Abstract
Aberrant activation of eIF4E signalling pathway is common in breast cancer and holds potential therapeutic options. In our work, galeterone as a chemical compound under clinical trials for the treatment of prostate cancer, was identified to be effective in targeting breast cancer cells via suppressing MNK-eIF4E and β-catenin. In despite of varying IC50, galeterone at nanomolar concentrations significantly decreased viability, proliferation and migration of a panel of breast cancer cell lines regardless of clinical subtypes and genetic mutations, and to a higher extent than in normal breast cells. Galeterone significantly enhanced the effects of chemotherapeutic drugs in reducing proliferation and viability but not migration. The in vivo efficacy of galeterone as single drug alone and its ability in augmenting chemotherapy’s efficacy were also shown in breast cancer xenograft mouse model. Mechanism analysis demonstrated that galeterone decreased MNK1/2 level and phosphorylation of eIF4E. In addition, galeterone decreased β-catenin level via promoting GSK-3β-mediated β-catenin degradation, and furthermore that Akt but not CK1 was involved in β-catenin degradation by galeterone. Rescue studies demonstrated that both MNK/eIF4E and β-catenin were responsible for anti-breast cancer activity of galeterone. Our study provides pre-clinical evidence to initialize clinical trials for breast cancer using galeterone in combination with chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most prevalent cancer affecting women and a leading cause of cancer-mortality globally [1]. Heterogeneity in breast cancer is extensive and mainly divided into four clinical subtypes based on cell surface receptors: estrogen receptor (ER)-positive (luminal), normal-like, epidermal growth factor receptor 2-(HER2-) enriched and basal like [2]. Triple negative breast cancer (TNBC) genotype lacks of HER2, ER and progesterone receptor (PR) expressions, and is the most aggressive subtype with limited treatment option [3, 4]. Although advances in cancer genomics provide tremendous insights into the genetic basis of breast cancer, the development of novel pharmacological therapies has not proceeded as rapidly. Novel therapeutic strategies are required to cater for better breast cancer clinical management.
Eukaryotic translation initiation factor 4E (eIF4E) is necessary for the translation of cap-dependent mRNA and preferentially enhances carcinogenesis associated mRNAs’ translation [5]. eIF4E dysregulation contributes to aberrant proliferation, survival, metastasis and resistance to therapy in cancer [6, 7]. MAPK-interacting kinases (Mnk) regulate eIF4E phosphorylation and activation via binding to phosphorylated eIF4E at Ser209 [8, 9]. Of note, MNK kinases are needed for eIF4E phosphorylation at Ser209 in cancerous but not normal cells [10]. Studies have demonstrated that inhibiting MNK-eIF4E axis is effective against aggressive breast cancer cells and increases their responsiveness to chemotherapy [11,12,13]. β-catenin activation is often the downstream consequence of MNK-eIF4E in cancer cells to promote growth, survival and chemoresistance [11, 14]. Aberrant activation of Wnt/β-catenin is associated with tumor progression and poor prognosis in patients with breast cancer and Wnt/β-catenin signaling pathway is a promising chemotherapeutic target [15]. Galeterone, formerly named TOK-001 or VN/124-1, is a steroid antiandrogen that has been under development for prostate cancer treatment due to its dual action as an androgen receptor (AR) antagonist and a CYP17A1 inhibitor [16]. Interestingly, recent studies reveal that galeterone and its analogs also inhibit MNK-eIF4E axis in prostate and pancreatic cancer cells [17, 18]. We hypothesized that galeterone might be a potent drug candidate for breast cancer. To address this hypothesis, we examined the efficacy of galeterone alone and its combination with chemotherapy drugs in breast cancer models. We also attempted to identify the mechanism of action of galeterone focusing on MNK-eIF4E and β-catenin signaling pathways.
Materials and methods
Cell culture procedures, generation of cell lines and drugs
For this study, cell lines related to human breast cancer origin were sourced from the Cell Bank of the Chinese Scientific Academy. Cell lines were authenticated using the human 9-Marker STR DNA profile analysis. These cell lines were cultured in DMEM media, with 10% fetal bovine serum (FBS, Hyclone) and 1% penicillin/streptomycin supplemented. They were kept at 37 °C, 5% CO2 atmosphere. Cell lines stably overexpressing the serine-to-alanine (S209A) and the serine-to-aspartic acid (S209D) of eIF4E were established by retroviral transduction in the presence of 6 µg/mL polybrene (Sigma) and viral particles were generated as previously described [19]. These mutants were cloned into the murine stem cell virus (MSCV)-internal ribosome entry site (IRES)-GPF construct. To select for stable clones, 1 µg/mL puromycin were added to the growth media. Paclitaxel, doxorubicin, galeterone and cisplatin were obtained from Selleck. Lithium chloride (LiCl) was obtained from Sigma.
Cell proliferation and viability processes and measurements
10,000 cells were seeded into 96-well-plate. After 24-h drug treatment, cell proliferative activities were measured using BrdU Cell Proliferation Assay Kit (Abcam). Combination studies were designed using Chou and Talalay’s method [20]. The concentrations of single drug required to produce 50% proliferation inhibition (IC50) were first established. The cells were then treated with an equipotent constant-ratio drug combination. Combination index (CI) was calculated using Calcusyn software. If CI is more than 1, the combination is antagonistic; if CI is equal to 1, the combination is additive; if CI is less than 1, the combination is synergistic. After 72-h drug treatment, viability was determined using CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega).
Boyden chamber migration assay
Cell migration assay was performed with 8 μm Falcon cell culture inserts (Cell Biolabs) according to manufacture’s instructions with modification. Briefly, 10,000 cells together with drugs were plated onto an insert in the upper chamber. DMEM containing 2%FBS and 10% FBS was placed into the upper and lower chamber, respectively. After 8-h incubation, cells on the upper layer of each insert was removed using a cotton swab. The migratory cells on the lower surface were then fixed and stained with Giemsa (Sigma). The quantity of cells that had migrated through the layer were quantified using light microscopy.
Western blot
After 24-h drug treatment, whole cells were lysed using RIPA buffer for total protein extraction. Cytoplasmic/nuclear fractionation was isolated using the protocol described previously [21]. Equal amount of proteins was loaded onto SDS-PAGE gel and separated by electrophoresis. Proteins were transferred to PVDF membrane and detected by western blot according to standard protocol. Antibodies were purchased from Cell Signaling.
TOPflash report assay
Cells were transfected with M50 Super 8 × TOPFlash plasmid (Addgene) or β-gal using Dharmafect Transfection Reagent. Transfected cells were treated with drugs for 24 h. β-catenin activity using luciferase assay (Promega) or the β-gal Enzyme Assay System were assessed according to manufacturer’s instructions. β-catenin activity was determined after normalization of TOPflash with β-gal activity.
Real time PCR conditions
Extraction of total RNAs was done using TRIzol Reagent (Ambion). cDNA was prepared using Superscript III First-Strand Synthesis System (Invitrogen). Real-time PCR was performed using the iQ5 Multicolor Real-Time Detection System (Bio-Rad). The sequences of the primers were LEF1 (F: TGA GTG CAC GCT AAA GGA GA and R: ATA ATT GTC TCG CGC TGA CC); Cyclin D (F: ATG GCC AGC GGG AAG AC and CCG TCC ATG CGG AAG ATC); c-Myc (F: 5′-AAT GAA AAG GCC CCC AAG GTA GTT ATC C-3′and R: 5′-GTC GTT TCC GCA ACA AGT CCT CTT C-3′) and Axin2 (F: 5′-TTA TGC TTT GCA CTA CGT CCC TCC A-3′and R: 5′-CGC AAC ATG GTC AAC CCT CAG AC-3′). The relative mRNA expression levels of targeted genes were normalized with β-actin.
Breast cancer xenograft in SCID mouse
All animal related work was carried out in compliance with the institutional guidelines and procedures approved by the Animal Care and Use Committee of Wuhan University. Male 4–6 weeks old severe combined immunodeficiency mice (SCID; Hunan SJA Animal Laboratory Co. Ltd) were subcutaneously inoculated with 100 μL of 107 MDA-MB-231 cell suspension and housed in a pathogen-free environment. Tumour size was estimated by the formula length × width2/2. When tumors reached approximately 200 mm3, the mice were randomly divided into different groups: vehicle (80%/20%, saline/DMSO), single drug alone or combination. Mice were weighed and monitored for signs (e.g., fur and abnormal behaviour) of possible toxicity due to treatment. After 1-month drug treatment, mice were euthanized using CO2.
Statistical analyses
Data presented in the study are expressed as mean and standard deviation (SD), unless defined otherwise. Statistical analyses comparing two categorical variables were performed using the unpaired Student’s t test. P-value < 0.05 was considered to be statistically significant.
Results
Galeterone is active against breast cancer cells
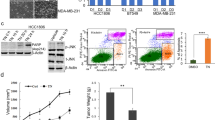
We firstly selected a panel of different cell lines that display breast cancer heterogeneity. Cells lines were selected with different histopathological characteristics and differ in molecular profiles. Based on their gene expression profiles, MCF-7, MDA-MB-231, SkBr-3, MDA-MB-468 falls into the four distinct subtypes: luminal, claudin-low, human epithelial growth factor receptor 2 (HER2) enriched and basal-like [22]. Based on the clinical subtypes, MDA-MB-231, MDA-MB-68, Hs 578T and BT-549 are triple negative and SkBr-3 is HER-positive [23]. We examined the effectiveness of galeterone as single drug alone on viability, growth and migration of these breast cancer cell lines. We also used normal breast cells as normal control. Exposure to galeterone at 0.25 to 16 μM dose-dependently decreased viability of six breast cancer cell lines (Fig. 1a). Galeterone inhibited proliferation in these cells as assessed by labelling BrdU (a proliferative marker), with IC50 from 0.5 to 4 μM (Fig. 1b and Supplementary Table 1). We found that galeterone also significantly inhibited proliferation of normal breast cells, but to a lesser extent than in breast cancer cells (Supplementary Fig. 1 and Supplementary Table 1). The IC50 of galeterone on normal breast cells are 9.5 μM and 15.7 μM, which is > tenfold higher than the IC50 of the most sensitive breast cancer cell lines tested in our study. In addition, galeterone inhibited breast cancer cell migration with IC50 > 4 μM (Fig. 1c, d). The results demonstrated that (1) galeterone at nanomolar concentration is active against breast cancer cells regardless of subtypes and genetic profiles; (2) IC50 of galeterone varies among breast cancer cell lines, with MDA-MB-231 being most sensitive and MCF-7 most resistant; (3) galeterone has preferential anti-proliferative activity to breast cancer cells compared to normal counterparts; (4) galeterone is more effective in inhibiting growth and survival than cell migration.
Galeterone is effective against breast cancer cells. Galeterone dose-dependently decreases viability (a) and proliferation (b) of a panel of breast cancer cell lines. c Representative migration image of breast cancer cells exposed to control and galeterone. d Galeterone inhibits migration of multiple breast cancer cell lines. Migrated cells in five different random fields per well were counted
The combination of galeterone and chemotherapeutic drugs achieves greater efficacy than drug alone in breast cancer cells
As a drug candidate for breast cancer treatment, it was important to address the issue of whether the combination of galeterone with chemotherapeutic agents can achieve greater efficacy than single drug alone. We tested three commonly used chemotherapy drugs on three breast cancer cell lines including the most sensitive and the most resistant to galeterone and used two approaches to address this question. One approach is to design the combination study using drugs at concentration that result in < 50% inhibition in breast cancer cells as single drug alone in order to achieve the liner range of combinatory effects. We found that cisplatin, paclitaxel or doxorubicin alone led to 30–40% viability reduction and growth (Fig. 2a–f and Supplementary Fig. 2). The combination of galeterone with cisplatin, paclitaxel or doxorubicin led to 70–90% inhibition on growth and viability, demonstrating that anti-proliferative and pro-apoptotic effects were greater when two drugs were added. Interestingly, the combination was not significantly better than the single drug alone in inhibiting migration (Fig. 3g–i). Another approach is to use the Chou-Talalay method based on an equipotent constant-ratio combination of both drugs [20]. The combination index (CI) of galeterone with cisplatin, paclitaxel or doxorubicin were less than 1 in all three breast cancer cell lines (Supplementary Table 2), indicating that the combination of galeterone and chemotherapy is synergistic in inhibiting proliferation.
Galeterone augments chemotherapy efficacy in breast cancer cells. Galeterone significantly augments the anti-proliferative (a–c) and anti-survival (d–f) effects of cisplatin and paclitaxel in cells. g–i Galeterone does not affect the anti-migratory effect of cisplatin and paclitaxel in breast cancer cells. Galeterone at 0.5, 2 and 8 μM was used in MDA-MB-231, Hs 578 T and MCF-7, respectively. Paclitaxel at 20 nM and cisplatin at 60 nM were used in combination studies. *p < 0.05, compared to paclitaxel or cisplatin alone
Galeterone inhibits MNK/eIF4E/β-catenin in breast cancer cells. a Galeterone decreases the level of MNK1, MNK2, p-eIF4E, p-Akt, p-β-catenin and β-catenin in MDA-MB-231 cells. b Galeterone decreases cytoplasmic and nuclear β-catenin levels. Purify of nuclear and cytoplasmic preparations was evaluated by WB for acetylated histone H3 (Ac-H3) and actin, respectively. Galeterone significantly decreases β-catenin activity (c), mRNA (d) and protein (e) levels of Axin2, c-Myc, LEF1 and Cyclin D in breast cancer cells. *p < 0.05, compared to control
Galeterone acts on breast cancer cells in an eIF4E and β-catenin-dependent manner.
Studies highlighted that the anti-cancer activity of galeterone is attributed to its ability in inhibiting MNK-eIF4E axis [17, 18]. Given the importance of eIF4E in breast cancer, we examined whether MNK-eIF4E inhibition is the mechanism of galeterone’s action in breast cancer. In agreement with previous findings, we observed decreased levels of MNK1, MNK2 and p-eIF4E but not total eIF4E in breast cancer cells exposed to galeterone (Fig. 3a). In addition, we observed decreased level of β-catenin in breast cancer cells after galeterone treatment (Fig. 3a). We then examined the phosphorylation of β-catenin at S33/37/T41which indicate GSK-3β phosphorylation sites for subsequent degradation; at S45 which indicates casein kinase 1(CK1) phosphorylation site for subsequent phosphorylation by GSK-3β; and at S552 which indicates nuclear localization. We found that galeterone increased p-β-catenin (S33/37/T41) and decreased p-β-catenin (S552) without affecting p-β-catenin (S45) (Fig. 3a), suggesting that galeterone promotes GSK-3β-mediated β-catenin degradation and decreases β-catenin accumulation in the nucleus in breast cancer cells. Furthermore galeterone-induced β-catenin degradation via GSK-3β is not associated with CK1 activation. It is well known that Akt phosphorylates β-catenin at Ser552 and increases its transcriptional activity. Consistent with the decreased p-β-catenin (S552), we observed the decreased p-Akt (S473) after galeterone treatment. We further performed WB using lysates isolated from the cytoplasm and nucleus to determine the nuclear/cytoplasmic distribution of β-catenin. As expected, galeterone decreased both cytoplasmic and nuclear β-catenin (Fig. 3b). Consistent with the decreased β-catenin, galeterone significantly decreased β-catenin activity as assessed by a β-catenin reporter assay (Fig. 3c) and reduced transcription of multiple β-catenin-targeted genes, including c-Myc, Axin2, Cyclin D and LEF1 (Fig. 3d). As expected, protein levels of c-Myc, Axin2, Cyclin D and LEF1 were decreased in breast cancer cells after galeterone treatment (Fig. 3e).
To further ascertain that eIF4E-β-catenin is the target of galeterone in breast cancer, we used retroviral transduction to generate MDA-MB-231 cells overexpressing the phosphomimetic form (S209) and the nonphosphorylatable form (S209A) of eIF4E. As shown in Fig. 4a, overexpression of eIF4E S209D but not S209A reversed the inhibitory effect of galeterone on β-catenin activity, demonstrating that β-catenin inhibition is the consequence of eIF4E inhibition by galeterone. In addition, overexpression of eIF4E S209D but not S209A reversed the inhibitory effect of galeterone on growth and viability (Fig. 4b, c). Our results indicate that galeterone inhibits MNK-eIF4E-β-catenin axis, and furthermore that this is dependent on eIF4E phosphorylation at S209.
Overexpression of eIF4E and stabilization of β-catenin reverses galeterone’s anti-breast cancer activities. Overexpression of eIF4E(S209D) but not eIF4E(S209A) significantly reverses the inhibitory effects of galeterone on β-catenin activity (a), growth (b) and survival (c) in MDA-MB-231 cells. d Galeterone reversed decreased β-catenin and c-Myc level but not p-eIF4E in cells in the presence of LiCl (50 μM). Galeterone is less effective in inhibiting proliferation (e) and survival (f) in cells in the presence of LiCl (50 μM) *p < 0.05, compared to control or Vector
To understand whether the β-catenin pathway contributes to the antitumor activity of galeterone, we performed rescue studies using lithium chloride (LiCl, Wnt/β-catenin activator via inhibiting β-catenin degradation) [24]. We demonstrated that LiCl stabilized β-catenin level even in the presence of galeterone (Fig. 4d). In the cells treated with LiCl, galeterone was less effective in decreasing c-Myc level, inhibiting proliferation and survival (Fig. 4d–f). In contrast, LiCl did not affect the inhibitory effect of galeterone on p-eIF4E. This indicates that β-catenin pathway is the downstream of eIF4E and contributes to the antitumor activity of galeterone in breast cancer.
Galeterone inhibits breast cancer growth and augments efficacy of chemotherapy in vivo
We finally investigated in vivo efficacy of galeterone alone and its combination with chemotherapy drugs. We generated breast cancer xenograft mouse model by subcutaneously injecting MDA-MB-231 cells to mice flank. After development of palpable tumor, we started drug treatment and monitored tumor size and signs of possible toxicity due to treatment. We did not observe significant weight loss and abnormality in appearance or behavior in mice, suggesting that the treatment given to mice are not toxic. Notably, oral galeterone at 150 mg/kg significantly inhibited breast cancer growth in vivo (Fig. 5a). The combination of galeterone with cisplatin was much more effective in inhibiting breast cancer growth than cisplatin alone (Fig. 5b). The greater efficacy was also observed when galeterone and paclitaxel were combined.
Galeterone significantly augments chemotherapy efficacy in breast cancer growth mice. a Galeterone at 150 mg/kg but not 100 mg/kg significantly inhibits MDA-MB-231 cell growth in vivo. Galeterone was administrated by oral gavage once per day. b Combination of galeterone with cisplatin or paclitaxel is significantly more effective in inhibiting breast cancer growth in mice than cisplatin or paclitaxel alone. Mice bearing MDA-MB-231 xenografts were treated with vehicle, galeterone (Gal, 150 mg/kg per day, PO), cisplatin (Cis, 1 mg/kg once per week, i.p), paclitaxel (Pac, 1 mg/kg twice per week, i.p), combination of galeterone with cisplatin or paclitaxel. *p < 0.05, compared to vehicle. #p < 0.05, compared to cisplatin or paclitaxel
Discussion
Evidence on the biological effects of suppression of MNK-eIF4E has supported the notion that this signaling pathway is a prime candidate for cancer therapy development [25, 26]. Particularly, eIF4E is overexpressed in a high proportion of breast cancers and its phosphorylation is further increased in response to chemotherapy, thus making eIF4E as a sensitizing therapeutic target [11, 13]. Galeterone, 3ß-((hydroxy)-17-(1H-benzimidazole-1-yl) androstane-5,16-dione, attracted attention initially as a potent CYP17 (a key enzyme in steroid biosynthesis) inhibitor for prostate cancer treatment [27]. The targets of galeterone extend beyond CYP17, as studies have demonstrated that galeterone acts as an AR antagonist to both full length and mutant AR [28, 29]. It is now recognized that galeterone also potently inhibits MNK-eIF4E axis in various types of cancer cells [17, 18].These highlight target complexity of galeterone and the utility of galeterone as a means to treat cancer. Here, we are the first to report that galeterone is a promising drug candidate in combination with chemotherapeutic agents to improve clinical management of breast cancer.
The six cell lines we selected for the demonstration of the biological effects of galeterone model breast cancer with different clinical subtypes and molecular background. As expected, treatment of breast cancer cells with nanomolar concentrations of galeterone inhibits cell proliferation, viability and migration, regardless of their heterogeneity in molecular and cellular features (Fig. 1). Most breast cancer cells express AR, but the clinical value of AR expression is inconclusive. Although AR expression is often related to low malignancy in breast cancer, many findings suggest that AR contributes to therapy-resistance [30]. Although galeterone is known as an AR inhibitor, galeterone displays strong antiproliferative activities against AR-negative prostate cancer cells [17]. This is supported by our observation that MCF-7 which is AR positive is more resistant than MDA-MB-231 which is AR negative to galeterone treatment (Fig. 1 and Supplementary Table 1). This suggests that galeterone acts on breast cancer cells via AR-independent manner. The preferential toxicity of galeterone to breast cancer cells while sparing normal breast cells suggests the therapeutic window of galeterone. This is also supported by our finding that galeterone significantly inhibits breast cancer growth without causing toxicity in mice (Fig. 5a).
A subject of major interest in cancer drug development is combination therapy which could produce higher rates of treatment response and allow lower doses of each drug to reduce toxicity. Galeterone has been shown to synergize with gemcitabine in pancreatic cancer [18]. Our findings show that the combination of galeterone and chemotherapy drugs results in greater efficacy than chemotherapy alone in breast cancer cells (Fig. 2a–f and Supplementary Table 1). Of note, we demonstrated the same in breast cancer xenograft mouse model at dose of each drug that is not toxic to mice (Fig. 5).
Our mechanism analysis indicates that (1) both MNK/eIF4E and β-catenin are responsible for anti-breast cancer activity of galeterone; (2) MNK/eIF4E might not regulate β-catenin directly but via other factors that galeterone interacts with; (3) β-catenin is the downstream of eIF4E; (4) galeterone decreases β-catenin level via promoting GSK-3β-mediated β-catenin degradation, and furthermore that Akt but not CK1 was involved in β-catenin degradation by galeterone. These are supported by the previous findings on the inhibitory effects of galeterone on MNK-eIF4E axis in prostate and pancreatic cancer [17, 18]. In addition, hyperactive MNK1 phosphorylation of eIF4E confers tamoxifen resistance in breast cancer [31]. Although eIF4E (S209D) overexpression significantly reversed the inhibitory effect of galeterone on β-catenin activity, overexpression of eIF4E (S209D) hardly changed the β-catenin activity (Fig. 4a). We speculate that this might be because eIF4E is not the exclusive regulator of β-catenin activity in breast cancer and eIF4E overexpression alone is not sufficient to increase the basal level of β-catenin activity. As galeterone remarkably decreased β-catenin activity, eIF4E overexpression reversed the decreased β-catenin activity in the presence of galeterone. Another possibility might be that MNK/eIF4E does not regulate β-catenin directly. MNK/eIF4E might regulate β-catenin via other factors that galeterone interacts with. Our findings suggest that Akt downregulation might be the upstream regulator of reduced β-catenin level via GSK3β activation in galeterone-treated cells (Fig. 3a).
Our work demonstrates that galeterone inhibits MNK/eIF4E and β-catenin, and synergizes with chemotherapy drugs in breast cancer. The efficacy of galeterone and its combination with gemcitabine for patients with metastatic pancreatic cancer are currently under clinical trial (ClinicalTrials.gov Identifier: NCT 04098081). Our findings provide rational of testing galeterone in combination with chemotherapy drug for breast cancer under clinical settings.
References
Dubey AK, Gupta U, Jain S (2015) Breast cancer statistics and prediction methodology: a systematic review and analysis. Asian Pac J Cancer Prev 16(10):4237–4245
Ribnikar D, Ratosa I, Perhavec A, Amir E (2017) General overview and treatment recommendations for young women with breast cancer. Rev Invest Clin 69(2):77–93. https://doi.org/10.24875/ric.17002175
Foulkes WD, Smith IE, Reis-Filho JS (2010) Triple-negative breast cancer. N Engl J Med 363(20):1938–1948. https://doi.org/10.1056/NEJMra1001389
Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L (2016) Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 13(11):674–690. https://doi.org/10.1038/nrclinonc.2016.66
Larsson O, Li S, Issaenko OA, Avdulov S, Peterson M, Smith K, Bitterman PB, Polunovsky VA (2007) Eukaryotic translation initiation factor 4E induced progression of primary human mammary epithelial cells along the cancer pathway is associated with targeted translational deregulation of oncogenic drivers and inhibitors. Cancer Res 67(14):6814–6824. https://doi.org/10.1158/0008-5472.CAN-07-0752
Bhat M, Robichaud N, Hulea L, Sonenberg N, Pelletier J, Topisirovic I (2015) Targeting the translation machinery in cancer. Nat Rev Drug Discov 14(4):261–278. https://doi.org/10.1038/nrd4505
Mamane Y, Petroulakis E, Martineau Y, Sato TA, Larsson O, Rajasekhar VK, Sonenberg N (2007) Epigenetic activation of a subset of mRNAs by eIF4E explains its effects on cell proliferation. PLoS ONE 2(2):e242. https://doi.org/10.1371/journal.pone.0000242
Altman JK, Szilard A, Konicek BW, Iversen PW, Kroczynska B, Glaser H, Sassano A, Vakana E, Graff JR, Platanias LC (2013) Inhibition of Mnk kinase activity by cercosporamide and suppressive effects on acute myeloid leukemia precursors. Blood 121(18):3675–3681. https://doi.org/10.1182/blood-2013-01-477216
Hou J, Lam F, Proud C, Wang S (2012) Targeting Mnks for cancer therapy. Oncotarget 3(2):118–131. https://doi.org/10.18632/oncotarget.453
Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R (2004) Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol 24(15):6539–6549. https://doi.org/10.1128/MCB.24.15.6539-6549.2004
Li Z, Sun Y, Qu M, Wan H, Cai F, Zhang P (2017) Inhibiting the MNK-eIF4E-beta-catenin axis increases the responsiveness of aggressive breast cancer cells to chemotherapy. Oncotarget 8(2):2906–2915. https://doi.org/10.18632/oncotarget.13772
Ramalingam S, Gediya L, Kwegyir-Afful AK, Ramamurthy VP, Purushottamachar P, Mbatia H, Njar VC (2014) First MNKs degrading agents block phosphorylation of eIF4E, induce apoptosis, inhibit cell growth, migration and invasion in triple negative and Her2-overexpressing breast cancer cell lines. Oncotarget 5(2):530–543. https://doi.org/10.18632/oncotarget.1528
Wheater MJ, Johnson PW, Blaydes JP (2010) The role of MNK proteins and eIF4E phosphorylation in breast cancer cell proliferation and survival. Cancer Biol Ther 10(7):728–735. https://doi.org/10.4161/cbt.10.7.12965
Lim S, Saw TY, Zhang M, Janes MR, Nacro K, Hill J, Lim AQ, Chang CT, Fruman DA, Rizzieri DA, Tan SY, Fan H, Chuah CT, Ong ST (2013) Targeting of the MNK-eIF4E axis in blast crisis chronic myeloid leukemia inhibits leukemia stem cell function. Proc Natl Acad Sci USA 110(25):E2298-2307. https://doi.org/10.1073/pnas.1301838110
Mukherjee N, Panda CK (2020) Wnt/beta-catenin signaling pathway as chemotherapeutic target in breast cancer: an update on pros and cons. Clin Breast Cancer. https://doi.org/10.1016/j.clbc.2020.04.004
Bastos DA, Antonarakis ES (2016) Galeterone for the treatment of advanced prostate cancer: the evidence to date. Drug Des Dev Ther 10:2289–2297. https://doi.org/10.2147/DDDT.S93941
Kwegyir-Afful AK, Bruno RD, Purushottamachar P, Murigi FN, Njar VC (2016) Galeterone and VNPT55 disrupt Mnk-eIF4E to inhibit prostate cancer cell migration and invasion. FEBS J 283(21):3898–3918. https://doi.org/10.1111/febs.13895
Kwegyir-Afful AK, Murigi FN, Purushottamachar P, Ramamurthy VP, Martin MS, Njar VCO (2017) Galeterone and its analogs inhibit Mnk-eIF4E axis, synergize with gemcitabine, impede pancreatic cancer cell migration, invasion and proliferation and inhibit tumor growth in mice. Oncotarget 8(32):52381–52402. https://doi.org/10.18632/oncotarget.14154
Kharas MG, Deane JA, Wong S, O’Bosky KR, Rosenberg N, Witte ON, Fruman DA (2004) Phosphoinositide 3-kinase signaling is essential for ABL oncogene-mediated transformation of B-lineage cells. Blood 103(11):4268–4275. https://doi.org/10.1182/blood-2003-07-2193
Chou TC (2010) Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 70(2):440–446. https://doi.org/10.1158/0008-5472.CAN-09-1947
Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, Jernigan KK, Meyers KC, Hang BI, Waterson AG, Kim K, Melancon B, Ghidu VP, Sulikowski GA, LaFleur B, Salic A, Lee LA, Miller DM 3rd, Lee E (2010) Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nat Chem Biol 6(11):829–836. https://doi.org/10.1038/nchembio.453nchembio.453
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lonning PE, Borresen-Dale AL (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98(19):10869–10874. https://doi.org/10.1073/pnas.191367098
Dai X, Cheng H, Bai Z, Li J (2017) Breast cancer cell line classification and its relevance with breast tumor subtyping. J Cancer 8(16):3131–3141. https://doi.org/10.7150/jca.18457
Clement-Lacroix P, Ai M, Morvan F, Roman-Roman S, Vayssiere B, Belleville C, Estrera K, Warman ML, Baron R, Rawadi G (2005) Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci USA 102(48):17406–17411. https://doi.org/10.1073/pnas.0505259102
Grzmil M, Seebacher J, Hess D, Behe M, Schibli R, Moncayo G, Frank S, Hemmings BA (2016) Inhibition of MNK pathways enhances cancer cell response to chemotherapy with temozolomide and targeted radionuclide therapy. Cell Signal. https://doi.org/10.1016/j.cellsig.2016.06.005
Bramham CR, Jensen KB, Proud CG (2016) Tuning specific translation in cancer metastasis and synaptic memory: control at the MNK-eIF4E axis. Trends Biochem Sci 41(10):847–858. https://doi.org/10.1016/j.tibs.2016.07.008
Njar VC, Kato K, Nnane IP, Grigoryev DN, Long BJ, Brodie AM (1998) Novel 17-azolyl steroids, potent inhibitors of human cytochrome 17 alpha-hydroxylase-C17,20-lyase (P450(17) alpha): potential agents for the treatment of prostate cancer. J Med Chem 41(6):902–912. https://doi.org/10.1021/jm970568r
Soifer HS, Souleimanian N, Wu S, Voskresenskiy AM, Collak FK, Cinar B, Stein CA (2012) Direct regulation of androgen receptor activity by potent CYP17 inhibitors in prostate cancer cells. J Biol Chem 287(6):3777–3787. https://doi.org/10.1074/jbc.M111.261933
Yu Z, Cai C, Gao S, Simon NI, Shen HC, Balk SP (2014) Galeterone prevents androgen receptor binding to chromatin and enhances degradation of mutant androgen receptor. Clin Cancer Res 20(15):4075–4085. https://doi.org/10.1158/1078-0432.CCR-14-0292
Giovannelli P, Di Donato M, Galasso G, Di Zazzo E, Bilancio A, Migliaccio A (2018) The androgen receptor in breast cancer. Front Endocrinol 9:492. https://doi.org/10.3389/fendo.2018.00492
Geter PA, Ernlund AW, Bakogianni S, Alard A, Arju R, Giashuddin S, Gadi A, Bromberg J, Schneider RJ (2017) Hyperactive mTOR and MNK1 phosphorylation of eIF4E confer tamoxifen resistance and estrogen independence through selective mRNA translation reprogramming. Genes Dev 31(22):2235–2249. https://doi.org/10.1101/gad.305631.117
Acknowledgements
This work was supported by Teaching Reform Research Project of Medical Department of Wuhan University (Grant No. 2019037); the Guide Foundation of Renmin Hospital of Wuhan University (Grant No. RMYD2018M78); Fundamental Research Funds for the Central Universities of China (Grant No. 2042019kf0102) and National Natural Science Foundation of China (Grant No. 81700599).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, Y., Liao, S., Wang, L. et al. Galeterone sensitizes breast cancer to chemotherapy via targeting MNK/eIF4E and β-catenin. Cancer Chemother Pharmacol 87, 85–93 (2021). https://doi.org/10.1007/s00280-020-04195-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04195-w