Abstract
Purpose
The phase III MARIANNE study investigated single-agent trastuzumab emtansine (T-DM1) and combination T-DM1 plus pertuzumab as the first-line treatment for human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (MBC). Pharmacokinetic properties of T-DM1 and pertuzumab in these patients and the potential for drug–drug interactions (DDIs) were assessed.
Methods
Pharmacokinetic samples of T-DM1-related analytes (T-DM1 conjugate, total trastuzumab, DM1) and pertuzumab were analyzed. Observed pharmacokinetic data were summarized for all analytes. Historical population pharmacokinetic models for T-DM1 conjugate and pertuzumab in HER2-positive MBC were used to derive empirical Bayes estimates of pharmacokinetic parameters.
Results
In MARIANNE (N = 375), mean ± standard deviation population pharmacokinetic model-predicted Cycle 1 Cmax for T-DM1 conjugate was 74.4 ± 10.1 µg/mL, Cycle 1 Ctrough was 1.34 ± 0.802 µg/mL, and area under the concentration–time curve from time zero to infinity after first dose (AUCinf) was 338 ± 69.5 µg*day/mL. These values were similar to other T-DM1 studies. Pharmacokinetics of T-DM1 conjugate and other analytes (total trastuzumab, DM1) were similar with or without pertuzumab. In the pertuzumab plus T-DM1 arm, mean model-predicted Cycle 1 pertuzumab Cmax, Ctrough, and AUCinf were 276 ± 50.0 µg/mL, 64.8 ± 17.9 μg/mL, and 4470 ± 1360 µg*day/mL, respectively. These values were similar to other pertuzumab studies.
Conclusions
Based on the population pharmacokinetic analysis of T-DM1 conjugate and pertuzumab, pharmacokinetics are similar across different lines of treatment and stages of disease including previously untreated MBC patients, and no DDIs were identified for combined use of T-DM1 and pertuzumab.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trastuzumab emtansine (T-DM1) is an antibody–drug conjugate comprised of the humanized monoclonal antibody trastuzumab conjugated to the highly potent cytotoxic agent DM1. T-DM1 binds the extracellular domain (subdomain IV) of human epidermal growth factor receptor 2 (HER2) and selectively delivers DM1 to HER2-overexpressing tumor cells [1,2,3]. Similar to trastuzumab, T-DM1 induces antibody-dependent cellular cytotoxicity, inhibits cell signaling through the phosphatidylinositol-3-kinase/AKT pathway, and inhibits HER2 shedding [1, 3]. Single-agent T-DM1 demonstrated superior efficacy in patients with previously treated HER2-positive metastatic breast cancer (MBC) [4, 5] and has been approved by the US Food and Drug Administration for the treatment of patients with HER2-positive MBC who have previously received trastuzumab and a taxane, separately or in combination [6]. Pertuzumab is a HER2-targeted monoclonal antibody that also binds the HER2 extracellular domain (subdomain II), leading to inhibition of ligand-dependent signaling by preventing HER2/HER2 dimerization and activation of antibody-dependent cell-mediated cytotoxicity [7, 8]. Pertuzumab plus trastuzumab is the current standard of care for treatment of patients with previously untreated HER2-positive MBC [9].
Efficacy and safety of T-DM1 in combination with pertuzumab were recently evaluated in a phase III clinical study (MARIANNE) [10]. MARIANNE (NCT01120184; TDM4788g/BO22589) was a randomized trial conducted to assess the efficacy and safety of T-DM1 as a single agent or in combination with pertuzumab in patients with first-line HER2-positive MBC. A total of 1095 patients with HER2-positive advanced breast cancer (unresectable, progressive or recurrent locally advanced, or previously untreated MBC) and no prior therapy for advanced disease were randomized to one of three treatment arms: trastuzumab plus taxane (control arm), T-DM1 plus placebo (hereafter referred to as T-DM1), or T-DM1 plus pertuzumab; all patients were treated until disease progression or unacceptable toxicity. While treatment with T-DM1 met criteria for non-inferiority of progression-free survival, it did not meet the superiority endpoint; the addition of pertuzumab did not provide any additional efficacy benefit versus T-DM1 alone [10].
The MARIANNE study provided an opportunity to evaluate the pharmacokinetic interactions and drug–drug interactions (DDI) of T-DM1 in combination with pertuzumab in the first-line MBC setting. Both pertuzumab and the trastuzumab component of T-DM1 bind to the HER2 receptor and can modulate HER2 biology [1,2,3], thereby creating the potential for DDIs. In addition to assessing pharmacokinetics and DDIs associated with combination T-DM1 plus pertuzumab, data from MARIANNE allowed us to evaluate pharmacokinetics of T-DM1 in the first-line setting (previously untreated MBC patients). In the setting of previously treated HER2-positive MBC, longer PFS and OS were observed when T-DM1 was administered as second-line therapy (versus administration in the third-line, fourth-line, or greater line setting), and PFS and OS decreased numerically with increasing numbers of prior treatment lines [11]. Similar findings have been observed with first-line versus later line use of chemotherapy in patients with MBC [12, 13]. The decreasing benefit with later lines of treatment is consistent with the natural evolution of the disease, and patients treated in later lines are likely to have more compromised health status and higher disease severity. In the analysis reported here, we compared the pharmacokinetic characteristics of T-DM1 and pertuzumab in the MARIANNE patient population with historical data [14,15,16]. The pharmacokinetics of both T-DM1 and pertuzumab has been characterized previously in phase I, II, and III studies through extensive sampling and population pharmacokinetic analysis [14,15,16]. In the historical analyses, pharmacokinetics of both drugs was described by linear two-compartmental models in the clinical dose range, and the effects of statistically significant covariates were identified. For T-DM1, the model was developed based on 671 MBC patients in five phase I to phase III studies [16]. For pertuzumab, the model was developed based on 481 cancer patients (including MBC patients) in 12 phase I to phase III studies [14].
In the current analysis, the historical models for T-DM1 and pertuzumab [8, 10] were used to derive the empirical Bayes estimates (EBEs) of pharmacokinetic parameters of the T-DM1 conjugate and pertuzumab in the MARIANNE study population. The observed data were compared with model expectations to assess whether pharmacokinetics in the first-line treatment of HER2-positive recurrent or locally advanced or MBC was similar to other patient populations in the historical models. The potential pharmacokinetic DDI risks between T-DM1 and pertuzumab in this patient population were assessed by within-study and cross-study comparisons. Observed pharmacokinetic exposures for all analytes (T-DM1 conjugate, pertuzumab, total trastuzumab, DM1) were also summarized. An additional objective of the pharmacokinetic assessment from the MARIANNE study was to provide exposure parameters for exposure–efficacy and exposure–safety correlation analysis, which will be reported separately. All of these assessments are important to support a potential label claim for the use of T-DM1 and/or T-DM1 + pertuzumab in the first-line treatment of MBC based on this pivotal phase III study, although the filing was not pursued due to the negative trial outcome [10].
Materials and methods
Patients, pharmacokinetic sampling, and bioanalysis
The MARIANNE patient population and study design have been reported previously [10]. The study was approved by relevant independent ethics committees or institutional review boards at each site and conducted in accordance with the 1964 Declaration of Helsinki. All patients provided written informed consent. Only sparse pharmacokinetic sampling of T-DM1 and pertuzumab was obtained in MARIANNE, from a subset of patients (approximately 50% of intent-to-treat population). The pharmacokinetic sampling schedule for both T-DM1 and pertuzumab was pre-dose and 15–30 min after the end of infusions at Cycles 1, 2, and 4. For T-DM1, two additional pharmacokinetic sampling timepoints, at 24–72 h after the end of infusions at Cycles 1 and 4, were also included.
For T-DM1, multiple analytes including T-DM1 conjugate, total trastuzumab, and DM1 were quantified. Concentrations of T-DM1 conjugate and total trastuzumab were determined from serum samples using validated enzyme-linked immunosorbent assays (ELISAs) at Pharmaceutical Product Development, LLC (Richmond, Virginia, USA). The T-DM1 conjugate assay measured all conjugated trastuzumab containing one or more covalently bound DM1 molecules, while excluding unconjugated trastuzumab. The total trastuzumab assay quantified all forms of conjugated and fully unconjugated trastuzumab. Both assays have a minimum quantifiable concentration of 0.06 µg/mL. To avoid cross-reaction between trastuzumab and pertuzumab, pertuzumab pharmacokinetics were measured by a validated ELISA using a monoclonal antibody specific for the complementarity determining region of pertuzumab as the coating agent, with a minimum quantifiable concentration of 0.15 µg/mL. A validated liquid chromatography–tandem mass spectrometry assay method was used to quantify DM1 concentration in human plasma samples, and performed by QPS Netherlands B.V. (formerly Xendo Drug Development B.V.). The lower limit of quantification was 0.738 ng/mL.
Post hoc analysis based on historical population pharmacokinetic models for T-DM1 conjugate and pertuzumab
Population pharmacokinetic analysis was performed using nonlinear mixed-effects modeling and the first-order conditional estimation method, with interaction. Model evaluation was implemented with NONMEM 7 (version 7.2; ICON Development Solutions, Hanover, MD).
EBEs of pharmacokinetic parameters for T-DM1 conjugate and pertuzumab were obtained based on existing population pharmacokinetic models for T-DM1 [16] and pertuzumab [14]. The historical population pharmacokinetic model for T-DM1 included the relationships with the following baseline covariates on clearance and central volume of distribution: clearance was impacted by body weight, albumin level, baseline sum of longest dimension of target lesions (TMBD), baseline trastuzumab concentration, aspartate aminotransferase level, and HER2 extracellular domain (ECD) concentrations; central volume of distribution was impacted by body weight. For the MARIANNE study, ECD levels were not collected. For baseline TMBD, the historical study used Response Evaluation Criteria In Solid Tumors (RECIST) version 1.0 while the current study used RECIST version 1.1. Importantly, TMBD values are quite different for the two versions of RECIST [17]. For RECIST version 1.0, the number of target lesions is up to five per organ and up to ten in total. For RECIST version 1.1, the number of target lesions is up to two per organ and up to five in total. Therefore, the impact of TMBD and ECD on clearance was not estimated in the MARIANNE study, and median values from the historical model for ECD and TMBD were used in this analysis. For pertuzumab, the historical model suggested that clearance was dependent on lean body weight and albumin level; central and peripheral volume of distribution was dependent on lean body weight.
Standard diagnostics were used to assess goodness-of-fit and predictive checks (visual predictive check [VPC] and numerical predictive check [NPC] [18]) were used to assess the predictive performance of the historical models. For VPC, 500 Monte Carlo simulation replicates of the original data set considering inter-individual and residual variability were generated and summarized; the observed data were overlaid on the VPC plot. For NPC, the percentages of observed data below and above pre-specified quantiles (5th and 95th, 25th and 75th, and 50th [median] percentiles, respectively) of the simulated data were summarized as an additional diagnostic of the model predictive performance. These comparisons were made for all observations combined and separately at each nominal collection time.
For comparison with historical pharmacokinetic data, individual post hoc parameter estimates were used to derive metrics of exposures, including the trough concentrations at the exact nominal time of 504 h (end of Cycle 1, 21 days post-first dose) after the first dose (Ctrough), the maximum concentration at the end of infusion of the first dose (Cmax), and area under the concentration–time curve from time zero to infinity after the first dose (AUCinf). The individual AUCinf was calculated using the nominal dose (3.6 mg/kg multiplied by individual body weight for T-DM1; and 840 mg loading dose for pertuzumab) divided by individual EBE clearance.
To make comparisons with historical data, similar estimates were derived for two phase III T-DM1 studies, EMILIA (NCT00829166) [5] and TH3RESA (NCT01419197) [4] and for two phase III pertuzumab studies, CLEOPATRA (NCT00567190) [19] and NeoSphere (NCT00545688) [20]. The EMILIA study compared single-agent T-DM1 with capecitabine plus lapatinib in patients with HER2-positive locally advanced or metastatic breast cancer that was previously treated with trastuzumab and a taxane [5]. In TH3RESA, single-agent T-DM1 was compared with treatment of physician’s choice in patients with HER2-positive locally advanced breast cancer or MBC that was previously treated with two or more HER2-directed regimens [4]. CLEOPATRA compared first-line treatment with pertuzumab, trastuzumab and docetaxel with placebo, trastuzumab and docetaxel in patients with HER2-positive MBC [19]. NeoSphere compared four neoadjuvant treatment regimens (trastuzumab plus docetaxel, pertuzumab and trastuzumab plus docetaxel, pertuzumab and trastuzumab, or pertuzumab plus docetaxel) [20].
Exposure metrics of T-DM1 conjugate were also compared between the T-DM1 and T-DM1 plus pertuzumab arms within the MARIANNE study, to assess the drug interaction potential between pertuzumab and T-DM1 conjugate. As the Ctrough is the pharmacokinetic parameter used for exposure–response analysis (reported separately), and is highly correlated with AUC considering only “every 3 week” dosing was tested in the MARIANNE study, we have chosen to report Ctrough in this paper.
For the population pharmacokinetic modeling of T-DM1 conjugate or pertuzumab, pharmacokinetic observations associated with an absolute value of the conditional weighted residual (CWRES) greater than 6 (i.e., |CWRES| > 6) were removed as outliers. Concentrations that were below the minimum quantifiable concentration or lower limit of quantification were imputed at half of the respective analyte’s lower limit for these assessments. Pharmacokinetic results were summarized and tabulated for evaluations across treatment groups (T-DM1 plus pertuzumab and T-DM1).
Summary of observed concentrations for T-DM1-related analytes: total trastuzumab and DM1
Observed data for all analytes (T-DM1 conjugate, total trastuzumab, DM1, pertuzumab) were summarized by each nominal timepoint. Since T-DM1 conjugate and pertuzumab pharmacokinetics were also analyzed by the population pharmacokinetic approach, only the observed concentrations for total trastuzumab and DM1 are presented here. The data were also compared between the T-DM1 and T-DM1 plus pertuzumab arms.
Results
Population pharmacokinetic analysis of T-DM1 conjugate in the MARIANNE study
The T-DM1 population pharmacokinetic dataset comprised 385 patients, which included 194 of 363 patients randomly assigned to the T-DM1 plus pertuzumab group (i.e., 53% of the intent-to-treat population), and 191 of 367 patients randomly assigned to the T-DM1 group (52% of the intent-to-treat population). This dataset contributed 2713 serum T-DM1 concentration records and 8592 T-DM1 dosing event records. Data exclusion rules (e.g., concentration records with negative/missing sample time, concentration records at unscheduled visit, duplicated concentration records at the same time) were applied to identify data records to be removed from the analysis. A total of 371 (4.3%) T-DM1 dosing and 253 (9.3%) T-DM1 concentration data records were flagged for exclusion; these records were associated with at least one exclusion rule. For ten patients (T-DM1, n = 4; T-DM1 plus pertuzumab, n = 6), exclusion of these dosing and pharmacokinetic records resulted in no remaining evaluable T-DM1 pharmacokinetic data, and these patients were excluded from the analysis.
For the individual EBE analysis based on the T-DM1 historical model [16], 26 pharmacokinetic observations were excluded following the initial evaluation for outliers (|CWRES| > 6). The diagnostic plots revealed general goodness-of-fit. Ctrough and Cmax observations were well characterized by the individual EBE estimates (Supplemental Fig. 1).
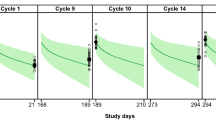
A VPC was used to evaluate the predictive performance of the existing model with respect to data from MARIANNE. In general, the model predicted the central tendency and variance of observed data well, although the Cycle 1 Ctrough was slightly over-predicted. There was no such notable bias, however, for the Cmax, 24 to 72 h post-infusion, or Cycle 3 Ctrough samples (Fig. 1a).
Visual predictive check for all T-DM1 observations. b Visual predictive check for all pertuzumab observations. Dashed line and gray shaded area represent simulated median and 90th percentiles using MARIANNE. Pink shaded area represents 95% CI of the simulated median. Circles and error bars represent median and 90th percentile of observations. Observations with nominal times at 504 and 1512 h (Ctrough); at 2, 506, and 1514 h (Cmax); and at 24–72 h post-first dose were split into 24, 48, 72 h and with nominal times at 1536 h post-first dose split at 1536, 1560, and 1584 h, based on closest actual time. *Observations with nominal times at 504 and 1512 h (Ctrough); at 2, 506, and 1514 h (Cmax); and at 24–72 h (24–72 h) post-first dose were split into 24, 48, 72 h, and with nominal times at 1536 h post-first dose split into 1536, 1560, and 1584 h, based on closest actual time. CI confidence interval, Cmax maximum concentration at the end of infusion of the first dose, Ctrough trough concentrations at the exact nominal time of 504 h after the first dose, T-DM1 trastuzumab emtansine
Comparison between the observed T-DM1 concentrations and the simulations was further investigated using an NPC. For all observations combined, both quartiles (25th and 75th percentiles) and median values of the observations were all within the range of ± 10% of the simulated values and the percentage of observations falling above or below these percentiles was near the nominal values. Even at the extremes of the 5th and 95th percentiles, the observations were within the range of ± 20% of the simulated values (Table 1).
The same general agreement between observed and simulated values held when the T-DM1 concentration data were evaluated for each nominal collection timepoint, with the exception of Cycle 1 Ctrough. Consistent with the VPC results, the NPC indicated a bias (model over-prediction) for the Cycle 1 Ctrough. For this timepoint, 75% of the observations were below the predicted median, nearly 50% of the observations were below the predicted 25th percentile concentration, and less than 5% of the observations were above the predicted 75th percentile concentration.
Population pharmacokinetic analysis of pertuzumab in the MARIANNE study
The pertuzumab population pharmacokinetics data set comprised 194 patients treated with T-DM1 plus pertuzumab contributing 1032 serum pertuzumab concentration records and 4707 pertuzumab dosing event records. Of these, 169 pertuzumab dosing and 232 pertuzumab concentration data records were flagged for exclusion, which resulted in the exclusion of six patients for whom no evaluable pertuzumab pharmacokinetics data remained. In addition, eight pharmacokinetic observations were identified during the outlier evaluation (|CWRES| > 6) and excluded. The goodness-of-fit plots revealed general goodness-of-fit (Supplemental Fig. 2). The VPC suggested that the model predicted the central tendency and variability of the observed data well (Fig. 1b). Comparison of the observed pertuzumab concentrations with the simulations was further investigated by NPC. For all observations combined, similar findings to those observed for T-DM1 conjugate were obtained (Table 1). The same general agreement between observed and simulated values held when the pertuzumab concentration data were evaluated for each nominal collection timepoint for Cmax, whereas the Ctrough concentrations were slightly over-predicted by the historical model.
Comparison of pharmacokinetic exposures simulated by individual EBEs of pharmacokinetic parameter estimates with historical data and within the MARIANNE study
T-DM1 conjugate: comparison with historical data
The Cycle 1 Cmax, Ctrough, and AUCinf simulated for T-DM1 conjugate in the MARIANNE study were generally consistent with those in the TH3RESA [21] and EMILIA studies [16]. For a total of 375 patients in the MARIANNE study, the T-DM1 conjugate Cmax was 74.4 ± 10.1 µg/mL, Ctrough was 1.34 ± 0.802 µg/mL, and AUCinf was 338 ± 69.5 µg*day/mL. For the 351 patients in TH3RESA study, the Cycle 1 Cmax was 76.3 ± 11.3 µg/mL, Ctrough was 1.66 ± 0.94 µg/mL, and AUCinf was 357 ± 62.8 µg*day/mL [21]. The pharmacokinetic exposures from the EMILIA study were within a similar range (Fig. 2). The pharmacokinetic exposure across these studies was considered to be within the inter-study variability, suggesting similar T-DM1 pharmacokinetics across different lines of treatment.
T-DM1 Cycle 1 exposures (aCmax, b AUCinf, c and Ctrough) simulated by empirical Bayes estimates of pharmacokinetic parameters: cross-study comparison of the MARIANNE study with historical studies EMILIA and TH3RESA. Predicted values are represented as circles. In the box and whisker plot, median values are designated by a black line within the box. Boxes indicate the inter-quartile range (IQR). Whiskers represent the observed data out to a maximum of ± 1.5*IQR. AUCinf area under the concentration–time curve from time zero to infinity after the first dose, Cmax maximum concentration at the end of infusion of the first dose, Ctrough trough concentrations at the exact nominal time of 504 h after the first dose, T-DM1 trastuzumab emtansine
Assessment of DDI potential: comparison of T-DM1 conjugate pharmacokinetics in the presence and absence of pertuzumab within the MARIANNE study
After the first dose of T-DM1, estimated by post hoc pharmacokinetic parameters for 186 patients in the T-DM1 arm, the mean T-DM1 conjugate Cmax was 74.4 ± 8.78 µg/mL, AUCinf was 336 ± 67.7 µg*day/mL, and Ctrough was 1.36 ± 0.832 μg/mL. For 189 patients in the T-DM1 plus pertuzumab arm, the mean T-DM1 conjugate Cmax was 74.4 ± 11.3 µg/mL, AUCinf was 339 ± 71.3 µg*day/mL, and Ctrough was 1.33 ± 0.773 μg/mL. These results suggested similar pharmacokinetics in the presence and absence of pertuzumab in this patient population (Fig. 3). This was consistent with the results published previously on the drug interaction potential of T-DM1 and pertuzumab [22], confirming a low potential for pertuzumab to impact T-DM1 pharmacokinetics when administered in combination to MBC patients.
T-DM1 empirical Bayes estimates of pharmacokinetic exposures (Cmax, AUCinf, and Ctrough): comparison between the T-DM1 and T-DM1 + pertuzumab arms for assessment of the impact of pertuzumab on T-DM1 pharmacokinetics. T-DM1 population pharmacokinetic model-predictions by treatment group: Cycle 1 aCmax, b AUCinf, and cCtrough. Predicted values are represented as circles. In the box and whisker plot, median values are designated by a black line within the box. Boxes indicate the inter-quartile range (IQR). Whiskers represent the observed data out to a maximum of ± 1.5*IQR. AUCinf area under the concentration–time curve from time zero to infinity after the first dose, CI confidence interval, Cmax maximum concentration at the end of infusion of the first dose, Ctrough trough concentrations at the exact nominal time of 504 h after the first dose, DDI drug–drug interaction, T-DM1 trastuzumab emtansine
Pertuzumab pharmacokinetic exposures in MARIANNE study: comparison with historical data
After the first dose of pertuzumab, estimated by post hoc pharmacokinetic parameters for 188 patients in the pertuzumab plus T-DM1 arm who had pertuzumab pharmacokinetics data available, the mean pertuzumab Cmax was 276 ± 50.0 µg/mL, AUCinf was 4470 ± 1360 µg*day/mL, and Ctrough was 64.8 ± 17.9 μg/mL. For 138 patients in the NeoSphere study, a combination of pertuzumab and trastuzumab was given, and the resulting mean pertuzumab Cmax was 283 ± 35.9 µg/mL, AUCinf was 4290 ± 1280 µg*day/mL, and Ctrough was 62.9 ± 17.9 μg/mL. Pertuzumab exposures were also similar to those observed for 20 patients in the CLEOPATRA study (Fig. 4). Overall, pertuzumab exposure was comparable across three separate studies: MARIANNE, NeoSphere and CLEOPATRA. These results suggest a low interaction potential for T-DM1 and pertuzumab when administered in combination, and pertuzumab PK is consistent across different stages of diseases (HER2-positive MBC versus early breast cancer).
Pertuzumab Cycle 1 exposures (aCmax, b AUCinf, c and Ctrough) simulated by empirical Bayes estimates of pharmacokinetic parameters: cross-study comparison of the MARIANNE study with historical studies CLEOPATRA and NeoSphere. Predicted values are represented as circles. In the box and whisker plot, median values are designated by a black line within the box. Boxes indicate the inter-quartile range (IQR). Whiskers represent the observed data out to a maximum of ± 1.5*IQR. AUCinf area under the concentration–time curve from time zero to infinity after the first dose, Cmax maximum concentration at the end of infusion of the first dose, Ctrough trough concentrations at the exact nominal time of 504 h after the first dose, T-DM1 trastuzumab emtansine
Summary of observed pharmacokinetic concentrations for total trastuzumab and DM1: comparison within MARIANNE study
For the other two T-DM1-related analytes (total trastuzumab and DM1), summaries of observed concentrations are shown in Supplemental Tables 1 and 2. Notably, nearly all Ctrough observations for DM1, at both Cycles 1 and 3 (i.e., Cycle 2 and Cycle 4 pre-dose), were below the lower limit of quantification.
Furthermore, for comparison between T-DM1 arm and T-DM1 plus pertuzumab arm, the geometric mean ratios for each analyte and at each collection timepoint (Cycle 1 Cmax and Ctrough [i.e., Cycle 2 pre-dose], Cycle 3 Ctrough [i.e., Cycle 4 pre-dose] and Cycle 4 Cmax) were near 1, and 95% confidence intervals included 1, indicating comparable exposures of total trastuzumab and DM1 in the presence and absence of pertuzumab.
Discussion
This analysis confirms that the historical MBC population pharmacokinetic models for T-DM1 conjugate and pertuzumab describe the pharmacokinetics of both T-DM1 and pertuzumab in the MARIANNE study population well. In addition, no DDIs were identified for the combined use of T-DM1 and pertuzumab. This analysis suggests that line of therapy may not largely impact T-DM1 conjugate pharmacokinetics, based on the comparison between T-DM1 pharmacokinetics in first-line patients (MARIANNE study) versus later line patients (EMILIA and TH3RESA studies).
When treated in later lines of therapy, patients may be expected to have more compromised health status and higher disease severity due to more advanced disease or prior therapies, and this could result in different pharmacokinetics (e.g., systemic clearance) for monoclonal antibodies, compared with those observed in patients treated in a first-line setting [23, 24]. The analysis of T-DM1 conjugate pharmacokinetics was based on the historical MBC population pharmacokinetic model for T-DM1 that was used to inform the prescribing information for T-DM1 in patients with HER2-positive MBC, who previously received trastuzumab and a taxane, separately or in combination. Although there was a slight trend in the MARIANNE study for lower median exposure for T-DM1 conjugate, the exposure ranges are largely overlapping with each other (Fig. 2). Thus, this magnitude of difference is not considered to be clinically relevant given the high variability in exposures.
Our findings for the cross-study pharmacokinetic comparison of T-DM1 are consistent with a prior population pharmacokinetic analysis of data from five phase I to phase III studies of patients with HER2-positive MBC (n = 671) [16]. This historical population pharmacokinetic analysis suggested that T-DM1 pharmacokinetic properties are similar across lines of therapy, based on the finding that the covariate of “prior systemic therapy in the locally advanced/metastatic setting” (yes/no, with 103 of 671 patients without prior systemic therapy in the locally advanced/metastatic setting) did not appear to affect clearance or volume of distribution in the central compartment (Vc).
Clinically relevant covariates identified from the previous population pharmacokinetic modeling were included in the current analysis [16]. As summarized in Supplemental Table 3, the values of baseline covariates were compared between the EMILIA, TH3RESA, and MARIANNE studies. Based on this comparison (Supplemental Table 3), the covariate “baseline trastuzumab concentrations” had a much lower value in the MARIANNE study (mean = 0.817 μg/mL) compared with EMILIA (mean = 10.8 μg/mL) and TH3RESA (mean = 5.46 μg/mL), as these patients were previously untreated for their metastatic disease. Indeed, based on baseline characteristics data, only 31.5% of patients (113 of 367 patients in the T-DM1 arm and 117 of 363 patients in the T-DM1 plus pertuzumab arm) had prior treatment with HER2-directed therapy in the MARIANNE study (trastuzumab or lapatinib in the neoadjuvant or adjuvant setting) [10], compared with 100% of patients who received prior trastuzumab in the EMILIA and TH3RESA studies (84.2% [417 of 495] of patients in EMILIA received prior trastuzumab for MBC or early stage breast cancer and 15.8% [78 of 495] received prior trastuzumab for early stage breast cancer only; all patients in the TH3RESA study received prior trastuzumab for advanced breast cancer) [4, 5]. Therefore, this covariate difference might explain the slight trend of lower exposure of T-DM1 conjugate observed in the MARIANNE study. It is worth mentioning that among the statistically significant covariates identified by the historical population pharmacokinetic model, two covariates were either not available or not adequate from the MARIANNE study for inclusion in the covariate analysis using the historical model: TMBD and ECD. First, tumor burden is a covariate used in the historical population pharmacokinetic model [16]; patients with high tumor burden have faster clearance and lower exposure. However, tumor burden was calculated differently for MARIANNE versus EMILIA and TH3RESA: the MARIANNE study used RECIST 1.1, while the EMILIA and TH3RESA studies used RECIST 1.0. For this reason, tumor burden could not be included in the population pharmacokinetic analysis of the MARIANNE study. Second, high HER2 gene amplification was not assessed as a covariate in the historical model [16]. Two other related covariates were assessed: baseline serum HER2 ECD concentration and HER2-positive centrally confirmed status (yes/no) [16]. HER2 ECD was identified as a statistically significant covariate; however, in the MARIANNE and TH3RESA studies, HER2 ECD concentrations were not collected at baseline [21, (Genentech data on file)]. Regardless, the pharmacokinetic difference of < 20% based on the mean value for Ctrough when compared with the EMILIA and TH3RESA studies is considered within the inter-patient variability.
For T-DM1, the Cycle 1 Ctrough appears slightly over-predicted by the historical model compared with the observed data for all samples (with or without pertuzumab), suggesting a potential existence of slight time-dependent clearance in which clearance decreases slightly over time. As a result, the Cycle 1 clearance might be slightly higher than the steady-state clearance. For the historical model, time-varying covariates were not used, as the model diagnostic plots did not suggest the existence of an apparent bias of residual versus time [16]. It is likely that the magnitude of this effect is small and may only be limited to Cycle 1. Hypothetically, the overall disease severity and health status for most patients who respond to therapy potentially improved over time and after repeated T-DM1 infusions (because of a decrease in TMBD following treatment). Based on non-compartmental analysis results from the historical data, T-DM1 pharmacokinetics did not appear to change over time (Genentech data on file), as similar pharmacokinetic parameters were observed in Cycle 1 and after several doses of T-DM1, again confirming that this effect is relatively small due to the inter-cycle variability of pharmacokinetic parameters. The overall VPC and NPC suggested good agreement between observed MARIANNE data and the historical model. The model-estimated Cycle 1 Ctrough can be considered a reliable estimate of trough concentration predicted by the patient clearance across the time period with pharmacokinetic data available, which is suitable for an exposure–response analysis (reported separately).
For pertuzumab pharmacokinetics, this analysis was based on the historical population pharmacokinetic model for pertuzumab that is based on pharmacokinetic data across clinical trials in a variety of solid tumors, including HER2-positive MBC, HER2-positive early breast cancer, non-small cell lung cancer, ovarian cancer, and prostate cancer. As shown in Fig. 4 by comparing to other HER2-positive MBC and early breast cancer studies, the ranges of exposure are highly overlapping between studies, with some slight differences in the median values. The magnitude of these differences is not considered to be clinically relevant, which is within the variability of the exposures.
There are some special considerations for the DDI assessment of an antibody–drug conjugate, given that it is a unique class of therapeutic proteins with small and large molecular components. In the in vivo setting, antibody–drug conjugates are catabolized into multiple clinically relevant analytes, which have distinct pharmacokinetic properties. This increases the complexity of an assessment of DDIs associated with antibody–drug conjugates. Based on the theoretical catabolism, metabolism and excretion pathways for antibody–drug conjugates, the combination drugs that affect these pathways, such as target-mediated disposition of a monoclonal antibody, cytochrome P450 mediated clearance of the small molecular component, may introduce DDI risk when given in combination with an ADC [25].
Although T-DM1 and pertuzumab both bind to HER2 receptors, when considering concomitant use it is important to note that they bind to distinct HER2 sub-domains without steric hindrance [22, 25,26,27]. Furthermore, the observation that both T-DM1 and pertuzumab exhibit linear pharmacokinetics at their clinically relevant doses suggests that HER2-mediated clearance plays a minor role in total clearance [22]. Similar to pertuzumab and other monoclonal antibodies, T-DM1 conjugate is mainly cleared by nonspecific proteolytic degradation. While this nonspecific pathway is attributed to the trastuzumab component of T-DM1 and overlaps with the pertuzumab clearance pathway, the DDI risk between T-DM1 and pertuzumab is considered low because this is a high-capacity pathway that is typically not saturable at therapeutic concentration ranges. Clearance pathways for the DM1 component of T-DM1 do not overlap with those of pertuzumab. Additionally, pertuzumab is not a cytokine inhibitor or stimulator. Therefore, pertuzumab is not expected to affect the activity of cytochrome P450. Altogether, the theoretical risk assessment of the drug interaction potential of concomitant T-DM1 and pertuzumab suggests a relatively low risk of drug interactions between T-DM1 and pertuzumab [22].
Consistent with this theoretical DDI risk assessment, we did not identify any DDIs for the combined use of T-DM1 and pertuzumab. The drug interaction potential of T-DM1 plus pertuzumab was also assessed in a phase Ib/II trial of 67 patients with HER2-positive, locally advanced or MBC [22], 21 of whom received treatment in the first-line MBC setting at the recommended dose of T-DM1 (3.6 mg/kg) [22]. This study showed that the pharmacokinetics of T-DM1-related analytes and pertuzumab were similar to historical studies, and the risk of drug interactions is low for this combination. The analyses presented in this paper confirmed a low risk for therapeutic protein–drug interactions associated with this combination.
Of note, the assessment of T-DM1 and pertuzumab pharmacokinetics in the MARIANNE patient population utilized a post hoc analysis instead of updating the historical population pharmacokinetic models that are available for T-DM1 and pertuzumab. This was adequate based on the prior population pharmacokinetic analysis, which showed that T-DM1 pharmacokinetic properties are similar across lines of therapy in breast cancer patients based on the historical population pharmacokinetic analysis [16], and that pertuzumab pharmacokinetic properties were similar across several solid tumor types (e.g., ovarian cancer, prostate cancer, lung cancer), and different lines of breast cancer [14].
Shrinkage of pharmacokinetic parameter estimates was possible although the EBE estimates described the observed pharmacokinetic data for T-DM1 and pertuzumab well, given that relatively sparse pharmacokinetic sampling was obtained from the MARIANNE study [28]. For each inter-individual variability parameter (ETA), the population distribution of the individual empirical Bayes estimates was compared with the distribution of the assumed normal variances (OMEGA values) from the historical model (Supplemental Fig. 3), to visualize the shrinkage for each ETA graphically. For T-DM1, considerable ETA shrinkage was associated with Vc (47.4%), volume of distribution in the peripheral compartment (Vp) (37.0%), and intercompartmental clearance (Q) (68.8%), whereas the ETA shrinkage for clearance (12.6%) was much less severe. Therefore, it was reasonable to consider the EBE of Ctrough, which is heavily influenced by the EBE of clearance, as a reliable exposure metrics for cross-study pharmacokinetic comparison and exposure–response analysis. For pertuzumab, the shrinkage for Vp was relatively considerable (44.1%), while for clearance and Vc the shrinkage was less severe: 16.6% and 17.8%, respectively. Therefore, the EBEs of Ctrough are considered reliable for pertuzumab. In addition, in general, the shrinkage for model predictions should be less around points of the pharmacokinetic curve where there are observed data such as Ctrough or Cmax.
In conclusion, in this analysis, T-DM1 pharmacokinetic properties were found to be consistent in patients with HER2-positive MBC across different lines (first-line setting versus later-line settings) [16], and pertuzumab pharmacokinetic properties were consistent in patients with HER2-positive MBC at different stages (previously untreated MBC versus early breast cancer in the neoadjuvant setting). The covariate relationships estimated previously appropriately described inter-individual variability in pharmacokinetic parameters in the current patient population. Based on EBE analysis using historical population pharmacokinetic models and MARIANNE covariate values, the pharmacokinetic exposures (AUCinf, Ctrough and Cmax) for T-DM1 conjugate and pertuzumab in the MARIANNE study were generally comparable with historical studies and within inter-study variability. The analysis also suggests that there are no apparent pharmacokinetic DDIs when T-DM1 and pertuzumab are used in combination, which is consistent with the conclusions made in a previous publication in MBC patients [22]. The T-DM1 conjugate pharmacokinetic exposures were similar between the T-DM1 arm and the T-DM1 plus pertuzumab arm.
References
Junttila TT, Li G, Parsons K, Phillips GL, Sliwkowski MX (2011) Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat 128(2):347–356. https://doi.org/10.1007/s10549-010-1090-x
Lewis Phillips GD, Li G, Dugger DL et al (2008) Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res 68(22):9280–9290. https://doi.org/10.1158/0008-5472.CAN-08-1776
Barok M, Tanner M, Koninki K, Isola J (2011) Trastuzumab-DM1 is highly effective in preclinical models of HER2-positive gastric cancer. Cancer Lett 306(2):171–179. https://doi.org/10.1016/j.canlet.2011.03.002
Krop IE, Kim SB, González-Martín A et al (2014) Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol 15(7):689–699. https://doi.org/10.1016/S1470-2045(14)70178-0
Verma S, Miles D, Gianni L et al (2012) Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367(19):1783–1791. https://doi.org/10.1056/NEJMoa1209124
Genentech, Inc., South San Francisco, CA (2016) KADCYLA® (ado-trastuzumab emtansine) for injection [package insert]
Baselga J, Swain SM (2009) Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer 9:463–475
Scheuer W, Friess T, Burtscher H et al (2009) Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res 69:9330–9336
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Version 4.2018. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site. Accessed Feb 14 2019
Perez EA, Barrios C, Eiermann W et al (2017) Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: primary results from the Phase III MARIANNE study. J Clin Oncol 35(2):141–148
Montemurro F, Ellis P, Anton A et al (2019) Safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive advanced breast cancer: primary results from the KAMILLA study cohort 1. Eur J Cancer 109:92–102
Bonotto M, Gerratana L, Iacono D, Minisini AM, Rihawi K, Fasola G, Puglisi F (2015) Treatment of metastatic breast cancer in a real-world scenario: is progression-free survival with first line predictive of benefit from second and later lines? Oncologist 20(7):719–724
Bakker JL, Wever K, van Waesberghe JH, Beeker A, Meijers-Heijboer H, Konings IR, Verheul HM (2015) What is the benefit of treatment with multiple lines of chemotherapy for patients with metastatic breast cancer? A retrospective cohort study. Cancer Epidemiol 39(6):848–853
Garg A, Quartino A, Li J et al (2014) Population pharmacokinetic and covariate analysis of pertuzumab, a HER2-targeted monoclonal antibody, and evaluation of a fixed, non-weight-based dose in patients with a variety of solid tumors. Cancer Chemother Pharmacol 74(4):819–829. https://doi.org/10.1007/s00280-014-2560-3
Girish S, Gupta M, Wang B et al (2012) Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody-drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother Pharmacol 69(5):1229–1240. https://doi.org/10.1007/s00280-011-1817-3
Lu D, Girish S, Gao Y et al (2014) Population pharmacokinetics of trastuzumab emtansine (T-DM1), a HER2-targeted antibody-drug conjugate, in patients with HER2-positive metastatic breast cancer: clinical implications of the effect of covariates. Cancer Chemother Pharmacol 74(2):399–410. https://doi.org/10.1007/s00280-014-2500-2
Kim JH, Min SJ, Jang HJ, Cho JW, Kim SH, Kim HS (2015) Comparison of RECIST 1.0 and RECIST 1.1 in patients with metastatic cancer: a pooled analysis. J Cancer 6(4):387–393
Brendel K, Comets E, Laffont C, Mentré F (2010) Evaluation of different tests based on observations for external model evaluation of population analyses. J Pharmacokinet Pharmacodyn 37(1):49–65
Swain SM, Kim S-B, Cortés J et al (2013) Overall survival benefit with pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer in CLEOPATRA, a randomised Phase 3 study. Lancet Oncol 14(6):461–471. https://doi.org/10.1016/S1470-2045(13)70130-X
Gianni L, Pienkowski T, Im YH et al (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13(1):25–32. https://doi.org/10.1016/S1470-2045(11)70336-9
Chen S-C, Quartino A, Polhamus D et al (2017) Population pharmacokinetics and exposure-response of trastuzumab emtansine in advanced breast cancer previously treated with ≥ 2 HER2-targeted regimens. Br J Clin Pharmacol 83(12):2767–2777
Lu D, Burris HA 3rd, Wang B et al (2012) Drug interaction potential of trastuzumab emtansine (T-DM1) combined with pertuzumab in patients with HER2-positive metastatic breast cancer. Curr Drug Metab 13(7):911–922
Ryman JT, Meibohm B (2017) Pharmacokinetics of monoclonal antibodies. CPT Pharmacomet Syst Pharmacol 6(9):576–588. https://doi.org/10.1002/psp4.12224
Mould DR (2015) The pharmacokinetics of biologics: a primer. Dig Dis 33(Suppl 1):61–69
Lu D, Sahasranaman S, Zhang Y, Girish S (2013) Strategies to address drug interaction potential for antibody-drug conjugates in clinical development. Bioanalysis 5(9):1115–1130
Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M (2009) Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res 69(24):9330–9336
Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX (2004) Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 5(4):317–328
Karlsson MO, Savic RM (2007) Diagnosing model diagnostics. Clin Pharmacol Ther 82(1):17–20
Acknowledgements
The authors acknowledge the investigators, patients, and their families who participated in these clinical trials. The authors are grateful for the assistance of Jia Kang, PhD, from Metrum Research Group, for her assistance in reviewing the data presented in this manuscript.
Funding
This study was funded by Genentech, Inc. Support for third-party writing assistance for this manuscript was provided by Genentech, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DL, CL, PA, S-CC, AQ, JYJ, and SG are employees of Genentech and own stock in F. Hoffmann-La Roche; MP and AS are employees of and own stock in F. Hoffmann-La Roche; MR, DP, and JF are salaried employees of Metrum Research Group, which was contracted by Genentech, Inc.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lu, D., Li, C., Riggs, M. et al. Pharmacokinetics of trastuzumab emtansine (T-DM1) as a single agent or in combination with pertuzumab in HER2-positive breast cancer patients with recurrent or locally advanced metastatic breast cancer. Cancer Chemother Pharmacol 84, 175–185 (2019). https://doi.org/10.1007/s00280-019-03852-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03852-z