Abstract
Purpose
Thiopurine drugs like 6-Mercaptopurine (6MP) are the cornerstone of maintenance therapy in acute lymphoblastic leukemia (ALL). A recently described variant in alpha-ketoglutarate dependent dioxygenase (FTO) gene has been reported to play an important role in thiopurine induced myelosuppression.
Methods
In this study, we genotyped a coding variant (p.Ala134Thr, rs79206939) and an intronic variant (rs16952570) of FTO in 174 Indian children (age ≤ 12 years) with ALL on maintenance phase of chemotherapy and examined correlation with the risk of thiopurine induced myelosuppression and hepatic toxicity.
Results
The prevalence of FTO-rs16952570 polymorphism was 18.4% (32/174) with 142 (82%) cases having TT genotype, 26 (15%) cases with TC genotype and 6 (3.4%) cases having CC genotype. FTO-rs79206939 was absent and non-polymorphic in our study group. The mean dose of 6-MP during 36 weeks of maintenance of TT, TC and CC carriers of FTO-rs16952570 was 53.7, 53.6 and 54.1 mg/m2/day. Number of patients tolerating starting dose of 60 mg/m2/day was significantly higher in CC (50%) than TT/TC (14%) genotype carrying cases (p = 0.014). However, no statistical significance was observed for total leukocyte count (TLC), absolute neutrophil count (ANC) as well as for platelets counts in patients harboring FTO-rs16952570 TT/TC/CC genotype at 4, 8, 12, 24 and 36 weeks after start of thiopurine therapy. Further, no significant correlation was noted between number of weeks of chemotherapy interruptions or episodes of febrile neutropenia and no evidence of hepatotoxicity was found with the genotype studied.
Conclusion
Polymorphism in FTO-rs16952570 did not show any correlation with thiopurine related toxicity in ALL patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute lymphoblastic leukemia is the most common childhood cancer, accounting for 25% of all malignancies in childhood, with peak incidence between two and five years of age [1]. The survival rates of childhood ALL have approached around 90% in recent studies due to better risk stratification involving genetic characteristics of leukemic cells, modification of therapy based on patient pharmacogenomics, and improved supportive care of patients [2].
The maintenance phase of treatment of ALL is the longest with oral thiopurines given for a period of 2–2.5 years. Thiopurines are metabolized through a series of reactions involving enzymes of the purine salvage pathway [3]. The thiopurine metabolism enzymes are known to harbor genetic variations that have long been implicated in the toxicity of thiopurines, the most significant being the single nucleotide polymorphism (SNP) involving the enzyme TPMT [4]. SNPs in TPMT are more prevalent in the Caucasian population and dose reductions based on TPMT levels have been incorporated in many leukemia protocols [5]. The other enzyme implicated in increasing the risk of thiopurine toxicity especially in Asian and Hispanic ancestry is NUDT15 [6,7,8]. NUDT15 polymorphism is observed in 12% in our population [9,10,11]. Around 30% of our ALL cohort has been observed to suffer from thiopurine toxicity. Therefore, there is a need of further studies and identification of other variants which may be responsible for thiopurine toxicity.
To identify novel genetic variants associated with toxicity to thiopurines, recent genome wide association studies have shown that a coding and an intronic variant in fat mass and obesity-associated (FTO) gene is significantly associated with the risk of thiopurine induced leukopenia in patients with inflammatory bowel disease (IBD) [12]. Another study has demonstrated a protective effect of the presence of intronic variant of FTO against thiopurine induced leukopenia in IBD [13]. Considering the controversial reports regarding this gene, we examined the frequency of FTO gene mutation in our cohort to evaluate its role in thiopurine toxicity. To our knowledge, this is the first study reporting the correlation between thiopurine induced toxicity in ALL patients with FTO gene variants.
Methods
Patients and treatment
Pediatric ALL patients (age ≤ 12 years) undergoing maintenance therapy were enrolled prospectively in the study. The patients were risk stratified and treated as per Indian Childhood Collaborative Leukemia Group 2015-ICICLE protocol (Clinical Trials Registry-India (CTRI) CTRI/2015/12/006434) [14]. Informed consent was obtained from the patients or their guardians. The study was approved by the hospital ethics committee.
The maintenance phase includes daily 6-mercaptopurine (6MP), weekly oral methotrexate (MTX) along with an intrathecal MTX at 3-monthly intervals as per the ICICLE protocol. 6MP and MTX was started at the recommended starting doses being 60 mg/m2/day and 20 mg/m2/, respectively. Blood sample for genotyping was drawn prior to starting maintenance therapy. The enrolled patients were followed with a complete blood count done every 2 weeks and liver function tests done 3 monthly. Side effect profile included; (1) myelosuppression (absolute neutrophil count-ANC < 750/mm3, and/or thrombocytopenia-platelets count < 75,000/mm3), (2) occurrence of febrile neutropenia (temperature higher than 38 °C with ANC < 500/mm3 or ANC showing a falling trend to reach < 500/mm3), (3) hepatotoxicity {an increase of the liver enzyme alanine transferase (ALT) to at least 5 times(> 44 U/L) and /or rise of serum bilirubin beyond 3 times the upper limit of normal} and (4) duration of treatment interruptions; were recorded for 36 weeks. Dose adjustment, if any, was done as per routine in the pediatric oncology clinic to maintain an ANC between 750 and 1500/mm3.
Genotyping
Approximately 2 ml of peripheral blood was collected from enrolled patients in sodium EDTA tubes. DNA was extracted using the QIAmp DNA blood kit (Qiagen Inc.) as per the manufacturer’s instructions. FTO (rs79206939& rs16952570) genotyping was performed by Real-Time PCR based on competitive allele-specific PCR. The SNP positive cases and randomly selected negative cases were further confirmed by Sanger sequencing using ABI 3500 genetic analyzer (Applied Biosystems).
Statistical analysis
Statistical analysis was performed using IBM SPSS software version 22.0. Allelic and genotypic frequencies were noted. Baseline characteristics, 6MP related toxicities and genotypes were compared using Student t test and two-sided Fisher’s exact test or Chi- Square test for continuous and categorical data respectively. The association of the different genotypes with 6MP related febrile neutropenia and hepatotoxicity was evaluated using the Mann Whitney U test. P value < 0.05 was considered as statistically significant.
Results
Patient demographics and clinical characteristics
A total of 200 children (age ≤ 12 years) undergoing maintenance phase chemotherapy according to ICICLE 2015 protocol in the study period between January 2016 and August 2021 were randomly enrolled in the study. Twenty six cases were excluded from the analysis due to insufficient DNA or loss of follow up. One hundred and seventy four patients underwent genotyping and were monitored for 6MP related toxicity profile for 36 weeks. The median follow up of the cohort was 29 months following start of maintenance therapy. The demographics and clinical characteristics of patients (n = 174) are listed in Table 1. The cohort comprised of patients with a mean age of 5 years (SD ± 3 years) with a male to female ratio of 2.4:1. One hundred and fifty cases were B-cell ALL (86%) and 24 were T-cell ALL (14%). According to ICICLE risk stratification 65 (37.3%) cases were standard risk, 32 (18.4%) were intermediate and 78 (44.2%) were high risk cases.
6MP dose and toxicity profile
The mean dose of 6MP in the studied cohort was 53.7 mg/m2/day (SD ± 6) and cumulative mean dose over 36 weeks was 12382 mg/m2. Sixty percent of cases tolerated more than 80% (n = 105) of planned dose of 6MP while only 2% (n = 3) of cases tolerated less than 50% of planned dose and the remaining 38% (n = 66) cases tolerated doses between 50 and 80% of planned dose. Fifty-eight out of 174 (33.3%) patients developed at least one neutropenic episode. The average daily dose of cases having at least one episode of neutropenia was 52.9 mg/m2/day compared to 54.1 mg/m2/day for cases having no neutropenia (p = 0.039). The mean weeks of off therapy in the cohort was noted to be 5 weeks for cases developing neutropenia compared to 2 weeks of off therapy for patients having no neutropenia (p = 0.02). The hepatotoxicity profile was monitored for 36 weeks. Though transaminases were mildly raised in 18 patients and serum bilirubin was abnormal in 3 cases, no patient required cessation of therapy owing to hepatotoxicity.
Genotype
Observed frequencies of the FTO variants are shown in Table 2. The coding variant p.Ala134Thr of FTO (rs79206939) was absent and non-polymorphic in our study cohort. The prevalence of FTO intronic variant (rs16952570) was 18.4% (n = 32) with 82% (n = 142) TT (wild type) carriers 82%, 15% (n = 26) TC carriers and CC carriers were 3% (n = 6) noted. The minor allele frequency (C) was 0.11.
Effect of FTO variant rs16952570 on 6MP related toxicity
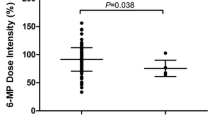
The mean dose of 6MP over 36 weeks of maintenance phase for FTO rs16952570 TT carriers was 53.7 mg/m2/day, for CC carriers was 54.1 mg/m2/day for TC carriers was 53.6 mg/m2/day (Table 2). Number of patients tolerating starting dose of 60 mg/m2/day was significantly higher in CC (50%) than TT/TC (14%) genotype carrying cases (p = 0.014). The total leukocyte count (TLC), absolute neutrophil count (ANC) and platelets count observed during 4, 8, 12, 24 and 36 weeks of follow up after start of thiopurine therapy in patients harboring FTO rs16952570 TT, TC and CC genotypes is shown in Fig. 1. However, no statistical difference could be sought. Further, a trend of lower platelets counts in CC patients, similar to that of TLC and ANC levels (Fig. 1) was also noted at different time points. No significant correlation was observed with hepatotoxicity among the 3 genotypes for a follow up of 36 weeks.
Discussion
Maintenance phase of chemotherapy seems to be one of the most important and most challenging phases of chemotherapy in ALL [15], with strict monitoring of counts being vital toward reducing the risk of relapse [16, 17]. It is also one of the most difficult phases of chemotherapy because of the wide variability in dosing in different protocols and inter-individual variations in 6MP/MTX bioavailability and pharmacokinetics [18]. All these factors in combination cause patients receiving identical doses per body surface area to experience very different systemic and intracellular drug exposures [19]. An estimated 20–25% of children being treated for ALL experience severe drug related toxicities [15]. Identification of the high risk children prone to severe adverse drug effects and tailoring therapy accordingly helps in reducing the therapy interruptions in the children and ensures good compliance ultimately resulting in the reduced rates of relapse [2, 15].
One of the most common reasons for interpatient variability in drug response is gene polymorphisms in thiopurine metabolism pathway. SNPs in the FTO gene have been recently implicated in thiopurine induced myelosuppression [12, 20]. An intronic variant (rs16952570) and a coding variant (rs79206939, p.A134T) have been found to be significantly associated with the risk of thiopurine induced myelosuppression [12, 13]. In the present study we evaluated the association of the presence of these variants and the risk of thiopurine toxicity in children with ALL undergoing maintenance phase of therapy. We found that coding variant (rs79206939, p.A134T) was non-polymorphic in our cohort. Similar report was provided by Chen S et al. in their study showing that this variant is not present in the Indian sub-population [13]. It has been reported that the FTO rs79206939 is absent in several 1000 Genome Project populations, namely European-Caucasians, Africans, and South Asians and low in East Asians (2.2%) [12]. This would explain the absence of this variant in our study population as it was an ethnically homogenous population comprising of only Indians. Thus, considering the low frequency of (rs79206939, p.A134T) across different ethnicity, its potential to be considered as biomarker for thiopurine generated leukopenia is very low, despite its association with myelosupression as reported previously [10].
The prevalence of non-coding variant of FTO rs16952570 in our study cohort was 18%. Similar frequency was reported by Chen et al. [13]. We further examined the association of FTO rs16952570 with thiopurine induced toxicity. We did not find any statistically significant difference in the dose of 6MP, febrile neutropenia episodes and weeks off therapy among the genotypes. In the study by Chen et al. the protective nature of FTO rs16952570 variant was suggested in their cohort of IBD patients [13]. Though we did note slightly higher mean dose of 6MP during 36 weeks of maintenance in CC carriers (54.1 mg/m2/day) and the number of patients tolerating starting dose (60 mg/m2/day or higher) higher in CC (50%) than TT/TC (14%) genotype carrying cases (p = 0.014), no further parameters was suggestive of the protective profile of this polymorphism in relation to myelosupression or hepatotoxicity in ALL patients.
We further looked at the TLC, ANC and platelets counts before and after 4, 8, 12, 24 and 36 weeks of start of maintenance therapy. We noted that the TT and TC alleles carrying patients had higher TLC, ANC and platelets counts compared to CC genotype throughout the period of follow up of 9 months (Fig. 1a, b, c). Peculiarly, patients with the CC genotype had lower ANC/TLC/platelets at baseline or starting of maintenance therapy. This is indicative of the fact that these patients may have lower threshold to chemotherapeutics agents that remains consistent though out the therapy and also contributes toward masking the 6-MP related myelosuppression. This data could be correlated to study by Kim et al. where they have shown that FTO rs16952570 is strongly linked to rs79206939, p.A134T and there is a strong association with thiopurine induced leukopenia [12]. Further, our data are contrary to the previous report by Chen et al. where they noted that CC carrying patients have significantly higher TLC, ANC and platelets counts than TT and TC variant carrying IBD patients before and after 4, 8, 12 weeks of thiopurine therapy [13]. There could be a number of reasons for the difference noted in the thiopurine related toxicity in genotypes of FTO between the two reports. One reason could the ethnicity of the two population, where this particular variant may be forming a haplotype with other SNPs resulting in different outcomes. Further, due to different protocols of treatment being followed, patients of these two cohorts may show different drug response and treatment related toxicity, as the combination of drugs and duration given to patient may vary.
Recently, FTO gene has been identified as RNA modifier and have been shown to govern normal haematopoiesis and leukemogenesis [21]. FTO has been reported to be upregulated in many tumors and its high expression represents an independent risk factor of shorter overall survival in multiple types of cancer patients [22,23,24,25]. The consistent lower TLC, ANC and platelets in CC carrying is suggestive of involvement of FTO rs16952570 in regulation of FTO protein expression in cells which may further result in alteration in haematopoiesis and possibly leukemogenesis. The role of FTO in the thiopurine metabolic pathway remains undefined and warrants further investigation considering its functional role as demethylase that may serve to regulate the nucleotide pools for DNA and RNA synthesis as well.
Data availability
Data will be made available on reasonable request.
References
Inaba H, Greaves M, Mullighan CG (2013) Acute lymphoblastic leukaemia. Lancet (Lond, Engl) 381:1943–1955
Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ, Reaman GH, Carroll WL (2012) Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol 30:1663–1669
Zaza G, Cheok M, Krynetskaia N et al (2010) Thiopurine pathway. Pharmacogenet Genom 20:573–574
Relling MV, Hancock ML, Rivera GK, Sandlund JT, Ribeiro RC, Krynetski EY, Pui CH, Evans WE (1999) Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J Natl Cancer Inst 91:2001–2008
Lennard L (2014) Implementation of TPMT testing. Br J Clin Pharmacol 77:704–714
Yang JJ, Landier W, Yang W et al (2015) Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol 33:1235–1242
Moriyama T, Yang W, Smith C, Pui CH, Evans WE, Relling MV, Bhatia S, Yang JJ (2022) Comprehensive characterization of pharmacogenetic variants in TPMT and NUDT15 in children with acute lymphoblastic leukemia. Pharmacogenet Genom 32:60–66
Wang Q, Mailloux J, Schwarz UI, Kim RB, Wilson A (2022) A novel NUDT15 variant identified in Caucasian TPMT wild type patients with inflammatory bowel disease and azathioprine-related myelotoxicity. Pharmacogenet Genom 32:39–41
Puangpetch A, Tiyasirichokchai R, Pakakasama S, Wiwattanakul S, Anurathapan U, Hongeng S, Sukasem C (2020) NUDT15 genetic variants are related to thiopurine-induced neutropenia in Thai children with acute lymphoblastic leukemia. Pharmacogenomics 21:403–410
Khera S, Trehan A, Bhatia P, Singh M, Bansal D, Varma N (2019) Prevalence of TPMT, ITPA and NUDT 15 genetic polymorphisms and their relation to 6MP toxicity in north Indian children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol 83:341–348
Wang DS, Yu CH, Lin CY et al (2021) Childhood acute lymphoblastic leukemia mercaptopurine intolerance is associated with NUDT15 variants. Pediatr Res 89:217–222
Kim HS, Cheon JH, Jung ES et al (2017) A coding variant in FTO confers susceptibility to thiopurine-induced leukopenia in East Asian patients with IBD. Gut 66:1926–1935
Chen S, Tan WZ, Sutiman N et al (2020) An intronic FTO variant rs16952570 confers protection against thiopurine-induced myelotoxicities in multiethnic Asian IBD patients. Pharmacogenom J 20:505–515
Das N, Banavali S, Bakhshi S et al (2022) Protocol for ICiCLe-ALL-14 (InPOG-ALL-15-01): a prospective, risk stratified, randomised, multicentre, open label, controlled therapeutic trial for newly diagnosed childhood acute lymphoblastic leukaemia in India. Trials. https://doi.org/10.1186/S13063-022-06033-1
Schmiegelow K, Nielsen SN, Frandsen TL, Nersting J (2014) Mercaptopurine/methotrexate maintenance therapy of childhood acute lymphoblastic leukemia: clinical facts and fiction. J Pediatr Hematol Oncol 36:503–517
Bhatia S, Landier W, Shangguan M et al (2012) Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: a report from the children’s oncology group. J Clin Oncol 30:2094–2101
Prognostic importance of 6-mercaptopurine dose intensity in acute lymphoblastic leukemia—PubMed. https://pubmed.ncbi.nlm.nih.gov/10216075/. Accessed 6 Sep 2022
Zimm S, Collins JM, Riccardi R, O’Neill D, Narang PK, Chabner B, Poplack DG (1983) Variable bioavailability of oral mercaptopurine. Is maintenance chemotherapy in acute lymphoblastic leukemia being optimally delivered? N Engl J Med 308:1005–1009
Lafolie P, Hayder S, Bjork O, Ahström L, Liliemark J, Peterson C (1986) Large interindividual variations in the pharmacokinetics of oral 6-mercaptopurine in maintenance therapy of children with acute leukaemia and non-Hodgkin lymphoma. Acta Paediatr Scand 75:797–803
Sato T, Takagawa T, Kakuta Y et al (2017) NUDT15, FTO, and RUNX1 genetic variants and thiopurine intolerance among Japanese patients with inflammatory bowel diseases. Intest Res 15:328–337
Yao L, Yin H, Hong M, Wang Y, Yu T, Teng Y, Li T, Wu Q (2021) RNA methylation in hematological malignancies and its interactions with other epigenetic modifications. Leukemia 35:1243–1257
Huang H, Wang Y, Kandpal M et al (2020) FTO-dependent N 6-methyladenosine modifications inhibit ovarian cancer stem cell self-renewal by blocking cAMP signaling. Cancer Res 80:3200–3214
Liu J, Ren D, Du Z, Wang H, Zhang H, Jin Y (2018) m 6 A demethylase FTO facilitates tumor progression in lung squamous cell carcinoma by regulating MZF1 expression. Biochem Biophys Res Commun 502:456–464
Li J, Han Y, Zhang H, Qian Z, Jia W, Gao Y, Zheng H, Li B (2019) The m6A demethylase FTO promotes the growth of lung cancer cells by regulating the m6A level of USP7 mRNA. Biochem Biophys Res Commun 512:479–485
Zejuan Li A, Weng H, Su R, Jin J, He C, Chen J (2017) FTO plays an oncogenic role in acute myeloid leukemia as a N 6-methyladenosine RNA demethylase accession numbers GSE34184 GSE30285 GSE76414 GSE84944 GSE85008. Cancer Cell 31:127–141
Acknowledgements
The study was funded by intramural special research grant for MD/DM from Postgraduate Institute of Medical Education & Research, Chandigarh.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, M., Bhaskar, D., Bhatia, P. et al. Evaluation of FTO polymorphism in 6-mercaptopurine related intolerance in children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol 92, 51–56 (2023). https://doi.org/10.1007/s00280-023-04546-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-023-04546-3