Abstract
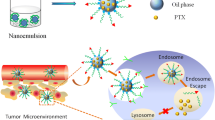
Lack of cancer-targeted delivery of chemotherapeutics is one of the major obstacles for successful cancer therapy. Nanostructured lipid carriers (NLC) have shown great promise in drug-delivery applications since they are highly scalable, biodegradable nanocarriers with high-drug-loading capacity. However, traditional method prepared NLC, the diameter of which range from 80 to 200 nm, is easily blocked and trapped in perivascular regions without further penetration. As a result, ultrasmall NLC with size under 100 nm or lower range are reported to be ideally tumor targeting carrier as it allows for superior tumor accumulation and permeation. Moreover, surface modification of NLC with folic acid (FA) could significantly increase the drug-delivery efficiency through active targeting effect. In our study, an ultrasmall NLC with FA modification (FA-NLC) was prepared to load docetaxel (DTX) for cancer therapy. Our results showed that DTX-loaded FA-NLC comprised of homogeneous particles with size around 30 nm. In addition, it exhibited great colloidal stability, satisfactory drug-loading efficiency, and high biocompatibility in vitro. Meanwhile, in vivo studies indicated that ultrasmall FA-NLC exhibited greater tumor retention and enhanced antitumor effect compared with control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer remains to be one of the most lethal diseases around the world, whereas the current cancer treatments are still far from perfection. Over the past decades, efforts have been made to develop a variety of tumor-targeted drug-delivery systems to overcome the serious side effects of chemotherapy [1,2,3,4]. Among them, nanocarriers have played irreplaceable role in obtaining efficient and safe therapeutic drug delivery [5, 6].
Solid lipid nanocarriers (SLN) are nanostructured drug carriers which comprise a variety physiological lipids, including fatty acids, steroids, etc. [7]. In the past two decades, SLN have been widely investigated as a preferable drug-delivery system due to their beneficial properties, such as ease of surface modifications and possibility for large-scale production [8, 9]. Nanostructured lipid carriers (NLC) are the second generation of SLN which basically comprised of solid lipid matrix with a certain content of liquid lipid [10]. Therefore, compared with SLN, NLC exhibit more crystal defects and display improved drug-loading capacity. Moreover, liquid lipid can also increase the flexibility of the prepared NLC which give raise to the possibility to generate smaller sized particles [11].
Tumor tissue has special physiological environments, including abundant vessels, broad interval of vessel walls, poor structure integrity, and lack of lymphatic drainage. The phenomenon that nanocarriers could target to the tumor tissue is commonly referred as enhanced permeability and retention effect [12, 13]. The in vivo performance of carriers, such as circulation time and biodistribution, are closely related with their size [14]. These parameters could be carefully adjusted by altering their particle size. Nanocarriers greater than 200 nm are preferably eliminated by the reticuloendothelial system (RES), while nanocarriers with size under 200 nm, especially those under 50 nm. Besides, cellular uptake efficiency is also highly dependent on the size [15]. For a given nanocarrier, the rate and doses of cellular uptake vary with its size. The small ones could be internalized much more efficiently by cells than the large counterparts [16]. Recently, more and more researches have demonstrated that ultrasmall particles with size under 50 nm or lower range can exhibit high tumor targeting as well as strong permeating ability for effective cancer therapy [17, 18].
Apart from size, drug-delivery efficiency of nanocarriers could be further facilitated by surface modification. It has been well recognized that surface modification of nanocarriers with polyethylene glycol (PEG) can greatly decrease their capture by RES [19]. Covalently modification with tumor-targeting ligands, such as folic acid (FA), could significantly improve the tumor-homing and tumor retention profiles of nanocarriers through binding with the over-expressed receptors on cancer cells [20, 21].
In our study, a commonly used anticancer drug, docetaxel (DTX), was employed to evaluate the drug-delivery efficiency of FA-modified NLC (FA-NLC). Firstly, DTX-loaded FA-NLC was prepared and its particle size, morphology as well as drug-loading capacity was investigated. Afterwards, the colloidal stability and hemolysis of FA-NLC were further studied. The in vitro intracellular uptake behavior was evaluated on HeLa cells which highly express folate receptor. Finally, the in vitro and in vivo antitumor efficiencies of DTX-loaded FA-NLC were studied in compare to commercially available formulation Taxotere. The results of above assays are encouraging, which establish theoretical and experimental basis for ultrasmall FA-NLC to be employed as a promising candidate in clinical cancer therapy.
Materials and methods
Materials
Glycerin monostearate (GM), Oleic acid (OA) and Docetaxel (DTX) were purchased from Sigma-Aldrich (St Louis, MO, USA). Folic acid (FA), methylthiazoletetrazolium (MTT) and coumarin 6 (C6) were offered by Thermo Fisher Scientific (Massachusetts, USA). Taxotere was obtained from Aventis Pharma (Frankfurt, German). Phosphatidylcholine (PC) was obtained from Lipoid GmbH (Ludwigshafen, German). Stearic acid–polyethylene glycol–folate (SA–PEG–FA) was purchased from Nanocs Inc. (New York, USA). Other chemicals involved in the study were analytically pure obtained from Shanghai Chemical Reagent Co. Ltd., (Shanghai, China) and used without further pretreatment.
Cell culture
Cervical cancer cells (HeLa) was cultured in folate-free DMEM medium (Sigma-Aldrich, USA) containing 10% fetal bovine serum (FBS), 100 U/mL of penicillin, and 100 μg/mL of streptomycin (Gibco, USA) at 37 °C using a humidified 5% CO2 incubator (311, Thermo scientific, USA).
Animal model
BALB/c nude mice and New Zealand rabbits were purchased from Shanghai Lab, Animal Research Center. All animal experiments were conducted in strict compliance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. In order to obtain HeLa tumor xenograft mice, approximately 1 × 106 of HeLa cells were incubated subcutaneously using 50% Matrigel (BD Biosciences) to the flank region of the mice. Tumor volume (V) was determined by measuring length (L) and width (W), and calculated as formula:\({\text{V}} = {\text{ L}} \times {\text{ W}}^{{2}} /{2}\).
Preparation and characterization of DTX-loaded FA-NLC
Glycerin monostearate (GM, 2 mg), phosphatidylcholine (PC, 3 mg), oleic acid (OA, 2 mg) and stearic acid–polyethylene glycol–folate (SA–PEG–FA, 3 mg) were added into 1 mL of ethanol and ultrasonicated (JY92-II, Ningbo Scientz Biotechnology Co., Ltd, China; 400 w, work 2 s and stand 3 s, 20 times) to obtain a transparent solution. To a preheated (70 °C) aqueous solution (10 mL), the mixture of lipid components was injected constantly (0.1 mL/s) via syringe under gentle agitation (500 rpm). The mixed preheated aqueous solution was also allowed to agitate for another 10 min after the addition of lipids. Afterwards, the solution was centrifuged at 20,000 rpm (Allegra 64R, Beckman Coulter, USA) for 10 min to remove the un-encapsulated DTX and the supernatant was collected and stored in 4 °C for further use. Preparation method of DiR-loaded nanoparticles is the same as that of DTX-loaded FA-NLC.
In order to quantify drug-loading efficiency, different weight ratios of drug to NLC, ranging from 0 to 20% were tested in order to optimize drug loading. Chemical drug DTX was loaded into NLC by physical encapsulation. Drug was dissolved with lipids in the organic phase, and proceeded as described above. After drug loading, the solution was centrifuged at 20,000 rpm (Allegra 64R, Beckman Coulter, USA) for 10 min. The supernatant was collected and freeze-dried to obtain white powder. The powder was then dissolved in acetonitrile with appropriate heating, filtered through 0.45 μm membrane (Millipore, USA), and then subjected to high-performance liquid chromatography (HPLC, 1100 series, Agilent, USA) analysis using a C18 analytical column (Agilent, USA) with a mobile phase of 50:50 water to acetonitrile, and a detection wavelength of 230 nm [17].
Size and zeta potential of the resulted nanoparticles were measured by dynamic light scattering (DLS) method using Nano S90 (Malvern Instruments Ltd., UK). Transmission electron microscopy (TEM, Hitachi 7700, Japan) was conducted to observe morphology of nanoparticles.
Drug release over time was investigated by dialyzing samples against phosphate buffer (PBS, pH 7.4). The amount of drug retained in the nanoparticle samples was assessed at 0, 1, 2, 4, 6, 12, 24, and 48 h. Samples were collected, lyophilized, and then analyzed by HPLC as described above.
Large size NLC (lNLC) was prepared as a control group in in vivo distribution assay. OA (1 mg), GM (6 mg), and SA–PEG–FA (3 mg) were added into 1 mL of ethanol and heated to obtain a transparent solution. To a preheated (70 °C) aqueous solution (10 mL), the mixture of lipid components was injected via pipette under gentle agitation (300 rpm). The follow steps are consistent with the preparation of ultrasmall FA-NLC as mentioned above.
Colloidal stability and hemolysis assay
Colloidal stability and hemolysis assay were performed similar to previous report [19]. Briefly, for colloidal stability test, the freshly prepared DTX-loaded FA-NLC was diluted with phosphate buffer saline (PBS, pH 7.4) at the volume ratio of 1:10. The change in particle size was recorded by Zetasizer Nano ZS90 at predetermined time intervals for up to 48 h. For hemolysis assay, Red blood cells (RBCs) were firstly obtained from New Zealand rabbit and diluted to 2% suspension with saline solution. Then, the RBCs were formulated as a 2% suspension with saline solution. The DTX-loaded FA-NLC was added into 2% RBCs suspension with the same volume to achieve the designated concentrations (0.1, 0.25, 0.5, 0.75, 1 mg/mL), and incubated at 37 °C for 1 h. Besides, RBCs suspension was also incubated with saline and distilled water under the same condition as negative (0% hemolysis) and positive controls (100% hemolysis), respectively. After that, all the samples were centrifuged at 3000 rpm for 10 min and the absorption values (represented the counts of released hemoglobin) of same volume of supernatants were measured at 545 nm by ultraviolet spectrophotometer (TU-1810, Purkinje, China).
Intracellular cellular uptake assay
HeLa cells were placed in the 24-well plates (Costar, USA) at a density of 3 × 104 cells/well. After 24 h of cell attachment, the medium was replaced by 1 mL of fresh serum-free medium which contained C6-loaded FA-NLC (the C6-loading in NLC was 0.2%). At the same time, another parallel group of cells were incubated with excess amount of free FA (1 mM) for 1 h before nanoparticles addition. After incubation, the cells were rinsed several times with PBS and observed under inverted fluorescence microscope (Axio Observer A1, Zeiss, Germany).
In vitro cytotoxicity assay
The cytotoxicity of blank FA-NLC and DTX-loaded FA-NLC was evaluated by standard MTT assay. Various concentrations of blank FA-NLC and DTX-loaded FA-NLC were prepared by adding into the serum-free culture medium. The cells (5 × 103 cells/well) were seeded in 96-well plates (Costar, USA), and cultured in 200μL of serum-free culture medium containing blank FA-NLC or DTX-loaded FA-NLC for 72 h at 37 °C. After incubation, the wells were washed with 1 × PBS and MTT (5 mg/mL) was added to into each well. After 4 h of staining, MTT formazan crystals were dissolved by 150μL of dimethyl sulfoxide (DMSO). Absorbance was read at 570 nm on a microplate reader (Bio-Rad, model 680, USA). Cell viability was calculated by the following formula:\({\text{Cell viability }}\left( \% \right) \, = \, A_{{{\text{vector}}}} /A_{{{\text{control}}}} \times {1}00\%\), where Avector and Acontrol were the absorbance of the cells treated with formulations and the serum-free culture medium (as a negative control), respectively [22].
In vivo distribution of nanoparticles
Tissue distribution assay was investigated in HeLa tumor xenograft mice nude mice model (Zhang et al. 2015b). When tumor volume reached about 100 mm3, the tumor xenograft mice were randomly divided into two groups. Group one was given DiR-loaded ultrasmall FA-NLC and group two was treated with DiR-loaded FA-lNLC both at same dose of DiR via the tail vein. At 48 h postinjection, NIR fluorescent images were captured by in vivo imaging system (FX PRO, Kodak, USA). Mice were sacrificed and the tumor, heart, liver, spleen, lung, and kidney of mice were collected to measure their individual fluorescence intensities. Kodak Molecular Imaging Software 5.X was applied to analyze the images.
In vivo antitumor assay
HeLa tumor xenograft mice were recruited to perform antitumor assay (n = 6) when the tumors were palpable to be 70–80 mm3. The treatment was initiated with the following: saline solution, Taxotere, and DTX-loaded FA-NLC. Both drug containing formulations were administered at 4 mg/kg of DTX. Treatments were administered every other day and lasted for a period of 14 days. Tumor volumes and body weight were measured before administration and the total volume was calculated by the equation as mentioned above. Mice were sacrificed at the end of the treatment and tumor tissues were subjected to hematoxylin–eosin (HE) staining and observed under inverted fluorescence microscope.
Statistical analysis
Values are expressed as the mean ± standard deviation. P < 0.05 was considered to indicate a statistically significant difference. Statistical significance was determined using two-tailed Student’s t-test or one-way analysis of variance using Origin 8.0 (OriginLab, Massachusetts, USA.).
Results and discussion
Characterizations of DTX-loaded FA-NLC
In order to optimize the loading of DTX into the FA-NLC, drug input to NLC weight ratios ranging from 5 to 20% were tested. After the removal of free drug by centrifugation, the drug loading was quantified by HPLC. As shown in Fig. 1a, increasing drug input ratio result in higher drug-loading content. This positive relation reached a balance at 15% drug input ratio. Thus, FA-NLC with 15% drug input was used for the following investigation unless otherwise stated.
Successful drug delivery requires the nanocarrier to remain stable under physiological environment without significant drug leakage and size change for enough time to allow their circulation to the targeted site. Drug-release study was conducted by dialyzing the DTX-loaded FA-NLC against PBS to mimic its release profile under physiological environment. DTX-loaded NLC showed much more sustained drug-release profile compared with free DTX. DTX release from NLC were relatively slow, 68% cumulative release in the first 24 h (Fig. 1b). The sustained-release pattern of DTX–NLC was ascribed to the controlled dissociation from compact configuration that reassembled with lipid. Colloidal stability assay was carried out to demonstrate the stability of DTX-loaded FA-NLC in the circulation, which is essential for nanocarriers to delivery sufficient drug to the targeted tissue. To estimate the colloidal stability of the as-prepared nanoparticles, the change in particle size under physiological environment (PBS, pH 7.4) was monitored for 24 h. The results were recorded and displayed Fig. 2a. After incubation with the PBS, the particle size of DTX-loaded FA-NLC had no significant changes compared with the initial one, and remained unchanged until the end of the test. The results of drug-release assay and colloidal stability assay indicated that NLC exhibited satisfactory stability in physiological environment and allow for controlled release of cargo packaged in the nanocarriers; thereby, leading to improved antitumor effects in vitro and in vivo.
As prepared DTX-loaded FA-NLC was opalescent. According to the results obtained by DLS, the DTX-loaded FA-NLC had a mean size of ~ 30 nm (Fig. 1a), which held great promise to across the multiple barriers and penetrate deeply into the tumor tissue for advanced in vivo performance [17]. Morphology observation (Fig. 1d) revealed by TEM also confirmed that DTX-loaded FA-NLC was ultrasmall particle with near spherical shape which showed uniformed size distribution. In our study, a drug-loaded formulation using the 15% input with a final drug loading of approximately 3.83% was employed.
Hemolysis as one of the key risks associated with the biocompatibility of materials intended for parenteral administration was also investigated to further confirm the safety profile of DTX-loaded FA-NLC for intravenous administration and medicinal applications [23]. As shown in Fig. 2b, although increased nanoparticle concentration could increase hemolysis, DTX-loaded FA-NLC displayed neglectable hemolysis (< 2%) even at the highest concentration (1 mg/mL). It was suggested upon intravenous administration, the nanoparticles would be greatly diluted by vast blood components that the actual plasmatic nanoparticle concentration would be much lower than 1 mg/mL [24]. As a result, our experiment indicated that DTX-loaded FA-NLC, which comprised of naturally derived materials, exhibited great hemocompatibility that might be a safe platform for further applications.
In vitro cellular uptake of DTX-loaded FA-NLC
It was reported that the FA ligands exposed toward the surface of the nanoparticles can selectively target FA receptor which excessively expressed in many tumor cells to enhance the tumor-homing profile of the modified ones [25]. Herein, we speculated that FA-NLC would provide an active-targeting platform for the delivery of DTX. The cellular uptake behavior of C6-loaded FA-NLC in HeLa cells was investigated. Cells were incubated with C6-loaded FA-NLC for different time intervals (3 h and 6 h) and the internalization of C6-loaded FA-NLC was visualized by inverted fluorescence microscope. As shown in Fig. 3, cells treated with C6-loaded FA-NLC showed strong green fluorescent signal in the cytoplasm and increased as a function of time, which indicated that the cellular uptake process was positively related to the incubation time. It was worth mentioning that pretreatment with excess FA resulted in significantly dropped fluorescent intensity in both tested time intervals when compared with untreated groups at the same condition. These results suggested that cellular uptake of C6-loaded FA-NLC was positively rely on FA receptor-mediated internalization which is beneficial for potential tumor-homing drug delivery.
In vitro cytotoxicity assay
In vitro cytotoxicity of DTX-loaded FA-NLC against HeLa cells was evaluated. A variety of concentrations of drug-free FA-NLC were firstly tested using MTT assay. As shown in Fig. 4a, compared with untreated HeLa cells, the viability of cells treated with blank FA-NLC remained unchanged even at the highest concentration of 100 μg/mL, which suggested that blank FA-NLC could be a highly biocompatible drug carrier with negligible cytotoxicity on cells. The following cytotoxicity assay using DTX-loaded FA-NLC was shown in Fig. 4b. Taxotere, a clinically applied formulation, was employed as control. It was concluded that Taxotere had strong cytotoxicity effect on HeLa cells and this effect was positively related to the DTX concentration. However, it was noted that DTX-loaded FA-NLC also had comparable cytotoxicity to Taxotere at all indicated concentrations, suggesting that the encapsulated DTX within FA-NLC could be sufficiently released after being internalized by HeLa cells.
In vivo antitumor efficacy of DTX-loaded FA-NLC
Given the active results from in vitro assay, we evaluated antitumor potential of DTX-loaded FA-NLC in a murine xenograft tumor model. Saline solution, as well as Taxotere were used as controls. As expected, the tumor volume of mice treated with saline rapidly increased, while tumor growth in Taxotere and DTX-loaded FA-NLC groups was inhibited. In particular, inhibition effect on tumor growth in DTX-loaded FA-NLC group was much higher than that in Taxotere treated one. In detail, the tumor volume of DTX-loaded FA-NLC group was 408.08 ± 67.29 mm3, when compared with 1221.80 ± 172.20 mm3 and 657.58 ± 36.75 mm3 in saline and Taxotere group, respectively. The HE staining in Fig. 5b also confirmed the conclusion obtained from tumor volume assay. It was observed that compared with saline group, cancer cells in both Taxotere and DTX-loaded FA-NLC groups showed necrocytosis to certain extent. In particular, DTX-loaded FA-NLC showed much stronger effect than Taxotere. In addition, the body weight variations of all mice were also recorded. As an indicator of physical condition, the body weight variations revealed interesting results. As shown in Fig. 5c, Taxotere treated mice suffered obvious body weight loss since Day 4 with a final body weight loss of 0.93 g, which indicated that the health condition of treated mice was greatly impaired. Unlike Taxotere that exerted significant side effects on subjects, DTX-loaded FA-NLC, like saline solution, exhibited no negative effect on body weight, suggesting the high biocompatibility of DTX-loaded FA-NLC.
The tumor volume (a), HE staining of tumor tissue (b), and body weight (c) analysis of HeLa tumor-bearing BALB/c nude mice after intravenous injection administration of different formulations (saline, Taxotere, and DTX-loaded FA-NLC). The measurement of tumor volumes and the injection of formulations were repeated every 2 days for two weeks. Dose: 4 mg/kg DTX per mouse. Data were expressed as mean ± S.D. (n = 6). **P < 0.01
In vivo distribution of nanoparticles
To assess the tumor-targeting ability of ultrasmall FA-NLC, biodistribution of DiR-loaded FA-NLC and DiR-loaded FA-lNLC were compared in xenograft tumor model by non-invasive near infrared optical imagining technique. As shown in Fig. 6a, the fluorescence signal in the tumor tissue of ultrasmall FA-NLC group is much higher than that of FA-lNLC group at 24 h after administration. After analysis of organ, fluorescent intensity from tumor in ultra-small FA-NLC group was almost 1.37 times greater than that of FA-lNLC group, while that from the normal organ sample was lower (Fig. 6b). In other word, more FA-NLC accumulated in tumor tissue compared with FA-lNLC. These results confirmed that ultrasmall FA-NLC could specifically be retained in the tumor site, which was attributed to targeting and penetration abilities facilitated by its small size.
Conclusion
In summary, we fabricated an ultrasmall FA-NLC for tumor-targeted delivery of DTX. FA-NLC possessed proper particle size and could remain colloidal stability under physiological environment. Therefore, the optimized formulation could accumulate at tumor tissue through enhanced permeability and retention and FA-mediated targeting. Furthermore, in vitro and in vivo investigation all confirmed the excellent tumor targetability, remarkable tumor inhibition efficacy, and laudable biosafety of this nanocarrier. These encouraging results suggested that the ultrasmall FA-NLC could be a promising platform for clinical applications in cancer-targeted therapy by improving tumor tissue penetration of loaded drug.
Abbreviations
- NLC:
-
Nanostructured lipid carriers
- DTX:
-
Docetaxel
- FA:
-
Folic acid
- SLNs:
-
Solid lipid nanocarriers
- RES:
-
Reticuloendothelial system
- PEG:
-
Polyethylene glycol
- GM:
-
Glycerin monostearate
- OA:
-
Oleic acid
- MTT:
-
Methylthiazoletetrazolium
- C6:
-
Coumarin 6
- PC:
-
Phosphatidylcholine
- SA-PEG-FA:
-
Stearic acid–polyethylene glycol–folate
- HeLa:
-
Cervical cancer cells
- FBS:
-
Fetal bovine serum
- V:
-
Tumor volume
- L:
-
Length
- W:
-
Width
- RBCs:
-
Red blood cells
- PBS:
-
Phosphate buffer saline
- HE:
-
Hematoxylin–eosin
References
Iannazzo D, Pistone A, Salamã M et al (2017) Graphene quantum dots for cancer targeted drug delivery. Int J Pharm 518:185–192
Lammers T, Subr V, Ulbrich K, Hennink WE, Storm G, Kiessling F (2010) Polymeric nanomedicines for image-guided drug delivery and tumor-targeted combination therapy. Nano Today 5:197–212
Liu Y, Lu W (2012) Recent advances in brain tumor-targeted nano-drug delivery systems. Expert Opin Drug Deliv 9:671–686
Zhang W, Zhang Z, Zhang Y (2011) The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res Lett 6:555
Chou CW, Chang SY, Chen YS, Su WC, Wu TH (2011) Herbal-loaded multifunctional nanocomposities as nanocarrier for cancer therpy: synthesis and quality. Adv Mater Res 311–313:1625–1629
Huang Y, He L, Song Z et al (2017) Phycocyanin-based nanocarrier as a new nanoplatform for efficient overcoming of cancer drug resistance. J Mater Chem B 5:3300–3314
Zur MHA, Schwarz C, Mehnert W (2000) Solid lipid nanoparticles (SLN) for controlled drug delivery–drug release and release mechanism. Eur J Pharm Biopharm 50:161–177
Mehnert W, Mäder K (2001) Solid lipid nanoparticles: Production, characterization and applications. Adv Drug Deliv Rev 47:165–196
Mosallaei N, Jaafari MR, Hanafibojd MY, Golmohammadzadeh S, Malaekehnikouei B (2013) Docetaxel-loaded solid lipid nanoparticles: preparation, characterization, in vitro, and in vivo evaluations. J Pharm Sci 102:1994–2004
Beloqui A, Solinís MÁ, Rodríguez-Gascón A, Almeida AJ, Préat V (2016) Nanostructured lipid carriers: promising drug delivery systems for future clinics. Nanomed Nanotechnol Biol Med 12:143
Saupe A, Wissing SA, Lenk A, Schmidt C, Müller RH (2005) Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC)–structural investigations on two different carrier systems. Bio Med Mater Eng 15:393
Nakamura Y, Mochida A, Choyke PL, Kobayashi H (2016) Nanodrug delivery: is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjug Chem 27:2225–2238
Tanaka T, Shiramoto S, Miyashita M, Fujishima Y, Kaneo Y (2004) Tumor targeting based on the effect of enhanced permeability and retention (EPR) and the mechanism of receptor-mediated endocytosis (RME). Int J Pharm 277:39–61
Yoo JW, Chambers E, Mitragotri S (2010) Factors that control the circulation time of nanoparticles in blood: challenges, solutions and future prospects. Curr Pharm Des 16:2298
Tang L, Gabrielson NP, Uckun FM, Fan TM, Cheng J (2013) Size-dependent tumor penetration and in vivo efficacy of monodisperse drug-silica nanoconjugates. Mol Pharm 10:883–892
Guo X, Wu Z, Li W et al (2016) Appropriate size of magnetic nanoparticles for various bioapplications in cancer diagnostics and therapy. Acs Appl Mater Interfaces 8:3092–3106
Dehaini D, Fang RH, Luk BT et al (2016) Ultra-small lipid-polymer hybrid nanoparticles for tumor-penetrating drug delivery. Nanoscale 8:14411–14419
Song J, Yang X, Jacobson O et al (2015) Ultrasmall gold nanorod vesicles with enhanced tumor accumulation and fast excretion from the body for cancer therapy. Adv Mater 27:4910
Tang D, Zhao X, Yang T, Wang C (2018) Paclitaxel prodrug based mixed micelles for tumor-targeted chemotherapy. Rsc Advances 8:380–389
Wang C, Bao X, Ding X et al (2015) A multifunctional self-dissociative polyethyleneimine derivative coating polymer for enhancing the gene transfection efficiency of DNA/polyethyleneimine polyplexes in vitro and in vivo. Polym Chem 6:780–796
Wang L, Li M, Zhang N (2012) Folate-targeted docetaxel-lipid-based-nanosuspensions for active-targeted cancer therapy. Int J Nanomed 7:3281–3294
Ding X, Xu X, Zhao Y et al (2017) Tumor targeted nanostructured lipid carrier co-delivering paclitaxel and indocyanine green for laser triggered synergetic therapy of cancer. RSC Adv 7:35086–35095
Slowing II, Wu CW, Vivero-Escoto JL, Lin SY (2009) Mesoporous silica nanoparticles for reducing hemolytic activity towards mammalian red blood cells. Small 5:57–62
Abbad S, Wang C, Waddad AY, Lv H, Zhou J (2015) Preparation, in vitro and in vivo evaluation of polymeric nanoparticles based on hyaluronic acid-poly(butyl cyanoacrylate) and D-alpha-tocopheryl polyethylene glycol 1000 succinate for tumor-targeted delivery of morin hydrate. Int J Nanomed 2015:305–320
Zeng YP, Luo SL, Yang ZY et al (2016) A folic acid conjugated polyethylenimine-modified PEGylated nanographene loaded photosensitizer: photodynamic therapy and toxicity studies in vitro and in vivo. J Mater Chem B 4:2190–2198
Acknowledgements
This work was supported by the grants received from youth innovation fund of the First affiliated Hospital of Zhengzhou University (YNQN2017172). All authors are thankful for the support received from the First affiliated Hospital of Zhengzhou University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, M., Pei, J., Ma, Z. et al. Docetaxel-loaded ultrasmall nanostructured lipid carriers for cancer therapy: in vitro and in vivo evaluation. Cancer Chemother Pharmacol 85, 731–739 (2020). https://doi.org/10.1007/s00280-020-04048-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04048-6