maizels RM et al (2010) CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med 207:2089–2096
Tawara I, Nieves E, Liu C, Evers R, Toubai T, Sun Y, Alrubaie M, Reddy P (2011) Host basophils are dispensable for induction of donor T helper 2 cell differentiation and severity of experimental graft-versus-host disease. Biol Blood Marrow Transplant 17:1747–1753
Leyva-Castillo JM, Hener P, Michea P, Karasuyama H, Chan S, Soumelis V, Li M (2013) Skin thymic stromal lymphopoietin initiates Th2 responses through an orchestrated immune cascade. Nat Commun 4:2847
Suurmond J, Stoop JN, Rivellese F, Bakker AM, Huizinga TW, Toes RE (2014) Activation of human basophils by combined toll-like receptor- and FcεRI-triggering can promote Th2 skewing of naive T helper cells. Eur J Immunol 44:386–396
Zhong W, Su W, Zhang Y, Liu Q, Wu J, Di C, Zhang Z, Xia Z (2014) Basophils as a primary inducer of the T helper type 2 immunity in ovalbumin-induced allergic airway inflammation. Immunology 142:202–215
Tang H, Cao W, Kasturi SP, Ravindran R, Nakaya HI, Kundu K, Murthy N, Kepler TB, Malissen B, Pulendran B (2010) The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol 11:608–617
Wakahara K, Van VQ, Baba N, Begin P, Rubio M, Delespesse G, Sarfati M (2013) Basophils are recruited to inflamed lungs and exacerbate memory Th2 responses in mice and humans. Allergy 68:180–189
Otsuka A, Nakajima S, Kubo M, Egawa G, Honda T, Kitoh A, Nomura T, Hanakawa S, Sagita Moniaga C, Kim B et al (2013) Basophils are required for the induction of Th2 immunity to haptens and peptide antigens. Nat Commun 4:1739
Gomez MR, Talke Y, Hofmann C, Ketelsen I, Hermann F, Reich B, Goebel N, Schmidbauer K, Dunger N, Bruhl H et al (2014) Basophils control T-cell responses and limit disease activity in experimental murine colitis. Mucosal Immunol 7:188–199
Kim S, Shen T, Min B (2009) Basophils can directly present or cross-present antigen to CD8 lymphocytes and alter CD8 T cell differentiation into IL-10-producing phenotypes. J Immunol 183:3033–3039
Khodoun MV, Orekhova T, Potter C, Morris S, Finkelman FD (2004) Basophils initiate IL-4 production during a memory T-dependent response. J Exp Med 200:857–870
Denzel A, Maus UA, Rodriguez Gomez M, Moll C, Niedermeier M, Winter C, Maus R, Hollingshead S, Briles DE, Kunz-Schughart LA et al (2008) Basophils enhance immunological memory responses. Nat Immunol 9:733–742
Chen K, Xu W, Wilson M, He B, Miller NW, Bengten E, Edholm ES, Santini PA, Rath P, Chiu A et al (2009) Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol 10:889–898
Charles N, Hardwick D, Daugas E, Illei GG, Rivera J (2010) Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med 16:701–707
Walker JA, Barlow JL, McKenzie AN (2013) Innate lymphoid cells—how did we miss them? Nat Rev Immunol 13:75–87
Walker JA, McKenzie AN (2013) Development and function of group 2 innate lymphoid cells. Curr Opin Immunol 25:148–155
von Moltke J, Locksley RM (2014) I-L-C-2 it: type 2 immunity and group 2 innate lymphoid cells in homeostasis. Curr Opin Immunol 31:58–65
Motomura Y, Morita H, Moro K, Nakae S, Artis D, Endo TA, Kuroki Y, Ohara O, Koyasu S, Kubo M (2014) Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity 40:758–771
Kim BS, Wang K, Siracusa MC, Saenz SA, Brestoff JR, Monticelli LA, Noti M, Tait Wojno ED, Fung TC, Kubo M et al (2014) Basophils promote innate lymphoid cell responses in inflamed skin. J Immunol 193:3717–3725
Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3:23–35
Van Dyken SJ, Locksley RM (2013) Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol 31:317–343
Lumeng CN, Bodzin JL, Saltiel AR (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117:175–184
Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW et al (2007) Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 447:1116–1120
Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM (2011) Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332:243–247
Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Roers A, Eming SA (2010) Differential roles of macrophages in diverse phases of skin repair. J Immunol 184:3964–3977
Daley JM, Brancato SK, Thomay AA, Reichner JS, Albina JE (2010) The phenotype of murine wound macrophages. J Leukoc Biol 87:59–67
Egawa M, Mukai K, Yoshikawa S, Iki M, Mukaida N, Kawano Y, Minegishi Y, Karasuyama H (2013) Inflammatory monocytes recruited to allergic skin acquire an anti-inflammatory M2 phenotype via basophil-derived interleukin-4. Immunity 38:570–580
Obata-Ninomiya K, Ishiwata K, Tsutsui H, Nei Y, Yoshikawa S, Kawano Y, Minegishi Y, Ohta N, Watanabe N, Kanuka H et al (2013) The skin is an important bulwark of acquired immunity against intestinal helminths. J Exp Med 210:2583–2595
Bleriot C, Dupuis T, Jouvion G, Eberl G, Disson O, Lecuit M (2015) Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity 42:145–158
Anthony RM, Rutitzky LI, Urban JF Jr, Stadecker MJ, Gause WC (2007) Protective immune mechanisms in helminth infection. Nat Rev Immunol 7:975–987
Mukai K, Matsuoka K, Taya C, Suzuki H, Yokozeki H, Nishioka K, Hirokawa K, Etori M, Yamashita M, Kubota T et al (2005) Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity 23:191–202
Anthony RM, Kobayashi T, Wermeling F, Ravetch JV (2011) Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature 475:110–113
Campbell IK, Miescher S, Branch DR, Mott PJ, Lazarus AH, Han D, Maraskovsky E, Zuercher AW, Neschadim A, Leontyev D et al (2014) Therapeutic effect of IVIG on inflammatory arthritis in mice is dependent on the Fc portion and independent of sialylation or basophils. J Immunol 192:5031–5038
Nakashima C, Otsuka A, Kitoh A, Honda T, Egawa G, Nakajima S, Nakamizo S, Arita M, Kubo M, Miyachi Y et al. (2014). Basophils regulate the recruitment of eosinophils in a murine model of irritant contact dermatitis. J Allergy Clin Immunol 134:100–107.e12
Cheng LE, Sullivan BM, Retana LE, Allen CD, Liang HE, Locksley RM (2015) IgE-activated basophils regulate eosinophil tissue entry by modulating endothelial function. J Exp Med 212:513–524
Acknowledgments
This work is supported by research grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology. The authors apologize for not citing all the relevant publications due to the space limitation.
Author information
Authors and Affiliations
Corresponding author
Additional information
This submission is related to Basophils and Mast Cells in Immunity and Inflammation - Dr Hajime Karasuyama
Rights and permissions
About this article
Cite this article
Yamanishi, Y., Karasuyama, H. Basophil-derived IL-4 plays versatile roles in immunity. Semin Immunopathol 38, 615–622 (2016). https://doi.org/10.1007/s00281-016-0568-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-016-0568-y