Abstract
Purpose
Preclinical studies support the JAK2–STAT3 signaling pathway as a key driver in CD44+ CD24− “stem-cell-like” breast cancer cells. Ruxolitinib is an orally bioavailable JAK1/2 inhibitor. We aimed to identify the recommended phase 2 dose (RP2D) of ruxolitinib in combination with paclitaxel in patients with HER2-negative metastatic breast cancer (MBC).
Methods
Eligible patients had HER2-negative MBC and had received ≤ 3 chemotherapy regimens for advanced disease. Patients received oral ruxolitinib (10–25 mg bid) in a 3 + 3 dose escalation design in combination with weekly paclitaxel 80 mg/m2 in a 3-week cycle. The primary objective was to determine the maximum tolerated dose (MTD) and the RP2D.
Results
Nineteen patients received protocol therapy (mean age 52 years). Eight (42%) had triple-negative breast cancer and 11 (58%) had hormone receptor-positive disease; 12 (63%) had visceral disease. Ten (53%) patients had not received prior treatment for advanced disease. Patients received a median number of 5 cycles of combination therapy (range 1–12) and five patients continued single-agent ruxolitinib. The MTD of ruxolitinib was 25 mg bid when combined with paclitaxel, and the RP2D for the combination was 15 mg bid. Thirteen (68%) patients required dose reductions or holds. Most frequent toxicities reported of any grade were neutropenia (50%) and anemia (33%). There were no grade 4/5 toxicities attributed to study drug. Four (21%) patients had PR, 12 (63%) had SD and three (16%) had PD as their best response.
Conclusion
The combination of ruxolitinib and weekly paclitaxel was well tolerated with evidence of clinical activity. Further analysis of this combination is ongoing (NCT02041429).
Trial registration
NCT02041429. Date of registration: January 22, 2014.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preclinical studies support the JAK2–STAT3 signaling pathway as a key driver in CD44+ CD24− “stem-cell-like” breast cancer cells [1]. JAK2–STAT3 pathway inhibition results in decreased viability of breast cancer cells and reduced tumor sizes in breast cancer xenograft models [1]. Preclinical data demonstrate a highly active JAK2/STAT3 pathway in inflammatory breast cancer (IBC) and greater than 95% of triple-negative IBC demonstrate high levels of activated STAT3 (pSTAT3), an indicator of activation of the JAK/STAT3 pathway [1, 2]. JAK2 inhibition has been shown to decrease the proliferation of IBC cell lines with high levels of pSTAT3 in vitro and reduce the growth of IBC tumors in xenograft models with high levels of pSTAT3 + [3].
Ruxolitinib is an orally bioavailable JAK1/2 inhibitor, previously evaluated in patients with both hematologic malignancies as well as solid tumors [4,5,6]. The agent is approved by the U.S. Food and Drug Administration for use in intermediate and high-risk myelofibrosis, as second-line therapy in JAK2-positive myeloproliferative disorders such as polycythemia vera, and steroid-refractory acute graft-versus-host disease [7,8,9]. The most common toxicities associated with ruxolitinib are thrombocytopenia, anemia, leukopenia, dizziness, bruising and headache, of which the majority are grade 1–2 and managed by dose reduction or interruption. In a study in advanced pancreatic cancer of combination full-dose capecitabine with ruxolitinib or placebo (15 mg bid), grade 3 or higher adverse events were similar in the ruxolitinib (74.6%) and placebo (81.7%) groups [6]. A recent phase II trial of single-agent ruxolitinib in patients with metastatic triple-negative breast cancer failed to meet the primary endpoints with no objective responses observed and a median progression-free survival of 1.2 months [4]. Expected hematologic toxicity was identified and no new toxicity signals were observed. Lack of efficacy could in part be explained by the absence of chemotherapy administered with the targeting agent ruxolitinib. Preclinical studies show that ruxolitinib synergistically interacts with paclitaxel in human ovarian cancer cells [10].
Here, we report the results of a phase I study of the combination of ruxolitinib and paclitaxel in patients with HER2-negative metastatic breast cancer (MBC). The biologic rationale for this combination was based on in vitro data [10] and after the recommended phase 2 dose (RP2D) was determined, the intended development plan was to proceed with a preoperative phase II study of ruxolitinib in combination with weekly paclitaxel for the treatment of triple-negative IBC led by the Translational Breast Cancer Research Consortium (TBCRC; NCT02876302).
Patients and methods
This study was approved by the Dana-Farber/Harvard Cancer Center Institutional Review Board (DF/HCC#13–494; NCT02041429) and informed consent was obtained from all subjects.
Patients
Patients were required to have histologically confirmed metastatic or unresectable HER2-negative breast cancer. Eligibility criteria included either measurable or evaluable disease; ≤ 3 prior chemotherapies for advanced disease; age ≥ 18 years; life-expectancy > 3 months; Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2; and adequate end organ function. Both men and women were allowed to participate and those who were pregnant were excluded.
Study design and objectives
We conducted a single-center (Dana-Farber Cancer Institute, Boston, USA), open-label, phase I study utilizing a 3 + 3 dose escalation design for ruxolitinib in combination with standard dose weekly paclitaxel in patients with HER2-negative MBC. If a dose-limiting toxicity (DLT) was observed in one of the three patients in a cohort, then three additional patients were added. If no further DLTs were observed, then the next cohort using a higher dose of ruxolitinib opened. The maximum tolerated dose (MTD) was identified as the level below the cohort where DLT occured in ≥ two patients within the cohort.
The primary objective of the study was to determine the MTD of ruxolitinib in combination with standard dose weekly paclitaxel. Because our goal was to test this combination in the preoperative setting where dose intensity of chemotherapy cannot be compromised, we selected the RP2D as the dose that could allow administration of more than 80% of the paclitaxel dose without need for dose reduction during 4 cycles (12 weeks) of combination therapy. Secondary objectives included evaluation of the safety and tolerability of ruxolitinib when administered in combination with paclitaxel, and to describe the response and progression of disease per RECIST v1.1. Disease response was assessed radiographically every 2 cycles (6 weeks).
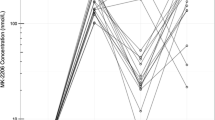
Given that pharmacokinetic (PK) and pharmacodynamic (PD) information is well known for ruxolitinib and paclitaxel [11, 12], and there is no plausible basis for PK or PD interaction, formal evaluation of PK/PD was not conducted, in accordance with the recommendations from the Clinical Trial Design Task Force of the NCI Investigational Steering Committee [13].
Treatment
Ruxolitinib was provided by Incyte Pharmaceuticals and commercially available paclitaxel was used as standard of care in MBC. In a 3 + 3 dose escalation design, oral administration of ruxolitinib was initiated at 10 mg bid with paclitaxel 80 mg/m2 IV on days 1, 8, and 15 of a 21-day cycle. Patients were pre-treated as per institutional standards. There were specified dose modification and delays for paclitaxel planned in the protocol.
Treatment for all patients consisted of repeating 21-day cycles with treatment continuing as long as the regimen was tolerated, and the patient did not meet discontinuation criteria. Patients who completed 4 cycles of combination paclitaxel and ruxolitinib and achieved partial response or stable disease had the opportunity to continue single-agent ruxolitinib at the same twice daily dose, with a treatment cycle remaining at 21 days. Single-agent ruxolitinib could continue until disease progression or unacceptable toxicity.
Assessments
Safety and tolerability were assessed by monitoring adverse events, measuring vital signs, physical examinations and clinical laboratory testing weekly for the first 3 weeks and then every cycle (i.e., 3 weeks). Adverse events were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Response was determined by radiographic disease assessments (RECIST v1.1) with computerized tomography (CT) of the chest, abdomen, and pelvis with oral and IV contrast ± bone scan every 2 cycles.
DLT was defined based on 2 cycles of therapy, and included the following: (a) delay in ability to administer paclitaxel for more than 2 weeks due to toxicity; (b) grade ≥ 3 non-hematologic, non-hepatic organ toxicity not attributed to disease progression or another clearly identified cause, with the exception of alopecia, grade 3 nausea, vomiting, or diarrhea that resolves to grade ≤ 1 within 3 days, grade 3 fasting hyperglycemia that resolves to grade ≤ 1 within 7 days and grade 3 fasting hyperglycemia within 3 days of glucocorticoid use; (c) grade ≥ 4 thrombocytopenia lasting > 24 h or associated with clinically significant bleeding; (d) grade ≥ 4 neutropenia lasting > 4 days or accompanied by fever (oral or tympanic temperature > 100.4 °F or 38.0 °C); (e) grade ≥ 4 anemia; and (f) grade ≥ 3 total bilirubin, hepatic transaminase (ALT or AST), or alkaline phosphatase (ALP) lasting > 72 h; patients with grade 2 hepatic transaminase at baseline as a result of liver metastases, only hepatic transaminase ≥ 10.0 X the upper limit of normal (ULN) lasting > 72 h will be considered a DLT and patients with grade 2 ALP at baseline as a result of bone or liver metastasis, only ALP ≥ 10 X ULN lasting > 72 h will be considered as DLT
Statistical methods
All patients who initiated treatment were included in the safety analyses. Characteristics, treatment and clinical outcomes were summarized descriptively. Time to progression was defined from date of registration until documented progression of disease by RECIST v1.1 or censored at the date of last re-imaging. The distribution of time to progression was estimated by Kaplan–Meier method.
Results
Patients
A total of 20 patients with HER2-negative MBC were enrolled between February 3, 2014 and May 6, 2014 (Table 1). One patient who enrolled at dose level 1 never received treatment on study, and therefore, was excluded from further analysis. The mean age was 52 years at enrollment and all patients had an ECOG performance status of either 0 (79%) or 1 (21%) at baseline. Eleven (58%) patients had hormone receptor-positive breast cancer and 8 (42%) had triple-negative (negative estrogen, progesterone and HER2 receptor) disease. Twelve (63%) patients had visceral disease at the time of enrollment. Twelve (63%) patients had received prior endocrine therapy in the metastatic setting. Nine (47%) patients had received prior lines of chemotherapy for the treatment of metastatic breast cancer, including 2 (11%) patients who received 2 prior lines and 2 (11%) who received 3 prior lines of chemotherapy in the advanced setting. Ten (53%) patients received taxanes in the peri-operative setting and 3 (16%) in the advanced setting.
Dose escalation and subject disposition
Ruxolitinib and standard dose weekly paclitaxel were well tolerated among treatment groups (Table 2). The MTD of ruxolitinib was 25 mg bid in combination with full-dose weekly paclitaxel. One DLT of grade 3 osteonecrosis of the jaw occurred at 20 mg bid and was attributed to bisphosphonate use. At 10 mg and 15 mg bid doses of ruxolitinib, there were no dose modifications for the first 4 cycles of ruxolitinib given with concurrent weekly paclitaxel. In the cohort treated with 20 mg bid ruxolitinib, 43% patients received full-dose ruxolitinib during 4 cycles. One patient in each cohort at 10, 15 and 20 mg bid doses had one dose reduction of paclitaxel due to grade 2 neuropathy (cycle 11), grade 3 anemia (cycle 5) and grade 3 hyponatremia (cycle 4), respectively. There were two paclitaxel dose reductions at the 25 mg dose level, one due to infection associated with neutropenia and another due to neutropenia. The RP2D for ruxolitinib in combination with full-dose weekly paclitaxel was determined to be 15 mg bid to allow for maximal consistent dosing of paclitaxel and ruxolitinib during 4 cycles (12 weeks) of combination therapy.
The median number of cycles of combination ruxolitinib and weekly paclitaxel received was 5 (range 1–12) (Table 3). Ten (53%) patients discontinued study treatment due to disease progression. Six patients (32%) discontinued study treatment within 4 cycles due to adverse events: one patient experienced grade 3 anemia, three patients reported grade 1–3 fatigue, one patient experienced grade 2 neutropenia and one patient discontinued treatment due to developing osteonecrosis of the jaw that met crtieria for a DLT. Five patients continued single-agent ruxolitinib after completing 4 cycles of combination therapy, for an additional cumulative total of single-agent ruxolitinib equaling 38 cycles. Two patients received one additional cycle (corresponding to a total of 5 cycles of ruxolitinib), one patient received three, one patient received four and one patient with triple-negative IBC completed an additional 29 cycles of single-agent ruxolitinib before discontinuation due to disease progression.
Safety
There were no grade 5 adverse events observed. There was one grade 4 adverse event (neutropenia) and one grade 3 DLT (osteonecrosis of the jaw) not attributed to study treatment. Table 4 lists the worst toxicities described by CTCAE v4.0, regardless of the attribution. The most common adverse events were neutropenia and anemia with eight patients experiencing grade 3/4 neutropenia and five patients experiencing grade 3/4 anemia (Table 4). The most frequent non-hematologic toxicities reported and attributed to ruxolitinib were edema (n = 4), transaminitis (n = 2), dyspnea (n = 1) and dyspepsia (n = 1), all being grade 1–2. Of the cumulative total of 111 cycles of combination ruxolitinib and paclitaxel received, 32 (29%) cycles had ruxolitinib doses withheld [21 (19%)] or reduced [11 (10%)]. Five (26%) patients discontinued paclitaxel due to toxicity, with anemia, neutropenia, fatigue, or neuropathy being the reasons reported.
Efficacy
Measurable disease was present in 15 of 19 patients, 4 (21% overall; 27% of those having measurable disease) patients had partial disease responses (PR) and 12 (63%) had stable disease (SD). Disease progression as best response was noted in three patients (16%). Patients who achieved a PR received a median of 8.5 cycles of combination therapy. The overall median time to tumor progression (TTP) was 25 weeks. Of the five patients who continued single-agent ruxolitinib following four cycles of combination therapy, four discontinued treatment due to progression of disease following a median duration of 3.5 cycles of ruxolitinib. Only one patient discontinued single-agent ruxolitinib because of toxicity. This occurred after one cycle and was due to the development of grade 1 edema.
Discussion
In this phase I trial, the combination of ruxolitinib and weekly paclitaxel was found to be tolerable, with a RP2D of ruxolitinib of 15 mg bid identified as the dose that allowed for maximal consistent dosing of paclitaxel and ruxolitinib during 4 cycles (12 weeks) of combination therapy. The most frequently observed adverse events were hematologic (neutropenia and anemia) which is consistent with the prescribing information for ruxolitinib, although can also be associated with paclitaxel [14]. No new major toxicity signals were identified. Within the limits of this phase I study, the median TTP was 25 weeks, with four (21%) patients achieving PR and 12 (63%) achieving SD. The majority of patients in this study group had received prior systemic treatment and 63% of patients had known visceral disease at time of enrollment.
Interestingly, one patient with triple-negative inflammatory breast cancer remained on study therapy for 40 cycles (11 combination, 29 ruxolitinib alone) before disease progression. Inflammatory breast cancer is an aggressive form of locally invasive breast cancer that accounts for 2–5% of all invasive breast cancer [15, 16]. Overall, 55–85% of patients with IBC present with local metastases to axillary and/or supraclavicular lymph nodes and 20–40% present with distant metastatic disease [17]. The JAK2–STAT3 signaling pathway has been identified as a key driver of CD44+CD24− “stem-cell-like” breast cancer cells, a hallmark of IBC, therefore supporting the investigation of JAK2 inhibition in the treatment of IBC [2].
The extent of locoregional disease in IBC necessitates the use of preoperative chemotherapy. However, optimal neoadjuvant chemotherapy regimens have yet to be determined. Inferior survival rates demand ongoing investigation into novel treatment regimens. Based on the safety results of this study, a phase II randomized trial of combination ruxolitinib and paclitaxel for the treatment of triple-negative IBC (NCT02876302) conducted by the TBCRC was initiated and is ongoing. Our hypothesis is that the addition of ruxolitinib to standard chemotherapy will target the JAK/STAT3 pathway, inhibit its activation leading to decrease in survival/activation of CD44+CD24− cells, and result in a decrease in the subsequent development of metastasis. The primary endpoint of this phase II trial is a biologic response, meaning whether the tumor demonstrates a reduction in pSTAT3 activity defined as a change in pStat3 scoring between baseline samples and post-ruxolitinib biopsy samples.
In summary, ruxolitinib in combination with standard dose weekly paclitaxel was well tolerated and warrants further investigation in the treatment of breast cancer, particularly in inflammatory breast cancer.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Marotta LL, Almendro V, Marusyk A, Shipitsin M, Schemme J, Walker SR, Bloushtain-Qimron N, Kim JJ, Choudhury SA, Maruyama R, Wu Z, Gonen M, Mulvey LA, Bessarabova MO, Huh SJ, Silver SJ, Kim SY, Park SY, Lee HE, Anderson KS, Richardson AL, Nikolskaya T, Nikolsky Y, Liu XS, Root DE, Hahn WC, Frank DA, Polyak K (2011) The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(−) stem cell-like breast cancer cells in human tumors. J Clin Invest 121(7):2723–2735. https://doi.org/10.1172/JCI44745
Xiao Y, Ye Y, Yearsley K, Jones S, Barsky SH (2008) The lymphovascular embolus of inflammatory breast cancer expresses a stem cell-like phenotype. Am J Pathol 173(2):561–574. https://doi.org/10.2353/ajpath.2008.071214
Overmoyer BA, Almendro V, Shuh S, Peluffo G, Park SY, Nakhlis F, Bellon J, Yeh E, Hirshfield-Bartek J, Jacene HA, Polyak K (2012) JAK2/STAT3 activity in inflammatory breast cancer supports the investigation of JAK2 therapeutic targeting. Cancer Res 72(3):24 (P4-06-01)
Stover DG, Alcazar CRGD, Brock J, Guo H, Overmoyer B, Balko J, Xu Q, Bardia A, Tolaney SM, Gelman R, Lloyd M, Wang Y, Xu Y, Michor F, Wang V, Winer EP, Polyak K, Lin NU (2018) Phase II study of ruxolitinib, a selective JAK1/2 inhibitor, in patients with metastatic triple-negative breast cancer. NPJ Breast Cancer 4(1):10. https://doi.org/10.1038/s41523-018-0060-z
Bauer TM, Patel MR, Forero-Torres A, George TJ Jr, Assad A, Du Y, Hurwitz H (2018) A phase Ib study of ruxolitinib + gemcitabine +/− nab-paclitaxel in patients with advanced solid tumors. Onco Targets Ther 11:2399–2407. https://doi.org/10.2147/OTT.S157331
Hurwitz HI, Uppal N, Wagner SA, Bendell JC, Beck JT, Wade SM, Nemunaitis JJ, Stella PJ, Pipas JM, Wainberg ZA, Manges R, Garrett WM, Hunter DS, Clark J, Leopold L, Sandor V, Levy RS (2015) Randomized, double-blind, phase II study of ruxolitinib or placebo in combination with capecitabine in patients with metastatic pancreatic cancer for whom therapy with gemcitabine has failed. J Clin Oncol 33(34):4039–4047. https://doi.org/10.1200/JCO.2015.61.4578
Harrison C, Kiladjian J-J, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, McQuitty M, Hunter DS, Levy R, Knoops L, Cervantes F, Vannucchi AM, Barbui T, Barosi G (2012) JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 366(9):787–798. https://doi.org/10.1056/NEJMoa1110556
Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, Catalano JV, Deininger M, Miller C, Silver RT, Talpaz M, Winton EF, Harvey JH, Arcasoy MO, Hexner E, Lyons RM, Paquette R, Raza A, Vaddi K, Erickson-Viitanen S, Koumenis IL, Sun W, Sandor V, Kantarjian HM (2012) A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 366(9):799–807. https://doi.org/10.1056/NEJMoa1110557
Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, Szer J, Wagner EM, Zuckerman T, Mahuzier B, Xu J, Wilke C, Gandhi KK, Socie G, REACH2 Trial Group (2020) Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med 382(19):1800–1810. https://doi.org/10.1056/NEJMoa1917635
Han ES, Wen W, Dellinger TH, Wu J, Lu SA, Jove R, Yim JH (2018) Ruxolitinib synergistically enhances the anti-tumor activity of paclitaxel in human ovarian cancer. Oncotarget 9(36):24304–24319. https://doi.org/10.18632/oncotarget.24368
Deisseroth A, Kaminskas E, Grillo J, Chen W, Saber H, Lu HL, Rothmann MD, Brar S, Wang J, Garnett C, Bullock J, Burke LB, Rahman A, Sridhara R, Farrell A, Pazdur R (2012) U.S. food and drug administration approval: ruxolitinib for the treatment of patients with intermediate and high-risk myelofibrosis. Clin Cancer Res 18(12):3212–3217. https://doi.org/10.1158/1078-0432.CCR-12-0653
Taxol (paclitaxel) (prescribing information). Princeton, NJ: Bristol-Myers Squibb Company; April 2011. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020262s051lbl.pdf Accessed 11 Feb 2021
Paller CJ, Bradbury PA, Ivy SP, Seymour L, LoRusso PM, Baker L, Rubinstein L, Huang E, Collyar D, Groshen S, Reeves S, Ellis LM, Sargent DJ, Rosner GL, LeBlanc ML, Ratain MJ (2014) Design of phase I combination trials: recommendations of the clinical trial design task force of the NCI investigational drug steering committee. Clin Cancer Res 20(16):4210–4217. https://doi.org/10.1158/1078-0432.CCR-14-0521
Incyte Corporation (2019) JAKAFI prescribing information. Wilmington, DE, USA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/202192s017lbl.pdf Accessed 11 February 2021
Hance KW, Anderson WF, Devesa SS, Young HA, Levine PH (2005) Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. J Natl Cancer Inst 97(13):966–975. https://doi.org/10.1093/jnci/dji172
Anderson WF, Chu KC, Chang S (2003) Inflammatory breast carcinoma and noninflammatory locally advanced breast carcinoma: distinct clinicopathologic entities? J Clin Oncol 21(12):2254–2259. https://doi.org/10.1200/JCO.2003.07.082
Walshe JM, Swain SM (2005) Clinical aspects of inflammatory breast cancer. Breast Dis 22:35–44
Funding
Incyte Corporation, Alapocas, Delaware.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
FL has received research grants from Pfizer, Immunomedics, Regeneron, Chugai, Tesaro, Calithera, Inivata and BMS and has participated on advisory boards from Pfizer (remunerated) and BMS, Astra Zeneca and Jounce (non remunerated). BO has received clinical trial support from Incyte, Eisai. SMT receives institutional research funding from AstraZeneca, Lilly, Merck, Nektar, Novartis, Pfizer, Genentech/Roche, Immunomedics, Exelixis, Bristol-Myers Squibb, Eisai, Nanostring, Cyclacel, Odonate, and Seattle Genetics; has served as an advisor/consultant to AstraZeneca, Lilly, Merck, Nektar, Novartis, Pfizer, Genentech/Roche, Immunomedics, Bristol-Myers Squibb, Eisai, Nanostring, Puma, Sanofi, Celldex, Paxman, Puma, Silverback Therapeutics, G1 Therapeutics, AbbVie, Anthenex, OncoPep, Outcomes4Me, Kyowa Kirin Pharmaceuticals, Daiichi-Sankyo, and Samsung Bioepsis Inc. RAF receives institutional funding from Eisai and Puma Biotechnology. ELM has served as a consultant/advisor to Novartis, Lilly, Sanofi, and Eisai. MMR reports research funding from Novartis, Pfizer, Ipsen, TerSera, Merck, Pierre Fabre, Roche, AstraZeneca, Bristol Myers Squibb, Bayer, Veridex; and consulting or advisory role for Ipsen, Bristol-Myers Squibb, Tolmar Pharmaceuticals.
Ethics approval
This study was approved by the Dana-Farber/Harvard Cancer Center Institutional Review Board (DFCI#13–494; NCT02041429).
Consent to participate
Informed consent was obtained from all subjects.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior presentation: San Antonio Breast Cancer Symposium 2016 (Overmoyer B, Regan M et al. Abstract P6-12-12: Phase I study of the JAK1/2 inhibitor ruxolitinib with weekly paclitaxel for the treatment of HER2 negative metastatic breast cancer (MBC). Cancer Res 2017; 77(4 Supplement): P6-12-12.
Rights and permissions
About this article
Cite this article
Lynce, F., Williams, J.T., Regan, M.M. et al. Phase I study of JAK1/2 inhibitor ruxolitinib with weekly paclitaxel for the treatment of HER2-negative metastatic breast cancer. Cancer Chemother Pharmacol 87, 673–679 (2021). https://doi.org/10.1007/s00280-021-04245-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-021-04245-x