Abstract
Purpose
High dose methotrexate (HDMTX) acute kidney injury (AKI) results in prolonged hospitalization and treatment delays. Using a pharmacologically-based approach, HDMTX was administered with standard combination therapy to patients with osteosarcoma; nephrotoxicity was assessed.
Methods
Patients were randomized by cycle to 4 h or 12 h HDMTX (12 g/m2) infusions administered with hydration, alkalization and leucovorin rescue. Urinalysis, AKI biomarkers, and estimated glomerular filtration rate using serum creatinine or cystatin C (GFRCr or GFRcysC) were obtained. Serum and urine methotrexate concentrations [MTX] were measured.
Results
Patients (n = 12), median (range) age 12.4 (5.7–19.2) years were enrolled; 73 MTX infusions were analyzed. Median (95% Confidence Interval) serum and urine [MTX] were 1309 (1190, 1400) µM and 16.4 (14.7, 19.4) mM at the end of 4 h infusion and 557 (493, 586) µM and 11.1 (9.9, 21.1) mM at the end of 12 h infusion. Time to serum [MTX] < 0.1 µM was 83 (80.7, 90.7) h and 87 (82.8, 92.4) h for 4 and 12 h infusions. GFRCr was highly variable, increased after cisplatin, and exceeded 150 ml/min/1.73 m2. GFRcysC was less variable and decreased at the end of therapy. AKI biomarkers were elevated indicating acute tubular dysfunction, however, did not differ between 4 and 12 h infusions. Radiographic and histological response were similar for patients receiving 4 h or 12 h infusions; the median percent tumor necrosis was > 95%.
Conclusions
Reducing peak serum and urine MTX concentration by prolonging the infusion duration did not alter risk of acute kidney injury. GFRcysC was decreased at the end of therapy. Proteinuria and elevations in AKI biomarkers indicate that direct tubular damage contributes to HDMTX nephrotoxicity.
Clinical Trial
NCT01848457.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteosarcoma is the most common bone cancer in children and adolescents under 20 years of age. Therapy includes pre-operative chemotherapy with methotrexate, doxorubicin (Adriamycin™) and cisplatin (MAP), surgical resection of the tumor, and adjuvant chemotherapy for a total of 8 months of treatment [1]. For patients with localized osteosarcoma, overall survival is 70%; for patients presenting with overt metastatic disease, prognosis is poor, despite intensive treatment [2, 3]. MAP causes substantial acute and long-term toxicity, including nephrotoxicity, cardiotoxicity, ototoxicity and neurotoxicity [4]. Prevention or early detection of toxicity may eliminate short-term treatment delays and dose reductions that can compromise efficacy, and may decrease morbidity for long-term survivors.
The incidence of severe nephrotoxicity from high dose methotrexate (HDMTX) in osteosarcoma is 1.8%, and is fatal in 4% of patients who experience acute, severe nephrotoxicity [5]. HDMTX-induced acute kidney injury (AKI) is a non-oliguric decrease in glomerular filtration rate (GFR) heralded by an acute rise in serum creatinine. It has been attributed to crystalline AKI from methotrexate precipitation in renal tubules [6, 7]. Methotrexate is renally excreted. Nephrotoxicity leads to decreased methotrexate clearance, resulting in prolonged exposure to high methotrexate concentrations and increased toxicity including life-threatening myelosuppression, mucositis, dermatitis, and hepatic dysfunction. Successful strategies to reduce the risk of HDMTX AKI include hydration, alkalinization, therapeutic drug monitoring and serial creatinine measurements [8]. Glucarpidase, a recombinant bacterial enzyme that cleaves circulating methotrexate into less toxic metabolites, can be used to rapidly decrease the serum methotrexate concentrations and, in conjunction with high dose leucovorin, ameliorate systemic toxicity after HDMTX AKI [9]. Less severe nephrotoxicity occurs more frequently [10], requires prolonged leucovorin rescue, and can compromise the antitumor effect of methotrexate [11] by delaying subsequent therapy and reducing dose intensity.
Infusion of HDMTX over 4 h may increase the risk for nephrotoxicity because of the high peak serum and urinary methotrexate concentrations. Vigorous hydration and alkalinization improve MTX solubility in urine [12]. At a pH of 7, MTX (pKa, 4.8 and 5.7) is more than 98% ionized, so further increases in the urine pH are not likely to improve solubility [13]. Prolonging HDMTX infusion lowers peak serum and urine concentrations and potentially reduces the risk of nephrotoxicity. The duration of exposure to a cytotoxic methotrexate concentration is the primary determinant the anti-tumor effect [14]. Prior studies have demonstrated an association between high peak (end of infusion) plasma methotrexate concentration and improved survival. However, the role of peak plasma methotrexate concentration in determining outcome was confounded because peak plasma concentrations were highly correlated with area under the curve (AUC) [15]. In addition, critical peak methotrexate concentrations varied depending on the dose and infusion duration. For methotrexate, 12 g/m2 infused over 4 h, peak concentrations > 1000 µM were associated with better survival, whereas for doses of 8–10 gm/m2, the cut point for peak concentration was 700 µM, indicating that an absolute peak concentration is not the critical determinant of outcome [16]. In other studies, no relationship was found between peak methotrexate concentration and survival [17, 18]. In one study, very high methotrexate exposures were associated with a poorer outcome [19]; and in a randomized clinical trial, disease-free survival in osteosarcoma patients was similar for 7500 mg/m2 of methotrexate infused over 6 h and 690 mg/m2 infused over 42 h [20].
More sensitive and specific biomarkers to accurately quantify changes GFR and to rapidly detect renal tubular damage may be useful for detecting and monitoring renal toxicity of nephrotoxic anticancer drugs. Current clinical laboratory tests of renal function in children with cancer do not detect early, subtle changes in GFR or renal tubular function that may indicate development of significant renal dysfunction. Limitations of serum creatinine (sCr) as a marker of GFR [21] include non-renal factors (diet, muscle mass, tubular secretion) that can alter the sCr but do not affect GFR. sCr can be within the normal range with a GFR as low as 40 ml/min per 1.73 m2 [22]. Cystatin C, a 13 kDa cysteine protease inhibitor produced by all nucleated cells, is freely filtered, reabsorbed and metabolized in proximal tubules but not normally excreted in urine. Serum cystatin C is used to estimate GFR. Cystatin C is not influenced by muscle mass, and compared to sCr, it has improved temporal discrimination in detection of kidney injury. Cystatin C is measured by an FDA approved nephelometry method in clinical laboratories [23].
There are a growing number of urine biomarkers to assess renal tubular damage or dysfunction. Kidney Injury Molecule-1 (KIM-1) is a 50 kDa trans-membrane protein expressed on proximal tubule cells. Urine KIM-1 is a marker of proximal tubular damage that may discriminate pre-renal azotemia from ischemia [24]. Neutrophil Gelatinase-Associated Lipocalin (NGAL) is a 25 kDa protein expressed in intestine lung, liver, and renal tubular epithelial cells. Urine NGAL is an early predictor of AKI and may discriminate pre-renal from intrinsic AKI. N-acetyl-β-d-glucosamidase (NAG) is a 130 kD enzyme within lysosomes of proximal tubular cells of the kidney. Urinary NAG concentrations are increased during oxidative stress and renal tubular damage regardless of development of AKI. Prospective systematic evaluation of glomerular and tubular function using these biomarkers may detect early, subtle, changes in GFR and tubular function and may be useful in assessing the mechanism of methotrexate induced nephrotoxicity and the impact of the interventions to attenuate nephrotoxicity [25].
Materials and methods
We conducted a pilot study to evaluate pharmacologically-based approaches to prevent nephrotoxicity and investigate AKI biomarkers for use in early detection of nephrotoxicity during MAP chemotherapy (Supplemental Fig. 1) for children and adolescents with newly diagnosed osteosarcoma. In addition, bone specific alkaline phosphatase (BSAP) was assessed as a biomarker of response.
Using a randomized cross over design (Supplemental Fig. 2) we evaluated the effect of pantoprazole on cisplatin related nephrotoxicity and ototoxicity[26] and assessed the effect of prolonging the HDMTX infusion duration, thereby lowering the risk of drug precipitation in renal tubules, on MTX AKI. The objective of this part of the trial was to identify more rational, pharmacologically-based drug delivery approaches to prevent HDMTX nephrotoxicity in patients with osteosarcoma by comparing AKI biomarkers (KIM-1, NAG, NGAL), glomerular function (cystatin C, creatinine) and serum and urine methotrexate concentrations after 12 g/m2 of methotrexate infused over 4 or 12 h.
To design this protocol, we used pharmacokinetic modeling to assess methotrexate urine concentrations with varying infusion duration (4 h vs 12 h). We fit a 2-compartment pharmacokinetic model to the mean plasma or serum MTX concentrations from 1045 infusions of 12 g/m2 over 4 h and simulated 12 h infusion (Fig. 1a) [15]. The urinary concentration–time profile (Fig. 1b) was estimated using the equation below and assuming that the rate of urine production was equal to the IV fluid hydration rate (100 ml/m2/h):
where Curine is the amount of methotrexate in urine, Ccentral is the amount of methotrexate in the central compartment, Vcentral is the volume of the central compartment, kel is the elimination rate constant and 0.9 accounts for the fraction of the methotrexate dose excreted in the urine unchanged. We hypothesized that, with normal renal function, longer infusion duration would not increase the time to achieve a serum methotrexate concentration less than 0.1 µM and urine methotrexate concentrations were predicted to be below the solubility limit, thereby reducing the risk of methotrexate precipitation in the renal tubules.
a To design the trial, model simulations of previously published plasma concentrations after methotrexate (12 g/m2) infused over 4 or 12 h. b Model simulations of urine concentrations after methotrexate (12 g/m2) infused over 4 or 12 h were performed. c Model simulations of plasma concentrations after methotrexate (12 g/m2) infused over 4 or 12 h using the population PK parameters (Table 2). d End of Infusion serum and urine methotrexate concentrations after methotrexate (12 g/m2) infused over 4 or 12 h
Patients
Children, adolescents and young adults less than 30 years of age with untreated high-grade, localized or metastatic osteosarcoma were eligible if they had normal serum creatinine for age and gender, a left ventricular shortening fraction greater than 28% by echocardiogram, an absolute neutrophil count greater than 1000/µl and platelet count greater than 100,000/µl. Patients who were pregnant, breastfeeding or unable to cooperate with research procedures were excluded. Informed consent and assent were obtained in accordance with institutional IRB requirements. The trial was registered and data released on clinicaltrials.gov (NCT1848457).
Safety and response evaluation
Chemotherapy related toxicities were categorized and graded according to the National Cancer Institute Common Terminology for Adverse Events (NCI CTCAE v.4). Treatment delays or missed chemotherapy doses to allow for recovery from toxicity, dose modifications for toxicity and deaths attributed to chemotherapy were adverse events of interest. Radiographic response to two cycles of neoadjuvant chemotherapy was assessed using the Response Evaluation Criteria for Solid Tumors, (RECIST v 1.1) and histologic response (percent necrosis) determined at the time of surgical resection. Serum bone specific alkaline phosphatase (BSAP) was measured using ELISA (Quidel, San Diego, CA) to investigate its use as biomarker of disease burden.
Serum and urine methotrexate concentrations
Serum methotrexate levels were obtained at the end of infusion, 24 h after the start of the infusion and every morning until the methotrexate concentration was less than 0.1 µM. Methotrexate concentrations were quantified in the cincial laboratory of the Children’s Hosptial of Philadelphia usig a CLIA certified fluroenscence polarization assay (Abbott TdX). Urine was obtained at the end of infusion and diluted tenfold with ammonium acetate buffer to stablize alkaline pH and aviod preciptation when frozen at − 70 °C. Urine methotrexate concentrations were measured using HPLC tandem mass spectroscopy [27, 28]. In addition, the solubility of 10, 20 and 40 mM methotrexate was assessed at pH 5, 6.5 and 7.5. Methotrexate concentrations (up to 8 infusions per subject) were analyzed with a population pharmacokinetic approach in the Phoenix Non-Linear Mixed Effect module (v. 8.3, Certara, Princeton, NJ) using FOCE-ELS algorithm. The structural model was a 2-compartment model with first-order elimination from the central compartment, and the model was parameterized in terms of clearances (CL, CL2) and apparent volumes of distribution (V, V2). The methotrexate concentration–time data for the 4 and 12 h infusion durations were modeled separately. The individual predicted urine methotrexate concentration were calculated from the end of infusion methotrexate concentration and compared to measured urine methotrexate concentration collected from spontaneous void near the end of infusion.
GFR and AKI biomarkers
GFRCr was estimated from serum creatinine performed in the clinical lab and GFRcysC from the cystatin C (immunonephelometry, Seimans BNII) prior to each cycle. The Schwartz formula [29] was used to calculate creatinine clearance and estimate GFRCr from sCr.
In addition, we used the following cystatin C based formula developed in the Children with Chronic Kidney Disease Cohort [22] to calculate GFRcycC:
Urinary KIM-1, NAG and NGAL were measured using commercially available assays (Meso Scale Delivery, Rockville, MD; BioVision, Milpitas, CA) and normalized to the urinary creatinine concentration (Jaffe method, Vitros 250 Chemistry Analyzer) measured in the same urine specimen (KIM-1/Cr, NAG/Cr, NGAL/Cr). For each HDMTX dose administered, the end of infusion, 24 h post infusion and 7-day post infusion values for each biomarker were analyzed (See Supplemental Table 1 for calendar of procedures for evaluation of methotrexate nephrotoxicity).
Statistical analysis plan
The urinary methotrexate concentrations at the end of each HDMTX infusion during cycles 1–4 were tabulated for the 4 and 12 h infusion durations and compared to the pH dependent methotrexate solubility to determine whether the solubility limit was reached. The end infusion urine MTX concentration was correlated with simultaneous serum concentration and with the severity of renal toxicity as measured by AKI biomarkers and GFR. Comparison of GFRcr and GFRCysC at baseline and end of therapy were compared using the Wilcoxon log rank test.
Results
Patients, safety and response
Between July 2013 and August 2015, twelve patients, median (range) age 12.4 (5.7–19.2) years, were enrolled; 73 MTX infusions were analyzed. Patient characteristics and baseline values are presented in Table 1.
Methotrexate associated mucositis and myelosuppression did not differ between the 4 h and 12 h infusion durations. One participant (subject #11) experienced HDMTX AKI during a 12 h infusion. The end of infusion (Cycle 1, day 22) methotrexate concentration was 930 µM in serum and 7.5 mM in urine. Within 24 h of the start of the methotrexate infusion the patient’s sCr was 1.4 mg/dL (3.5 × baseline), cystatin C was 1.0 (0.7 × baseline); NAG, NGAL and Kim-1 increased by 570%, 450%, and 350%, respectively. At 40 h, the patient’s serum methotrexate concentration was 40 µM; glucarpidase was administered. Hydration, alkalinization and leucovorin were continued until a serum methotrexate concentration less than 0.1 µM was achieved at 310 h. Cycle 1 day 29 HDMTX dose was omitted. Recovery of renal function to baseline occurred at 20 days for sCr and 40 days for cystatin C. This patient’s subsequent methotrexate doses were modified and the patient’s subsequent methotrexate concentration–time data were excluded from the population pharmacokinetic modeling.
Radiographic and histologic response was similar among patients who received 4 h or 12 h infusions pre-operatively (cycles 1 and 2). For the ten patients who underwent primary tumor resection, the median decrease in longest diameter was 4% (RECIST v1.1: stable disease in 9, progressive disease in 1) and the median percent tumor necrosis was > 95%.
The baseline median (range) BSAP was 132 (40- 689) U/l. The patients with metastatic disease at diagnosis appeared to have higher baseline BSAP compared to patients with localized disease 411 (133–557) U/l vs 108 (40–398) U/l, though the small number of patients with metastatic disease precluded statistical comparison. One patient with progression of disease did not have follow up BSAP measured. The percent decrease in BSAP during therapy did not differ among patients in complete continual response versus those who relapsed or died from progressive osteosarcoma (54% vs 62%).
At a median (range) follow up of 3.6 (2.4–4.6) years, all eight patients with localized osteosarcoma are alive, in remission; three of four with metastases at diagnosis have died from progressive disease.
Serum and urine methotrexate concentrations
The population pharmacokinetic model parameters for the 4 (n = 38) and 12 (n = 35) hour infusion durations are presented in Table 2. Simulations of serum methotrexate concentrations for the 4 h and 12 h methotrexate infusions using the population parameters are presented in Fig. 1c. Visual predictive checks for the model are presented in Supplemental Fig. 3. Patient serum and urine methotrexate concentrations are summarized in Table 3. There was no correlation between end of infusion serum and measured urine methotrexate concentrations (Fig. 1d). The model predicted end of infusion urine methotrexate concentration exceeded the measured end of infusion MTX concentration (Supplemental Fig. 4).
In addition, we determined in vitro the solubility of 10 mM, 20 mM and 40 mM methotrexate at pH 5, 6.5, and 7.5 at 37 °C. (Fig. 2). At the end of the infusion, the urine methotrexate concentration measured in a spontaneously voided urine specimen did not exceed this solubility threshold in any patient, including the patient who received glucarpidase. However, model predicted instantaneous urine methotrexate concentration at the end of 4 h and 12 h infusion exceeded 40 mM in 90% (66/73) of the infusions (Supplemental Fig. 4).
Renal function: GFR and biomarkers of AKI
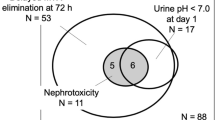
GFR was estimated using a creatinine-based formula as well as formula incorporating creatinine and cystatin C (Fig. 3). GFRcysC was less variable and demonstrated a decrease in glomerular filtration rate at the end of therapy. GFRCr was highly variable and appears to over-estimate renal function in children receiving chemotherapy with appropriate hydration as demonstrated by increase in GFRCr after cisplatin containing chemotherapy and tendency for GFRCr to exceed 150 ml/min per 1.73 m2. At the end of therapy GFRCr was greater than GFRcysC (P = 0.03). Urine biomarkers of AKI (Table 3) were elevated within 24 h of the start of the infusion indicating acute tubular dysfunction, however, urine biomarkers did not differ between 4 and 12 h infusions. Routine serial urinalysis demonstrated that urine pH was maintained at pH ≥ 7 for all infusions and 93% of HDMTX infusions were associated with acute transient proteinuria. Proteinuria (1 + to 3 +) was observed only at the end of infusion, thus, false positive proteinuria due to alkaline urine is unlikely. However, direct interference by methotrexate in the urine was not evaluated. Urinary biomarkers of AKI did not correlate with end of infusion serum and urine methotrexate concentrations (Supplemental Fig. 5) nor GFRCr and GFRcysC (Supplemental Fig. 6).
Discussion
We used a 2-compartment pharmacokinetic model and mean concentration–time data from a prior study in patients with osteosarcoma to simulate serum and urine methotrexate concentrations and determine an infusion duration that would result in a urine methotrexate concentration below the solubility limit at a urine pH ≥ 7. This pilot study used a randomized cross over design in which patients served as their own control to evaluate the impact of lengthening the infusion duration of HDMTX to 12 h in patients with newly diagnosed osteosarcoma. Supporting our hypothesis and predicted by the initial pharmacokinetic simulations, there was no difference in time to methotrexate clearance between 4 and 12 h infusion durations. We then fit a two-compartment model to the methotrexate concentrations from 73 infusions in patients enrolled on the trial.
In this small population, there was no difference in percent tumor necrosis, or the incidence of related toxicity. Urine biomarkers of AKI were elevated within 24 h of the start of the infusion indicating acute tubular dysfunction, however, urine biomarkers did not differ between 4 and 12 h infusions. Therefore, prolonged infusion duration did not appear to ameliorate nephrotoxicity. One patient receiving a 12 h infusion had clinically significant HDMTX AKI and required glucarpidase. This patient’s end of infusion serum methotrexate concentration was < 1000 µM, hydration and alkalinization were maintained, however, the acute rise in serum creatinine indicated AKI and the patient experienced delayed methotrexate excretion. High dose leucovorin and glucarpidase prevented myelosuppression, hepatic and dermatological toxicity. However, due to persistent elevations in creatinine and cystatin C, the subsequent dose of methotrexate was not administered. This indicates that peak methotrexate concentration and crystallization of drug in the urine is not the sole factor in HDMTX nephrotoxicity.
All children on this study received HDMTX and cisplatin. We performed serial measurement of serum creatinine and cystatin C and estimation of glomerular filtration. GFR estimated by the Schwartz formula was more variable and no cumulative decrease in GFR over the course of therapy was documented. GFR estimated using both serum creatinine and cystatin C (GFRcysC) was statistically significantly lower at the end of therapy compared to creatinine based GFR estimates and demonstrated a decrease compared to baseline. A limitation of our study is that we did not compare GFRCr or GFRcysC to GFR measured by inulin clearance or iohexol plasma clearance. However, our findings are supported by a meta-analysis of 12 studies including 1775 adults with cancer which concluded that Cystatin C was superior to creatinine for the detection of minor changes in GFR in early stages of renal insufficiency secondary to chemotherapy.[23] GFRCr, is estimated using a simple and validated equation based on serum creatinine, however, may be inferior to GFRcysC because of the limitations of serum creatinine measurement related to change in body mass, decreased nutritional status, and ample hydration in children with cancer receiving nephrotoxic chemotherapy.
Unlike the serum methotrexate concentrations which are an instantaneous measure, the end of infusion urine methotrexate concentration represents the concentration in the urine contained in the bladder since the prior void. Therefore, the instantaneous methotrexate concentration in urine predicted from the model exceeded the measured urine methotrexate concentration in 90% of the infusions analyzed. Our in vitro methotrexate solubility testing indicated that at urine pH 7.5, methotrexate at a concentration of 40 mM was soluble. For 4 h infusions, no measured end of infusion methotrexate concentrations exceeded 40 mM, however, the model predicted that 24% (9/38) of instantaneous urine methotrexate concentrations exceeded 40 mM. For 12 h infusions, all end of infusion urine methotrexate measurements as well as the model predicted instantaneous methotrexate concentration in urine were below the solubility threshold at pH 7.5. If the pH in the renal tubules were ≤ 5, the model predicts that urine methotrexate concentration would exceed the solubility threshold for many patients receiving 4 h or 12 h methotrexate (12 g/m2) infusions and these patients would be at risk for precipitation and crystalline AKI.
Pedrosa et al. [30] reported urinary KIM-1/Cr greater than 6.2 µg/g measured 24 h after cisplatin or methotrexate predicted persistent renal impairment with sensitivity of 73%, specificity 92%, and Receiver Operator Curve 0.72 (95% CI 0.55, 0.71). In our study, the median KIM-1/Cr post infusion exceeded this threshold. Urine KIM-1 is a marker of proximal tubular damage, clearance of renal tubular apoptotic bodies and tubular regeneration [31]. The observed proteinuria and elevations in AKI biomarkers, particularly KIM-1/Cr in our study, indicate that direct tubular damage contributes to HDMTX nephrotoxicity.
In summary, using a pharmacokinetic model we predicted serum and urine methotrexate concentrations for 4 h and 12 h infusion of HDMTX (12 g/m2) in children and adolescents with newly diagnosed osteosarcoma. However, reducing peak serum and urine methotrexate concentration by prolonging the infusion duration did not alter the risk of acute kidney injury in this study. Our data provides additional support for the use of serum cystatin C to estimate glomerular filtration rate. The measured end of infusion urine methotrexate concentration after 4 h or 12 h infusion did not exceed the predicted solubility limit in this cohort, including a patient with HDMTX AKI. However, instantaneous urine methotrexate concentrations calculated from the pharmacokinetic model based on measured end of infusion methotrexate concentrations indicate that 24% of 4 h infusions exceeded 40 mM. Additional studies into the precise mechanism of HDMTX nephrotoxicity are needed to develop strategies to reduce the risk of acute and chronic renal dysfunction.
References
Ritter J, Bielack SS (2010) Osteosarcoma. Ann Oncol 21(Suppl 7):vii320–vii325
Chou AJ et al (2009) Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma: a report from the Children’s Oncology Group. Cancer 115(22):5339–5348
Marina NM et al (2016) Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol 17(10):1396–1408
Janeway KA, Grier HE (2010) Sequelae of osteosarcoma medical therapy: a review of rare acute toxicities and late effects. Lancet Oncol 11(7):670–678
Widemann BC et al (2004) High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma. Cancer 100(10):2222–2232
Garneau AP, Riopel J, Isenring P (2015) Acute methotrexate-induced crystal nephropathy. N Engl J Med 373(27):2691–2693
Perazella MA (2015) The urine sediment as a biomarker of kidney disease. Am J Kidney Dis 66(5):748–755
Howard SC et al (2016) Preventing and managing toxicities of high-dose methotrexate. Oncologist 21(12):1471–1482
Ramsey LB et al (2018) Consensus guideline for use of glucarpidase in patients with high-dose methotrexate induced acute kidney injury and delayed methotrexate clearance. Oncologist 23(1):52–61
Ferrari S et al (2009) Sex- and age-related chemotherapy toxicity in patients with non-metastatic osteosarcoma. J Chemother 21(2):205–210
Hempel L et al (2003) Influence of high-dose methotrexate therapy (HD-MTX) on glomerular and tubular kidney function. Med Pediatr Oncol 40(6):348–354
Jacobs SA et al (1976) 7-Hydroxymethotrexate as a urinary metabolite in human subjects and rhesus monkeys receiving high dose methotrexate. J Clin Invest 57(2):534–538
Drost SA et al (2017) Outcomes associated with reducing the urine alkalinization threshold in patients receiving high-dose methotrexate. Pharmacotherapy 37(6):684–691
Treon SP, Chabner BA (1996) Concepts in use of high-dose methotrexate therapy. Clin Chem 42(8 Pt 2):1322–1329
Graf N et al (1994) Methotrexate pharmacokinetics and prognosis in osteosarcoma. J Clin Oncol 12(7):1443–1451
Ferrari S et al (1993) Serum methotrexate (MTX) concentrations and prognosis in patients with osteosarcoma of the extremities treated with a multidrug neoadjuvant regimen. J Chemother 5(2):135–141
Bacci G et al (2006) No correlation between methotrexate serum level and histologic response in the pre-operative treatment of extremity osteosarcoma. Anticancer Drugs 17(4):411–415
Zelcer S et al (2005) Methotrexate levels and outcome in osteosarcoma. Pediatr Blood Cancer 44(7):638–642
Crews KR et al (2004) High-dose methotrexate pharmacokinetics and outcome of children and young adults with osteosarcoma. Cancer 100(8):1724–1733
Krailo M et al (1987) A randomized study comparing high-dose methotrexate with moderate-dose methotrexate as components of adjuvant chemotherapy in childhood nonmetastatic osteosarcoma: a report from the Childrens Cancer Study Group. Med Pediatr Oncol 15(2):69–77
Stevens LA et al (2006) Assessing kidney function–measured and estimated glomerular filtration rate. N Engl J Med 354(23):2473–2483
Schwartz GJ et al (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20(3):629–637
He L et al (2019) The value of serum cystatin C in early evaluation of renal insufficiency in patients undergoing chemotherapy: a systematic review and meta-analysis. Cancer Chemother Pharmacol 83(3):561–571
Du Y et al (2011) Urinary biomarkers to detect acute kidney injury in the pediatric emergency center. Pediatr Nephrol 26(2):267–274
Ronco C, Bellomo R, Kellum JA (2019) Acute kidney injury. Lancet 394(10212):1949–1964
Fox E et al (2018) Pantoprazole, an inhibitor of the organic cation transporter 2, does not ameliorate cisplatin-related ototoxicity or nephrotoxicity in children and adolescents with newly diagnosed osteosarcoma treated with methotrexate, doxorubicin, and cisplatin. Oncologist 23(7):762-e79
Turci R et al (2000) Determination of methotrexate in human urine at trace levels by solid phase extraction and high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 14(3):173–9
Barbieri A et al (2006) Simultaneous determination of low levels of methotrexate and cyclophosphamide in human urine by micro liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 20(12):1889–93
Schwartz GJ, Gauthier B (1985) A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr 106(3):522–6
Pedrosa DC, Meneses NFGC, Wirtzbiki G, Moraes C, Martins A, Liborio A (2015) Urinary KIM-1 in children undergoing antineoplstic treatment: a prospective cohort study. Pediatr Nephrol 30:2207–2213
Parikh CR et al (2013) Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol 8(7):1079–88
Acknowledgements
This work was funded by Gateway for Cancer Research Foundation and Alex's Lemonade Stand Center of Excellence.
Funding
This clinical trial was funded by the Gateway for Cancer Research and Alex's Lemonade Stand Center of Excellence.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fox, E., Busch, C., DeBernardo, A. et al. A pharmacologically-based approach to high dose methotrexate administration to investigate nephrotoxicity and acute kidney injury biomarkers in children and adolescents with newly diagnosed osteosarcoma. Cancer Chemother Pharmacol 87, 807–815 (2021). https://doi.org/10.1007/s00280-021-04248-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-021-04248-8