Abstract
Purpose
While doses of carboplatin are mostly individualized according to the Calvert equation based on estimated Glomerular Filtration Rate (eGFR), there is still uncertainty regarding the best formula to predict GFR. Since Janowitz et al. recently proposed a new equation predicting GFR in cancer patients, we aimed to compare this equation to other carboplatin clearance (carboCL) predicting formulae.
Methods
The actual carboCL of 491 patients was compared to predicted carboCL according to the Calvert formula using several equations to predict GFR (Janowitz, Cockcroft–Gault, MDRD, CKD-EPI, CKD-EPI with cystatin C (CKD-EPI-cysC)); and according to two others that directly predict carboCL (Chatelut and Thomas). The formulae were compared on Mean Percentage Error (MPE), Mean Absolute Percentage Error (MAPE) and percentage of patients with a prediction error above 20% (P20).
Results
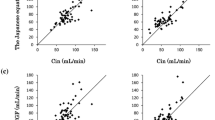
The MPE, MAPE and P20 were, respectively, within the ranges − 5.2 to + 5.9%; 14.0–21.2% and 23–46%. The MAPE and P20 of Calvert-CKD-EPI-cysC were the lowest. The performance of Calvert-CKD-EPI was better than that of other creatinine-based formulae although not significantly different from the Calvert–Janowitz formula. Among formulae based on creatinine only, Calvert-CKD-EPI and Calvert–Janowitz are the least influenced by patient characteristics.
Conclusion
Whereas CysC improves carboplatin CL prediction, the Calvert-CKD-EPI equation seems the most suitable creatinine-based formula to predict carboCL homogeneously in all subgroups of patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carboplatin is a widely used cytotoxic in combination therapies to treat many malignancies. Since Carboplatin is mainly eliminated by the kidneys, doses need to be adapted to the renal function. Several formulae have been proposed for individual dosing of carboplatin but the most frequently used is that published by Calvert et al. [1] with the following equation: Carboplatin Clearance (CL) [mL/min] = measured Glomerular Filtration Rate (GFR) [mL/min] + 25 [mL/min]. Since glomerular filtration rate is not measured in clinical practice, an estimation of GFR (eGFR) is used as a substitute. Most commonly, GFR is estimated by creatinine clearance using the Cockcroft–Gault formula [2]. Several other methods are available for estimating GFR: the MDRD eGFR equation was introduced in 1999 [3] then in 2009 the CDK-EPI formula [4] was shown to be more accurate (especially in patients who have a high GFR level) and is now recommended by the Kidney Disease Improval Global Outcome (KDIGO) guideline to estimate GFR [5].
Recently Janowitz et al. [6] proposed a new equation to estimate Glomerular Filtration Rate in cancer patients. They obtained their equation using 51Cr-EDTA GFR clearance measurements performed in 1977 patients, then validated it using two different data sets of 494 and 111 patients, using a method which is both original and accurate. They found a correlation between the square root of 51Cr-EDTA GFR and age, weight, Body Surface Area (BSA), sex and natural logarithmic-transformed creatinine.
They observed that their new model was the most accurate and among the least biased of seven published models for estimating GFR. Moreover they showed that the variance of their formula was constant in different subpopulations since the residual error was not dependent on serum creatinine level, age, or morphological characteristics of patients. Thus, they compared the accuracy of a carboplatin dose calculated from the Calvert equation using measured 51Cr-EDTA GFR, to that calculated from the same equation using various eGFR predicting formula. In this way, their new formula produced the least number of patients with an absolute dosing error above 20% in comparison to all other formulae for estimating GFR. However, in their study, no pharmacokinetic carboplatin data were available, thus the performance of the Calvert equation using this new eGFR to predict Carboplatin CL remains to be assessed. For this reason, our study aimed to assess the performance of Calvert’s equation using the Janowitz formula as a substitute for GFR by comparing predicted carboplatin CL to actual carboplatin CL. The performance of the corresponding new equation was compared to those of other carboplatin CL predicting formulae.
Patients and methods
Data collection
The data of 491 patients were collected from a subset of three different previously published clinical studies [7,8,9]. These protocols were approved by the ethical committee of Toulouse I and informed consent was obtained from each patient.
Carboplatin administration, blood sampling, and platinum analysis
Carboplatin was used as monotherapy or in combination with various drugs for different tumors at standard doses [7, 8] or as high dose (HD) in combination with etoposide [9]. It was administered as a daily 30-min or 1-h infusion in 5% dextrose, then four blood samples were taken: before administration, 5 min before the end of infusion, 1 and 4 h after the end of infusion. These samples were selected according to a limited sampling strategy developed previously [10]. After immediate centrifugation at 1500 g for 10 min at 4 °C, the plasma was separated and ultrafiltered using the Amicon MPS1 micropartition system with YM-T membrane à 4 °C for 15 min at 1500 g. Carboplatin levels in the plasma ultrafiltrate were measured by means of flameless atomic absorption spectrophotometric analysis according to a previously described method [11].
Pharmacokinetic analysis, determination of individual carboplatin clearance
Individual carboplatin CL were obtained using the NONMEM software [12] (version VI, level 2.0) according to a two-compartment pharmacokinetic model and first-order conditional estimation with interaction (FOCE-i) method. The carboplatin concentration versus time data of each patient were combined with those of a database composed of 143 patients with rich sampling [10]. Individual POSTHOC values of clearance were obtained for each patient (no covariate was considered for the typical value of CL). This approach to estimate individual carboplatin CL has been previously validated prospectively [10]. The low residual variability corresponding to every run (i.e., always lower than 20%) also confirms the robustness of the approach.
Assessment of different formulae for predicting carboplatin CL
Predicted carboplatin clearance (pCL) was calculated for each patient using seven different formulae. Five were based on Calvert’s formula using Janowitz [1] (Calvert–Janowitz), Cockcroft–Gault [2] (Calvert-CG), BSA adjusted CKD-EPI [4] (Calvert-CKD-EPI), BSA adjusted CKD-EPI creatinine-cystatin equation [13] (Calvert-CKD-EPI_cys) or BSA adjusted MDRD [3] (Calvert-MDRD) equation to predict Glomerular Filtration Rate (eGFR). Two others directly predict carboplatin CL: Chatelut formula [14] (with adjusted ideal body weight instead of actual body weight in overweight patients as previously published [15]), and modified Thomas formula [7], the latter including serum cystatin C level. The equations used in the different models are given in Table 1.
The relative difference between each predicted clearance and individual clearance (iCL) was calculated to assess the individual percentage error (IPE) in the following manner: IPE = [(pCL – iCL)/iCL] × 100. The absolute value of relative difference (|pCL-iCL|/iCL) × 100 was also calculated.
The bias of the predicting formulae was assessed as the mean of percentage error (MPE).
The precision was assessed as the mean absolute percentage error (MAPE) and as the percentage of patients with a MAPE over 20% (P20) [16].
As age, glomerular filtration rate (eGFR), body mass index (BMI), and sex are known to be significantly associated with carboplatin clearance, we decided to report their influence on precision and bias (Tables 4 and 6) according to the quartiles of age in our data and following the clinically accepted categories of BMI [17] and eGFR [18]. Hence the population was split into subgroups as follows: subgroups according to age: below the 1st quartile (≤ 44 y), within the interquartile range (44 year < age ≤ 67 year) and greater than the 3rd quartile (> 67 year); subgroups according to eGFR (estimated using BSA adjusted CKD-EPI formula): eGFR ≤ 60 ml/min; 60 ml/min < eGFR ≤ 90 ml/min, and eGFR > 90 ml/min; subgroups according to BMI: underweight (BMI < 18 kg/m2), normal weight (18 kg/m2 ≤ BMI < 25 kg/m2) overweight (25 kg/m2 ≤ BMI < 30 kg/m2) and obese (BMI ≥ 30 kg/m2), and subgroups according to sex (men vs women). The Kruskal–Wallis test was performed to test for significant differences in the MPE and MAPE of each formula between the different subgroups. To account for multiple testing, a p value less than 0.01 was considered significant.
A repeated-measures ANOVA was used to compare MPE and MAPE obtained between all formulae and pairwise comparisons were adjusted for multiple testing using Tukey’s method [19].
The Cochran’s Q test for paired nominal data was used to compare P20 obtained between all formula and post-hoc pairwise comparisons were then performed using the McNemar test adjusted for multiple testing (Benjamini–Hochberg method [20]).
Statistical analyses were performed with R version 3.6.0 [21] and Stata 15 [22].
Results
Patients
The main patient characteristics are shown in Table 2. The 491 patients (295/196 females/males, respectively) were between 20 and 87 years old, with a Scr level between 25 and 433 µM. The median body weight was 65 kg, 55 (11%) patients were obese (BMI > 30 kg/m2) and 38 (8%) underweight (BMI < 18.5 kg/m2). Carboplatin clearance ranged between 38 and 230 mL/min, and was over 200 mL/min for only 9 (2%) patients.
Precision
MAPE for each formula is shown in Table 3 as well as 2-by-2 comparisons of all the formulae. A one-way repeated measures ANOVA showed that overall there are statistically significant differences between the seven formulae in terms of precision (MAPE) with p < 0.0001. The Calvert-CKD-EPI-CysC formula has the lowest MAPE compared to all other formulae and this value is significantly lower than all other formulae except for Calvert-CKD-EPI and Thomas. Among the creatinine- based formulae, the Calvert CKD-EPI has the lowest MAPE, significantly lower than that of Calvert-MDRD and Chatelut formulae. The Chatelut formula has significantly higher MAPE than all the other formulae.
MAPE according to different subgroups are displayed in Table 4. Calvert-CKD-EPI-CysC, Calvert–Janowitz and Calvert-MDRD do not seem influenced by any patient characteristics. Age and sex influence the precision of both Thomas and Chatelut formula, while eGFR influences the precision of both Calvert-CKD-EPI and Calvert-CG formulae with better precision for higher eGFR in both cases. BMI was not found to influence the precision of any formula.
P20 for each formula as well as pairwise comparisons of all formulae are shown in Table 5. The P20 of the Calvert-CKD-EPI-CYsC formula was significantly lower than that of all other formulae. Among the creatinine-based formulae, the P20 of Calvert-CKD-EPI is significantly lower than all the others.
Bias
MPE are shown in Table 6. The MPE derived from all formulae except for Calvert CKD-EPI_cysC and Calvert-MDRD were significantly different from 0 (95% CI does not contain 0) demonstrating the existence of bias. A one-way repeated measures ANOVA showed that there are statistically significant differences between the seven formulae in terms of MPE with p < 0.0001.
Calvert–Janowitz and Calvert-CKD-EPI are the only two formulae for which bias is not influenced by age, eGFR level, BMI or sex.
The Calvert–Janowitz formula tends to underestimate carboplatin CL whatever the subgroup, Calvert-MDRD formula tends to overestimate carboplatin CL, while other formulae show different biases depending on the subgroups.
Discussion
The Calvert formula is widely used for individual dosing of carboplatin with creatinine clearance estimated by CG formula instead of GFR [23]. Since CG formula has some well-known limits such as an overestimation in overweight patients [24] and a poor accuracy in patients with a normal renal function [25], the question of the best GFR estimator remains.
Janowitz et al. recently proposed a new formula for GFR prediction developed in a population of cancer patients. We compared the performance of the Calvert formula based on the eGFR according to Janowitz et al. and different GFR predicting methods as well as two methods developed to directly predict carboplatin CL. Among the formulae tested all were based on serum creatinine level and two were also based on plasma cystatin C level. This analysis is based on the largest data set of carboplatin pharmacokinetic data ever used for such an evaluation.
Precision
The Calvert-CKD-EPI-CysC is clearly the best formula which is the least biased, with the lowest MAPE and the lowest P20. Moreover its precision is not influenced by any patient characteristics. This emphasizes the improvement of carboplatin CL predicting equations when taking into account both creatinine and cystatin C. Cystatin C is freely filtrated at renal level, not secreted, and is reabsorbed by tubular epithelial cells but subsequently catabolized so that it does not return to the blood flow (reviewed by Newman 2002 [26]). Despite this, the determination of cystatin C is not routinely recommended to assess kidney function [5]. Few hospitals currently assay cystatin C, thus we did not focus only on cystatin C-based formulae but broadened our study to other formulae.
Our results show that amongst all the creatinine-based formulae, the lowest MAPE was observed with Calvert-CKD-EPI formula, even though its MAPE value was not significantly different from that of Calvert–Janowitz-and Calvert-CG, it was significantly lower than the Calvert-MDRD and Chatelut formulae.
The precision of Calvert–Janowitz was comparable to Calvert-CKD-EPI, Calvert-CG and Calvert-MDRD, but better than the Chatelut formula. Janowitz et al. found their eGFR formula to be better than CG within the Calvert formula. Here with fewer patients the difference we observed was not statistically significant.
Considering the most accurate formulae, Calvert-CKD-EPI-CysC and Calvert–Janowitz were the only formulae for which the precision was not influenced by any patient characteristics.
We found an influence of eGFR level on the accuracy of Calvert-CG and Calvert-CKD-EPI. The GFR-predicting formulae CG, MDRD CKD-EPI and CKD-EPI-cysC are known to be less accurate for patients with normal renal function than for patients with renal failure [27,28,29] but unexpectedly, in our study, the better the renal function, the better the accuracy of carboplatin prediction. Although GFR prediction is better for renal failure, carboplatin CL prediction is worse, which means non-renal carboplatin CL is not well predicted. The performance of Calvert–Janowitz formula was not statistically associated to eGFR but we also found a worse accuracy for patients with low eGFR compared to those with a good renal function.
Considering the percentage of patients with a MAPE over 20% confirms that Calvert-CKD-EPI-CysC shows the best precision of all formulae and Calvert-CKD-EPI the best precision of creatinine-based formulae.
Bias
All formulae except for Calvert-CKD-EPI-CysC and Calvert-MDRD showed a MPE statistically different from 0, the bias was different between all formulae. Calvert–Janowitz tends to underestimate carboplatin CL, but unlike the others, its bias is not influenced by age, sex, BMI, or renal function.
The overestimation of both the Chatelut and Thomas formulae in young male patients with a good renal function has already been described, and an alternative formula has been proposed for this population [9]. Since we used the initial formula, the high proportion of young male patients (n = 89) may explain the poor performance of the Chatelut formula in the overall population and the worse performance of the Thomas formula compared to Calvert-CKD-cysC equation. Moreover raw predicted carboplatin CL was taken into account in our study, while in clinical practice it is capped to 200 mL/min, so the overestimation we observed is not in the same range in clinical practice.
As expected, we found a trend towards overestimation of carboplatin CL in overweight patients with the Calvert-CG formula since we used actual weight in the CG formula [30]. While a GFR underestimation by MDRD formula has been described in obese patients when eGFR is normalized to 1.73 m2 [31], we found an overestimation of carboplatin CL using Calvert-MDRD formula based on individual BSA-adjusted MDRD equation.
Conversely, we found an underestimation of carboplatin CL in obese patients with both the Calvert-CKD-EPI and Calvert-CKD-EPI-cysC formulae despite the consideration of BSA for individual eGFR. This is consistent with the GFR underestimation observed in obese patients using both CKD-EPI-cysC [32] and CKD-EPI formulae even if underestimation is reduced by BSA [33].
Conclusion
The present analysis confirms the benefit of considering the plasma cystatin C level to predict carboplatin CL. If we consider only the methods based on serum creatinine but not cystatin C, the eGFR from CKD-EPI seems the best estimator of GFR within the Calvert formula. This formula shows the best precision and its performance is not influenced by patients’ characteristics. The performance of the Calvert–Janowitz formula though not significantly better than that of other creatinine-base formulae seems comparable to Calvert-CKD-EPI.
Availability of data and materials
Main data are described in the manuscript, raw data are available on request.
Change history
13 April 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00280-021-04268-4
References
Calvert AH, Newell DR, Gumbrell LA, O’Reilly S, Burnell M, Boxall FE et al (1989) Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 7:1748–1756. https://doi.org/10.1200/JCO.1989.7.11.1748
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. https://doi.org/10.1159/000180580
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Akbari A, Clase CM, Acott P, Battistella M, Bello A, Feltmate P et al (2015) Canadian society of nephrology commentary on the KDIGO clinical practice guideline for CKD evaluation and management. Am J Kidney Dis 65:177–205. https://doi.org/10.1053/j.ajkd.2014.10.013
Janowitz T, Williams EH, Marshall A, Ainsworth N, Thomas PB, Sammut SJ et al (2017) New model for estimating glomerular filtration rate in patients with cancer. J Clin Oncol 35:2798–2805. https://doi.org/10.1200/JCO.2017.72.7578
Schmitt A, Gladieff L, Lansiaux A, Bobin-Dubigeon C, Etienne-Grimaldi M-C, Boisdron-Celle M et al (2009) A universal formula based on cystatin C to perform individual dosing of carboplatin in normal weight, underweight, and obese patients. Clin Cancer Res 15:3633–3639. https://doi.org/10.1158/1078-0432.CCR-09-0017
Thomas F, Séronie-Vivien S, Gladieff L, Dalenc F, Durrand V, Malard L et al (2005) Cystatin C as a new covariate to predict renal elimination of drugs: application to carboplatin. Clin Pharmacokinet 44:1305–1316. https://doi.org/10.2165/00003088-200544120-00009
Moeung S, Chevreau C, Broutin S, Guitton J, Lelièvre B, Ciccolini J et al (2017) Therapeutic drug monitoring of carboplatin in high-dose protocol (TI-CE) for advanced germ cell tumors: pharmacokinetic results of a phase II multicenter study. Clin Cancer Res 23:7171–7179. https://doi.org/10.1158/1078-0432.CCR-17-1344
Chatelut E, Pivot X, Otto J, Chevreau C, Thyss A, Renée N et al (1990) A limited sampling strategy for determining carboplatin AUC and monitoring drug dosage. Eur J Cancer Oxf Engl 2000(36):264–269
LeRoy AF, Wehling ML, Sponseller HL, Friauf WS, Solomon RE, Dedrick RL et al (1977) Analysis of platinum in biological materials by flameless atomic absorption spectrophotometry. Biochem Med 18:184–191
Beal SL, Sheiner LB (1982) Estimating population kinetics. Crit Rev Biomed Eng 8:195–222
Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T et al (2012) Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367:20–29. https://doi.org/10.1056/NEJMoa1114248
Chatelut E, Canal P, Brunner V, Chevreau C, Pujol A, Boneu A et al (1995) Prediction of carboplatin clearance from standard morphological and biological patient characteristics. J Natl Cancer Inst 87:573–580
Bénézet S, Guimbaud R, Chatelut E, Chevreau C, Bugat R, Canal P (1997) How to predict carboplatin clearance from standard morphological and biological characteristics in obese patients. Ann Oncol 8:607–609
Stevens LA, Zhang Y, Schmid CH (2008) Evaluating the performance of equations for estimating glomerular filtration rate. J Nephrol 21:797–807
WHO Expert Consultation (2004) Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet Lond Engl 363:157–163. https://doi.org/10.1016/S0140-6736(03)15268-3
Levey AS, Coresh J (2012) Chronic kidney disease. Lancet Lond Engl 379:165–180. https://doi.org/10.1016/S0140-6736(11)60178-5
Tukey JW (1977) Exploratory data analysis. Addison-Wesley Publishing Company, Reading, PA
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 57:289–300
R Core Team (2019) R : a language and environment for statistical computing. Release 3.6.0. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org
Stata Corp (2017) Stata statistical software: release 15. StataCorp LLC, College Station
Blankenship P, Waddell JA (2013) Survey of carboplatin dosing strategies in oncology practices. J Am Pharm Assoc JAPhA 53:420–422. https://doi.org/10.1331/JAPhA.2013.12053
Ekhart C, Rodenhuis S, Schellens JHM, Beijnen JH, Huitema ADR (2009) Carboplatin dosing in overweight and obese patients with normal renal function, does weight matter? Cancer Chemother Pharmacol 64:115–122. https://doi.org/10.1007/s00280-008-0856-x
Ekhart C, de Jonge ME, Huitema ADR, Schellens JHM, Rodenhuis S, Beijnen JH (2006) Flat dosing of carboplatin is justified in adult patients with normal renal function. Clin Cancer Res 12:6502–6508. https://doi.org/10.1158/1078-0432.CCR-05-1076
Newman DJ (2002) Cystatin C. Ann Clin Biochem 39:89–104. https://doi.org/10.1258/0004563021901847
Inker LA, Wyatt C, Creamer R, Hellinger J, Hotta M, Leppo M et al (1999) Performance of creatinine and cystatin C GFR estimating equations in an HIV-positive population on antiretrovirals. J Acquir Immune Defic Syndr 2012(61):302–309. https://doi.org/10.1097/QAI.0b013e31826a6c4f
Craig AJ, Samol J, Heenan SD, Irwin AG, Britten A (2012) Overestimation of carboplatin doses is avoided by radionuclide GFR measurement. Br J Cancer 107:1310–1316. https://doi.org/10.1038/bjc.2012.393
Cathomas R, Klingbiel D, Geldart TR, Mead GM, Ellis S, Wheater M et al (2014) Relevant risk of carboplatin underdosing in cancer patients with normal renal function using estimated GFR: lessons from a stage I seminoma cohort. Ann Oncol 25:1591–1597. https://doi.org/10.1093/annonc/mdu129
Nelson WK, Formica RN, Cooper DL, Schwartz PE, Rutherford TJ (2012) An analysis of measured and estimated creatinine clearance rates in normal weight, overweight, and obese patients with gynecologic cancers. J Oncol Pharm Pract 18:323–332. https://doi.org/10.1177/1078155211435714
Verhave JC, Fesler P, Ribstein J, du Cailar G, Mimran A (2005) Estimation of renal function in subjects with normal serum creatinine levels: influence of age and body mass index. Am J Kidney Dis 46:233–241. https://doi.org/10.1053/j.ajkd.2005.05.011
Fan L, Inker LA, Rossert J, Froissart M, Rossing P, Mauer M et al (2014) Glomerular filtration rate estimation using cystatin C alone or combined with creatinine as a confirmatory test. Nephrol Dial Transplant 29:1195–1203. https://doi.org/10.1093/ndt/gft509
Chew-Harris JSC, Chin PKL, Florkowski CM, George P, Endre Z (2015) Removal of body surface area normalisation improves raw-measured glomerular filtration rate estimation by the chronic kidney disease epidemiology collaboration equation and drug dosing in the obese. Intern Med J 45:766–773. https://doi.org/10.1111/imj.12791
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
This study was performed on data from clinical trials conducted in accordance with the Declaration of Helsinki, the studies’ protocols were approved by the ethical committee of Toulouse I and informed consent was obtained from each patient.
Consent for publication
This manuscript does not contain any individual person’s data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
White-Koning, M., Paludetto, M.N., Le Louedec, F. et al. Formulae recently proposed to estimate renal glomerular filtration rate improve the prediction of carboplatin clearance. Cancer Chemother Pharmacol 85, 585–592 (2020). https://doi.org/10.1007/s00280-019-04020-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-04020-z