Abstract
Backgrounds
Many patients with gastric cancer relapse during or early after adjuvant chemotherapy. The standard treatment for early relapse patients is a second-line chemotherapy (SLC) based on irinotecan, taxanes, or a platinum-based chemotherapy. The platinum-containing biweekly irinotecan plus cisplatin (IRI/CDDP) combination was assumed to be promising in several reports of clinical trials as SLC. TRICS trial, a randomized phase III study of IRI/CDDP vs. IRI in platinum-naïve gastric cancers refractory to S-1 monotherapy, revealed that both irinotecan-based chemotherapies were effective and well tolerated.
Methods
This study analyzed 108 patients in the TRICS trial who experienced early relapse. Patients receiving IRI/CDDP (IRI, 60 mg/m2; CDDP, 30 mg/m2, q2w) versus IRI (150 mg/m2, q2w) were compared regarding overall survival (OS), progression-free survival (PFS), overall response rate (ORR), and safety.
Results
The OS was 14.0 (95% confidence interval [CI]: 11.0–21.2) and 14.0 (95% CI: 10.7–16.5) months for IRI/CDDP and IRI, respectively (hazard ratio [HR]: 0.782; 95% CI: 0.515–1.188, P = 0.249). No significant differences were observed for PFS (5.0 vs. 4.5 months, respectively; HR: 0.802; 95% CI: 0.543–1.185, P = 0.268) or ORR (19.6% [95% CI: 9.4–33.9%] vs. 23.3% [95% CI: 11.8–38.6%], respectively). The incidence of grade 3–4 anemia was higher for IRI/CDDP than for IRI (20% vs. 0%, respectively; P = 0.0006).
Conclusion
Our study showed no significant survival differences between IRI/CDDP and IRI in platinum-naïve patients who relapsed during or within 6 months after S-1 adjuvant therapy; therefore, IRI may be a good option in this population.
Clinical trial information
UMIN 000002571.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
S-1 monotherapy has been considered the standard adjuvant treatment after curative gastrectomy in stage II or III gastric cancer patients in Japan [1]. However, many patients relapse during or early after adjuvant chemotherapy [2, 3]. Exploratory analysis of the ACTS-GC trial showed that 160 out of 515 patients experienced recurrence after adjuvant S-1 chemotherapy within 5 years after surgery, and 121 (75.6%) patients received chemotherapy after recurrence [3]. These findings indicate the need for effective treatment options for early relapse after S-1 adjuvant chemotherapy. It is common practice to administer second-line chemotherapy (SLC) not only to patients with advanced gastric cancer (AGC) after failure of first-line chemotherapy, but also to patients with gastric cancer that recurs during or within 6 months after completion of adjuvant chemotherapy [4,5,6].
Patients with early relapse after adjuvant therapy sometimes undergo SLC regimens with irinotecan or taxanes [4,5,6]. Also, the standard treatment for early relapse after adjuvant chemotherapy with fluoropyrimidines alone is considered to be platinum-based chemotherapies, such as XP, CapeOX, and FOLFOX [7,8,9]. However, there are few reports on the efficacy of platinum-based chemotherapy in this setting. As a result, it is not known whether to preferentially administer SLC regimens (irinotecan, taxanes) or platinum-based regimens in these cases. Treatment with irinotecan plus cisplatin may combine the strengths of both approaches.
Recently, the TRICS trial was conducted to compare the efficacy and safety of biweekly irinotecan (60 mg/m2) plus cisplatin (30 mg/m2) (IRI/CDDP) with biweekly irinotecan (150 mg/m2) (IRI) in platinum-naïve patients with AGC refractory to S-1 monotherapy [10]. Both irinotecan-based chemotherapies resulted in favorable long-term survival and were generally well tolerated. However, there was no significant difference in overall survival (OS) between IRI/CDDP and IRI monotherapy in patients with progressive AGC previously treated with S-1 monotherapy (median survival 13.9 vs. 12.7 months, respectively).
Therefore, in this exploratory subgroup study of the TRICS trial, we compared the effects of IRI/CDDP and IRI in platinum-naïve patients who developed recurrence during or early after completion of adjuvant therapy with S-1.
Patients and methods
Patients
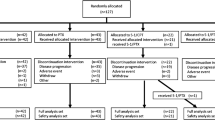
A total of 168 patients were enrolled in the TRICS trial between July 2007 and December 2011. The design, inclusion/exclusion criteria, treatment procedure, and other information concerning the TRICS trial have been previously reported [10]. 5 of the 168 patients refused to undergo treatment before the start of the assigned treatment. Of the remaining 163 patients, we analyzed 108 who had developed early relapse. Early relapse patients were defined as patients who developed recurrence during adjuvant chemotherapy or within 6 months after the completion of adjuvant chemotherapy. Patients with early recurrence after adjuvant therapy following R0 resection for Stage IV disease (n = 2) or R1 resection for positive peritoneal cytology (CY1) (n = 5) were included in this analysis.
The trial was conducted in accordance with the Declaration of Helsinki and registered with UMIN-CTR, UMIN 000002571. All patients provided written informed consent after having been informed about the purpose and investigational nature of the study. The institutional review boards or ethics committees of all participating centers reviewed and approved the protocol.
Study design and statistical methods
Comparisons of IRI/CDDP with IRI in early relapse groups were made based on the following efficacy endpoints: OS, progression-free survival (PFS), overall response rate (ORR), and adverse events. OS and PFS curves were constructed as time-to-event plots using the Kaplan–Meier method and were compared using the log-rank test, and hazard ratios (HRs) were estimated by Cox regression models. The ORR was analyzed in patients with at least one measurable lesion at baseline using Response Evaluation Criteria in Solid Tumors (RECIST 1.0), and was calculated as the best response in terms of complete response (CR) + partial response (PR) at any evaluation time. The disease control rate (DCR) was calculated as the best response in terms of CR + PR + stable disease (SD) at any evaluation time. We used Fisher’s exact test to compare the ORR and DCR between the two treatment groups, and a logistic regression model to assess the interaction between the ORR and DCR in both groups. The confidence coefficient for the confidence interval (CI) of the median OS, HR, ORR, and DCR was set to 95% (P < 0.05). Adverse events were evaluated using the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. All clinical data were held centrally at the data center of the Epidemiological & Clinical Research Information Network (ECRIN), a non-profit organization, and analyzed using SAS for Windows version 9.3 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
In total, 108 patients were considered eligible for this evaluation. The initial patient characteristics between the two arms were well matched (Table 1). 55 patients (51%) were allocated to the IRI/CDDP arm and 53 (49%) to the IRI arm.
Survival
The median OS was 14.0 (95% CI: 11.0–21.2) months in the IRI/CDDP arm and 14.0 (95% CI: 10.7–16.5) months in the IRI arm (Fig. 1). Thus, IRI/CDDP combination therapy did not significantly reduce the risk of death compared with IRI monotherapy (HR: 0.782; 95% CI: 0.515–1.188, P = 0.249). Similarly, no difference was observed in the median PFS (5.0 vs. 4.5 months, respectively; HR: 0.802; 95% CI: 0.543–1.185, P = 0.268; Fig. 2).
Response
Of the 55 patients in the IRI/CDDP group, 46 of whom had measurable disease, 3 achieved CR, 6 achieved PR, and 24 achieved SD. Of the 53 patients in IRI group, 43 of whom had measurable disease, 1 achieved CR, 9 achieved PR, and 19 achieved SD. The ORR was 19.6% (95% CI: 9.4–33.9) in the IRI/CDDP arm and 23.3% (95% CI: 11.8–38.6) in the IRI arm (Table 2). The DCR was 71.7% (95% CI: 56.5–84.0%) in the IRI/CDDP arm and 67.4% (95% CI: 51.5–80.9%) in the IRI arm (Table 2). No significant difference was observed between arms in either the ORR (P = 0.797) or the DCR (P = 0.818).
Adverse events
The proportions of patients who developed any type of toxicity are shown (Table 3). The incidence of grade 3–4 adverse events was low in both arms. No deaths resulted from toxicity. The IRI/CDDP arm showed significantly higher grade 3–4 anemia than the IRI arm (20% vs. 0%, respectively; P = 0.0006). Leukopenia and thrombocytopenia of any grade were more frequently observed in the IRI/CDDP arm, whereas diarrhea and increased levels of serum total bilirubin and serum C-reactive protein were more common in the IRI arm.
Compliance
The median number of courses was five (range, 1–31) in the IRI/CDDP arm and seven (range, 1–39) in the IRI arm (P = 0.183). Treatment delays occurred more frequently in the IRI/CDDP arm (35/55 [64%] vs. 23/53 [43%]; P = 0.0531). The most common reason for discontinuation was disease progression in both the IRI/CDDP and IRI arms (30/55 [55%] vs. 42/53 [79%], respectively; P = 0.0081).
Post-protocol therapy
Post-protocol therapy was administered in 72% (39/54) and 78% (40/51) of patients in the IRI/CDDP and IRI arms, respectively (P = 0.504). There was no significant difference between arms in the specific regimens selected.
Discussion
This exploratory subgroup study of the randomized phase III TRICS trial focused on platinum-naïve patients with AGC who showed recurrence during or early after completion of adjuvant therapy, and is the first report comparing the efficacy and safety of IRI/CDDP and IRI in this population. Our study did not show that IRI/CDDP combination therapy was superior to IRI monotherapy (HR: 0.782; 95% CI: 0.515–1.188, P = 0.249), but both regimens demonstrated favorable long-term survival (OS 14.0 vs. 14.0 months, respectively; PFS 5.0 vs. 4.5 months, respectively). The response rates were favorable given the second-line setting (ORR 19.6% vs. 23.3%, respectively). The incidence of grade 3–4 adverse events was low in both arms.
Biweekly IRI/CDDP combination therapy was developed to reduce IRI-associated diarrhea and febrile neutropenia by decreasing the dose of IRI [11, 12]. This treatment previously showed promising efficacy and a manageable toxicity profile [12]. Moreover, Higuchi et al. reported that biweekly IRI/CDDP significantly prolonged PFS (HR: 0.68) compared with IRI, but did not demonstrate an overall survival benefit (HR: 1.00) in 130 patients with metastatic or recurrent gastric cancer that progressed after S-1-based first-line chemotherapy [6]. In their study, the incidence of grade 3 or worse adverse events did not differ between the two groups. Serum creatinine elevation of any grade was more common in the IRI/CDDP group than the IRI group (25% vs. 8%, respectively; P = 0.009), but the opposite was true for diarrhea of any grade (17% vs. 42%, respectively; P = 0.002). This study showed similar efficacy and toxicity profiles as the study of Higuchi et al., especially concerning the incidence of diarrhea (IRI/CDDP, 24% vs. IRI, 45%; P = 0.0254). Consequently, the combination of IRI/CDDP may be a reasonable alternative as a SLC to replace IRI monotherapy.
An exploratory analysis of the ACTS-GC trial showed that in a subpopulation of patients with a recurrence-free interval (RFI) < 6 months, the median times from recurrence to death were 13.4 months and 6.8 months in patients whose regimens did or did not include S-1, respectively [3]. In a retrospective analysis of this same subpopulation by Shitara et al., S-1 plus cisplatin was not shown to be effective in patients with recurrent gastric cancer, especially those with an RFI of < 6 months after adjuvant S-1 chemotherapy [13]. In their report, patients with an RFI of < 6 months had a significantly lower ORR (5.0% vs. 37.5%, respectively), shorter PFS (2.3 vs. 6.2 months, respectively), and shorter OS (7.3 vs. 16.6 months, respectively) than patients with an RFI of ≥ 6 months.
Two prospective studies evaluated capecitabine plus cisplatin (XP) chemotherapy specifically in patients who failed adjuvant chemotherapy. First, a phase II study evaluated the efficacy of XP in 32 patients with gastric cancer that recurred before or after 6 months after adjuvant chemotherapy with doxifluridine or 5-fluorouracil (5-FU)-containing regimens [8]. This trial compared 13 patients with an RFI of > 6 months and 19 with an RFI ≤ 6 months, and demonstrated that response rates (39% vs. 21%, respectively; P = 0.427), time-to-treatment failure (8.3 vs. 5.4 months, respectively; P = 0.072), and OS (14.1 vs. 9.3 months, respectively; P = 0.075) were higher in patients with an RFI > 6 months, although the differences were not statistically significant. Second, a single-arm phase II trial (XParTS I) evaluated the efficacy of XP treatment in 40 AGC patients who relapsed during or within 6 months after S-1-containing adjuvant chemotherapy [14]. This trial showed that XP achieved favorable OS (13.7 months), PFS (4.4 months), and ORR (26.7%), results that were similar to those of this study. Patients who relapse early after adjuvant chemotherapy might have better survival than patients with progressive disease following first-line chemotherapy. Thus, the platinum-containing XP regimen may be a good option for patients with AGC who relapse within 6 months after S-1-based adjuvant therapy. Taken together with the results of this study, the platinum-based chemotherapy such as XP or IRI/CDDP may be a good option for early relapse after adjuvant chemotherapy with fluoropyrimidine alone.
While the TRICS trial was ongoing, the RAINBOW trial reported that ramucirumab plus paclitaxel combination therapy resulted in improved OS and PFS compared to weekly paclitaxel alone (OS, 9.6 vs. 7.4 months, respectively; PFS, 4.4 vs. 2.9 months, respectively) [15]. This led to the combination of ramucirumab with PTX being adopted as the current standard second-line chemotherapy for AGC. However, patients who relapsed during or within 6 months after adjuvant therapy were not eligible for the RAINBOW trial.
Our study had the following limitations. The exploratory subgroup analysis in this study was not pre-planned; therefore, the results should be interpreted with caution. Furthermore, application of the present results may be limited, as combination therapies containing platinum, such as CapeOX, are currently standard adjuvant treatments [16, 17]. Lastly, the current standard second-line chemotherapy for AGC is the combination of ramucirumab with PTX [15]. Future prospective randomized studies of ramucirumab with PTX vs. IRI/CDDP or XP should be conducted in patients with AGC who develop recurrence during or early after the completion of adjuvant therapy.
In conclusion, our study did not show significant survival differences between IRI/CDDP and IRI in platinum-naïve patients who relapsed during or within 6 months after S-1 adjuvant therapy. Thus, IRI could be a good alternative for patients with early recurrence who are vulnerable to paclitaxel.
References
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Furukawa H, Yamaguchi T et al (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820
Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T et al (2011) Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 29:4387–4393
Ito S, Ohashi Y, Sasako M (2018) Survival after recurrence in patients with gastric cancer who receive S-1 adjuvant chemotherapy: exploratory analysis of the ACTS-GC trial. BMC Cancer 18(1):449. [Epub ahead of print]
Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T et al (2013) Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 31:4438–4444
Higuchi K, Tanabe S, Shimada K, Hosaka H, Sasaki E, Nakayama N et al (2014) Biweekly irinotecan plus cisplatin versus irinotecan alone as second-line treatment for advanced gastric cancer: a randomised phase III trial (TCOG GI-0801/BIRIP trial). Eur J Cancer 50:1437–1445
Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J et al (2014) Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 15:78–86
Kang HJ, Chang HM, Kim TW, Ryu MH, Sohn HJ, Yook JH et al (2005) Phase II study of capecitabine and cisplatin as first-line combination therapy in patients with gastric cancer recurrent after fluoropyrimidine-based adjuvant chemotherapy. Br J Cancer 92:246–251
Park YH, Kim BS, Ryoo BY, Yang SH (2006) A phase II study of capecitabine plus 3-weekly oxaliplatin as first-line therapy for patients with advanced gastric cancer. Br J Cancer 94:959–963
De Vita F, Orditura M, Matano E, Bianco R, Carlomagno C, Infusino S et al (2005) A phase II study of biweekly oxaliplatin plus infusional 5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment of advanced gastric cancer patients. Br J Cancer 92:1644–1649
Nishikawa K, Fujitani K, Inagaki H, Akamaru Y, Tokunaga S, Takagi M et al (2015) Randomised phase III trial of second-line irinotecan plus cisplatin versus irinotecan alone in patients with advanced gastric cancer refractory to S-1 monotherapy: TRICS trial. Eur J Cancer 51:808–816
Sato A, Kurihara M, Matsukawa M, Shimada K, Yamazaki T, Nakamachi M et al (2001) Preliminary study of fortnightly irinotecan hydrochloride plus cisplatin therapy in patients with advanced gastric and colorectal cancer. Cancer Chemother Pharmacol 47:380–384
Koizumi W, Kurihara M, Satoh A, Takiuchi H, Tanabe S, Shimada K et al (2005) Phase I/II study of bi-weekly irinotecan plus cisplatin in the treatment of advavced gastric cancer. Anticancer Res 25:1257–1262
Shitara K, Morita S, Fujitani K, Kadowaki S, Takiguchi N, Hirabayashi N et al (2012) Combination chemotherapy with S-1 plus cisplatin for gastric cancer that recurs after adjuvant chemotherapy with S-1: multi-institutional retrospective analysis. Gastric Cancer 15:245–251
Nishikawa K, Tsuburaya A, Yoshikawa T, Takahashi M, Tanabe K, Yamaguchi K et al (2018) A phase II trial of capecitabine plus cisplatin (XP) for patients with advanced gastric cancer with early relapse after S-1 adjuvant therapy: XParTS-I trial. Gastric Cancer 21:811–818
Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y et al (2014) Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 15:1224–1235
Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH et al (2011) Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 379:315–321
Fuse N, Bando H, Chin K, Ito S, Yoshikawa T, Tsuburaya A et al (2017) Adjuvant capecitabine plus oxaliplatin after D2 gastrectomy in Japanese patients with gastric cancer: a phase II study. Gastric Cancer 20:332–340
Acknowledgements
We thank the investigators who enrolled patients in this trial. Furthermore, we deeply appreciate all patients who participated in the trial. This work was supported in part by the non-profit organization Epidemiological & Clinical Research Information Network (ECRIN).
Funding
This work was supported, in part, by the non-profit organization Epidemiological & Clinical Research Information Network (ECRIN). [No grant numbers apply].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kazuhiro Nishikawa has received honoraria from Chugai, Taiho, Yakult, Eli Lilly, Tsumura, and EA Pharma, and research funding from Yakult and Taiho, outside the submitted work. Takaki Yoshikawa has received lecture fees from Chugai, Taiho, Yakult, Eli Lilly and Ono, and for advisory work for Ono and MSD, outside the submitted work. Masato Nakamura has received honoraria from Chugai, Taiho, Merk, Takeda, Yakult, Eli Lilly, Bayer, Ono, and Otsuka Pharma, outside the submitted work. Satoshi Morita has received honoraria from Chugai and Taiho, outside the submitted work. Junichi Sakamoto has received consultant fee from Takeda, and Honoraria from Tsumura, Nihon Kayaku, and Chugai, outside the submitted work. All remaining authors have declared no conflicts of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nishikawa, K., Murotani, K., Fujitani, K. et al. A study of second-line irinotecan plus cisplatin vs. irinotecan alone in platinum-naïve patients with early relapse of gastric cancer refractory to adjuvant S-1 monotherapy: exploratory subgroup analysis of the randomized phase III TRICS trial. Cancer Chemother Pharmacol 83, 867–874 (2019). https://doi.org/10.1007/s00280-019-03802-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03802-9