Abstract
Irinotecan-induced mucositis is a major oncological problem. Goblet cells secrete mucus, protecting the intestinal mucosa, with secretion altered during mucositis. The enteric nervous system is involved in regulating gut motility and secretion. The aim of this study was to determine whether enteric neural cells and goblet cells are altered following irinotecan treatment. Tumour-bearing Dark Agouti rats were administered a single dose of 175 mg/kg of irinotecan intraperitoneally and 0.01 mg/kg atropine subcutaneously. Experimental and untreated control rats were killed at times 6, 24, 48, 72, 96 and 120 h after treatment. Jejunum and colon samples were formalin fixed. Haematoxylin and eosin staining, Alcian Blue–PAS staining, and immunohistochemistry with S-100 antibody (neural cell marker) were carried out. Statistical analyses were carried out using Kruskal–Wallis test with Dunns post test, Mann Whitney U test, and nonlinear regression. Total goblet cells decreased at 72 h compared with controls in the colon (p < 0.05). The percentage of cavitated goblet cells decreased compared to all other time points at 120 h in the colon. The number of S-100-positive cells in the submucosal plexus decreased in the colon (p = 0.0046) and in the myenteric plexus of the jejunum and colon (p = 0.0058 and p = 0.0022, respectively), on comparing treated with control. Enteric ganglia in the myenteric plexus of the jejunum decreased at 24 h and 96 h. Irinotecan-induced mucositis is associated with increases in mucus secretion and enteric neural cell change. These changes may contribute to the pathophysiology of mucositis through the dysregulation of neural signalling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alimentary mucositis (AM) is a severe, dose-limiting, toxic side effect of cytotoxic chemotherapy and radiotherapy, affecting the entire alimentary tract from mouth to anus [7, 8], and occurring in more than 40% of standard-dose chemotherapy patients and in 100% of high-dose chemotherapy patients [15, 16, 26]. Symptoms include mouth ulceration, abdominal pain, nausea, vomiting, abdominal bloating, and diarrhoea [14, 16, 36]. Alimentary mucositis often leads to reductions in treatment, which may lead to reduced survival [7, 35] and the need for nutritional adjuncts, including fluid replacement, liquid diets, and total parenteral nutrition, translating to a substantial cost increase per cycle of chemotherapy [7, 8]. Currently, there are no effective preventions or treatments for AM [17, 33, 37].
Mucin expression and secretion have been shown to be associated with mucositis [6, 30, 39,40,41,42,43]. Mucins are high molecular weight acidic glycoproteins secreted by goblet cells [13, 28, 38] and consist of two structurally and functionally different subfamilies: secretory gel forming (Muc2, Muc5AC) and transmembrane mucins (Muc1, Muc3, Muc4). Muc2 and Muc4 expression have both been shown to significantly decrease in the jejunum following irinotecan administration. Muc2 was shown to also be significantly decreased [39] in the colon following irinotecan administration [39]. Furthermore, Muc2 knockout (Muc2−/−) mice have been shown to have significantly reduced weight compared to wild-type mice in a methotrexate-induced mucositis model, with a mortality rate of 75% in the Muc2−/− mice compared to 0% in wild-type mice [6]. Mucins mediate between the luminal contents and the mucosa by protecting the mucosa from bacterial overgrowth, penetration and digestion, and providing attachment sites for commensal bacteria [28, 38]. Mucins also help facilitate lumen to epithelial signalling [4]. Sulphated mucins increase the viscosity and decrease bacterial digestion of the mucin barrier [29]. This may decrease mucin barrier loss during mucositis, decreasing mucosal damage and bacterial translocation from the lumen. Mucus composition and mucin expression have not yet been investigated in a tumour-bearing model of mucositis; therefore, the aims of this study are to determine changes in mucin composition during mucositis.
Dark Agouti mammary adenocarcinoma (DAMA)-bearing rats have been shown to have significantly worse mucositis (with increased mortality, increased severity and incidence of diarrhoea, and increased histological markers of damage) compared to tumour-naïve rats with higher levels of diarrhoea and an increased mortality [1]. Mammary adenocarcinomas have also been shown to have an increased IL-6 level [34], which may alter mucin secretion and subsequently affect mucin barrier composition [19].Therefore, the aims of this study are to determine changes in mucin composition and expression of muc2 and muc4 during mucositis in the tumour-bearing model.
Methods
Ethics
Ethical approval for the use of animals was obtained from the Animal Ethics Committees of the Institute of Medical and Veterinary Science (IMVS) and the University of Adelaide (approval number M-2010-118A), and complied with the National Health and Medical Research Council, Australia Code for the care and use of animals for scientific purposes (2013).
Tumour passage
The Dark Agouti mammary adenocarcinoma (DAMA) model used in this study has been used extensively [2, 9, 11, 12] as previously described [11]. Briefly, tumours were diced, homogenised, and filtered through sterile gauze. The tumour suspension was spun three times at 1100 rpm for 3 min each time, with the pellet resuspended in fresh phosphate-buffered saline (PBS) each time. A viable cell count was carried out using 0.4% v/v trypan blue.
Experimental design
All experiments were carried out in female DA rats, weighing between 150 g and 170 g. The rats were single-housed in an animal facility regulated at 22 ± 1 °C and subject to a 14:10 h light–dark cycle. All rats received tumour inoculum of 4.0 × 106 cells in 0.5 mL sterile PBS subcutaneously into each flank. Tumours were established for 10 days prior to rats receiving irinotecan. Rats were monitored four times daily, and if any rat showed a dull ruffled coat with accompanying dull and sunken eyes, was cold to touch with no spontaneous movement and a hunched appearance, or had a tumour size greater than 10% of its body weight, it was euthanised.
Forty-one rats were randomly assigned to groups according to time point, with final numbers able to be used for analysis as follows: control (6), 6 h (5), 24 h (5), 48 h (5), 72 h (8), 96 h (5), 120 h (6). Each experimental rat received 0.01 mg/kg of atropine subcutaneously (sc) to reduce cholinergic reaction, followed by 175 mg/kg irinotecan intraperitoneally (ip). Irinotecan (supplied by Pfizer, Kalamazoo, Michigan, USA) was administered in a sorbitol/lactic acid buffer (45 mg/ml sorbitol, 0.9 mg/ml lactic acid, pH 3.4), required for activation of the drug, at time designated 0 h. Control rats received sorbitol/lactic acid buffer (vehicle) and atropine only. Rats were killed using deep anaesthesia with 3% isofluorane in 100% O2, followed by exsanguination and cervical dislocation at times 6 h, 24 h, 48 h, 72 h, 96 h, and 120 h post-irinotecan treatment.
The gastrointestinal tract (GIT) was dissected out from the pyloric sphincter to the rectum, and separated into the small intestine (pyloric sphincter to ileocaecal sphincter) and colon (ascending colon to rectum). The small intestine (SI) was flushed with chilled, sterile distilled water, and 1 cm samples were collected at approximately 25%, 50%, and 75% of the length for histology. The colon was also flushed with chilled sterile, distilled water. Samples (1 cm) were collected at approximately 50% of the length for histology. Samples for histology were fixed in 10% neutral buffered formalin, processed and embedded in paraffin. Samples were also collected for molecular analysis. Mucosa was scraped from the small intestinal pieces of tissue and stored at − 80 °C for RNA extraction later using Aurum™ Total RNA Mini Kit (Bio-Rad, California, USA).
Analysis of goblet cells
Goblet cells and mucins were analysed histologically using Alcian Blue/PAS staining, as previously described [39]. Briefly, sections were dewaxed in xylene and rehydrated in a graded series of ethanol. Staining was carried out in Alcian Blue (1% Alcian Blue 8GX (CI 74,240) in 3% glacial acetic acid) for 5 min. Sections were then rinsed in distilled water, then oxidised with 1% periodic acid. Washing was carried out in running tap water, prior to rinsing in distilled water. Sections were treated for 15 min in Schiff’s reagent, then washed for 7 min in running tap water. Sections were dehydrated, cleared, and mounted. Analysis was carried out under 20× magnification. Total goblet cells and paired goblet cells (adjacent goblet cells with no enterocytes between, representing enterocyte cell death) were counted for each section in at least 15 complete villi and crypts. Paired goblet cells were expressed as a percentage of total goblet cells.
Analysis of sulphated mucins
Sulphated mucins were analysed in intestinal goblet cells using high iron diamine staining. Briefly, sections were dewaxed in xylene and rehydrated through a graded series of ethanols and stained in high iron diamine solution (2.4% N,N-dimethyl-meta-phenylenediamine dihydrochloride, 0.4% N,N-dimethyl-para-phenylenediamine dihydrochloride, 2.8% v/v 10% ferric chloride) for 18–24 h. Sections were then washed in running tap water for 2 min and couterstained in 1% Alcian Blue in 3% glacial acetic acid for 5 min. Sections were then washed in running tap water, rehydrated, cleared, and mounted. Sections were then analysed under 20X magnification. Sulphated goblet cells were counted per crypt (minimum of 15 complete villi and crypts per section).
Immunohistochemistry
To investigate whether mucin expression is affected by chemotherapy, anti-Muc2 (Santa Cruz Biotechnology, California, USA) and anti-Muc4 (Thermo Fisher, Carlsbad, California, USA) antibodies were used. Sections (4 µm) were placed onto silane-coated slides (HD Scientific, Sydney, New South Wales, Australia) and heated to 60 °C for 2 h on a heating block. Sections were dewaxed in xylene and rehydrated through graded ethanols and distilled water, followed by PBS. Endogenous peroxidases were blocked with 3% hydrogen peroxide in methanol for 1 min. Non-specific antibody binding was blocked with blocking solution (Ultra Streptavidin HRP kit, Signet, Dedham, Massachusetts, U.S.A) for 30 min. After washing in PBS, endogenous avidin and biotin were blocked using the Avidin and Biotin kit (Vector Laboratories, Burlingame, California, USA).
Sections were incubated with either rabbit polyclonal anti-Muc2 antibody (1:700, 0.3 µg/mL) for 1 h at room temperature (RT) or mouse monoclonal anti-Muc4 antibody (1:100, 0.5 µg/mL) for 18 h at 4 °C. Sections were washed in PBS (3 × 5 min), incubated with Linking reagent (Ultra Streptavidin HRP kit) for 30 min, then washed in PBS (3 × 5 min). Labelling reagent was then applied for 30 min, then washed in PBS (3 × 5 min). Staining was visualised using 3,3′-diaminobenzidine (DAB) and counterstained using haematoxylin, before dehydrating and coverslipping. Total positive cells for Muc2 and Muc4 were counted in a minimum of 15 complete villi and crypts for each section. Staining intensity was also used to analyse change in expression, based on a previously validated technique (Bowen, Gibson et al. [10]), where 0 = negative, 1 = weak, 2 = moderate, 3 = strong, and 4 = very intense.
RNA isolation
RNA was extracted from small intestinal mucosal scrapings with Aurum™ Total RNA Mini Kit (Bio-Rad, California, USA), according to the manufacturer’s instructions. Briefly, mucosal scrapings (40 mg) from the jejunum and colon were added to a 2 mL RNase-free tube. Lysis solution was added immediately and the solution was vortexed for 1 min. The lysate was centrifuged for 3 min and the supernatant transferred to a 2 mL capped microcentrifuge tube and mixed with 700 µL of 60% ethanol. The lysate was decanted into a RNA binding column and wash tube, and centrifuged for 1 min. The filtrate was discarded, and 700 µL of low stringency wash solution was added, and centrifuged for 30 s. The binding column was treated with DNAse at room temperature for 25 min, then washed with high stringency washing solution and centrifuged for 30 s. The filtrate was discarded, and the binding column washed with 700 µL low stringency washing solution, and centrifuged for 1 min. The filtrate was discarded, and the binding column was centrifuged for 2 min to dry. Elution solution was added to the binding column in a 1.5 mL capped microcentrifuge tube to elute the RNA. RNA was analysed with a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, Del., USA) to determine purity and concentration.
cDNA conversion
RNA was converted to cDNA using the iScript™ cDNA Synthesis Kit (Bio-Rad, California, USA). Briefly, 4 µL 5X iScript reaction mix, 1 µL iScript reverse transcriptase, and 1 µL of RNA template were added. Samples were made up to 20 µL with nuclease-free water. Samples were then incubated for 5 min at 25 °C, then 30 min at 42 °C and 5 min at 85 °C. The transcribed samples were analysed using a NanoDrop (NanoDrop Technologies, Wilmington, Del., USA) and diluted to create a working 100 ng/µL cDNA solution.
Real-time PCR
Real-time PCR was performed using a Rotor-Gene Q (Qiagen, Sydney, Australia). The amplification mixes contained 2 µL of cDNA, 5 µL of 2X SYBR green (Bio-Rad Laboratories Inc. USA), 3 µL nuclease-free water and 0.5 µL of forward and reverse primers (GeneWorks, Adelaide, Australia), making a final volume of 10 µl. Thermal cycling conditions were individually optimised for each primer set (Table 1). Ubiquitin C (UBC) and beta-2-microglobulin (B2M) were used as housekeeping genes for relative quantification for each gene of interest. All experiments were repeated in triplicate, with experimental thresholds (Ct) calculated by Rotor Gene 6 program. Pfaffl quantification [25] was used, based on primer efficiencies, to determine the fold change compared to control rats.
Statistical analysis
Statistical analyses were performed using Kruskal–Wallis test for non-parametric data with secondary Dunn’s comparative test to determine the difference between mean ranks, using GraphPad Prism 6.0 (Graphpad software, Califorina, USA). Non-parametric linear regressions were also analysed using StataIC12 (StataCorp LP, Texas, USA). Differences between mean ranks were determined to be significant at p < 0.05. Cohen’s D tests to determine clinical significance were also carried out as a measure of effect size. The effect was considered small if d > 0.20, moderate if d > 0.50, and large if d > 0.80 [5].
Results
Goblet cells
Jejunum
Percentage of paired goblet cells
Paired goblet cells were analysed as an indicator of enterocyte death, as goblet cells are not usually adjacent. The percentage of paired goblet cells (of total goblet cells) in the villi of the jejunums of vehicle control rats was 1.2 ± 0.5%. The percentage of paired goblet cells for irinotecan-treated rats increased significantly (p = 0.008) at 72 h (32.1 ± 3.9%, d = − 5.2, large effect) and 96 h (20.0 ± 5.2%, d = − 3.0, large effect) (Figs. 1, 3). Paired goblet cells could not be determined in crypts due to the close proximity of crypt epithelial cells.
Sulphated goblet cells
Sulphated goblet cells were demonstrated with high iron diamine staining. In vehicle control rats, the numbers of sulphated goblet cells in the jejunum were 4.2 ± 0.9 and 2.3 ± 0.5 per villus and crypt, respectively. In the villi of irinotecan-treated rats, sulphated goblet cells were lowest at 6 h (3.6 ± 1.0, d = 0.2, small effect) and highest at 96 h (6.0 ± 2.2, d = − 0.5, small effect). In the crypts, sulphated goblet cells were lowest at 72 h (1.2 ± 0.6, d = 0.8, medium effect, data not shown) and highest at 96 h (3.2 ± 1.0, d = − 0.5, small effect). The number of sulphated goblet cells in the colon of vehicle control rats was 4.1 ± 1.0 per crypt. In irinotecan-treated rats, the number of sulphated goblet cells was lowest at 6 h following treatment (1.4 ± 0.7, d = 1.3, large effect) (Fig. 2).
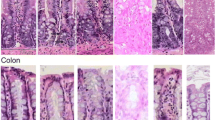
High Iron diamine staining. a Jejunum. b Colon (original magnification 20×). c Counts of goblet cells containing sulphated mucins in the villi of the jejunum. d Counts of goblet cells containing sulphated mucins in the crypts of the jejunum. e Counts of goblet cells containing sulphated mucins in the crypts of the colon
Mucin composition and distribution in goblet cells
Muc2 expression
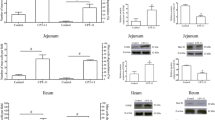
Immunohistochemistry was used to investigate the number of Muc2-positive cells in the intestine. In the jejunums of vehicle control rats, the numbers of Muc2-stained goblet cells were 15.1 ± 2.0 and 4.8 ± 0.8 (mean ± SEM) per villus and crypt, respectively. In irinotecan-treated rats, the numbers of Muc2-stained goblet cells declined in the villi at 6 h (9.6 ± 0.7, d = 1.7, large effect) and in the crypts at 48 h (0.6 ± 0.16, d = 3.8, large effect). The numbers of Muc2-stained goblet cells were highest in the villi and crypts at 96 h (20.7 ± 3.1 and 9.2 ± 1.6, respectively, d= − 0.9 and − 1.6, respectively, large effects). The mean ranks of Muc2-stained goblet cells in the villi and crypts deviated significantly across groups (p = 0.0181 and p = 0.0007, respectively) (Fig. 3).
Immunohistochemistry of Muc2 positively stained goblet cells. a Jejunum, villus, and crypt sections. b Jejunum, counts of Muc2-positive goblet cells per villus or crypt. c Colon, crypt sections. d Colon, counts of Muc2-positive goblet cells per crypt (original magnification 20×) (*denotes statistical significance, where p < 0.05)
In the colon, the number of Muc2-stained goblet cells was 11.4 ± 1.5 per crypt in vehicle control rats. In irinotecan-treated rats, the number of Muc2-stained goblet cells decreased significantly at 72 h (4.0 ± 1.0, p < 0.05, d = 2.3, large effect). The mean ranks of Muc2-stained goblet cells in the colon also deviated significantly across groups (p = 0.0255, Fig. 3).
Real-time PCR was used to compare gene expression of Muc2 between vehicle control and irinotecan-treated rats in the jejunum, based on the immunohistochemistry results. Muc2 gene expression did not change following irinotecan treatment (Fig. 4).
Muc4 expression
Immunohistochemistry was used to investigate the number of Muc4-positive goblet cells. In the jejunum, the numbers of Muc4-stained goblet cells were 13.4 ± 3.1 and 5.1 ± 1.1 per villus and crypt, respectively. In irinotecan-treated rats, the numbers of Muc4-stained goblet cells were highest in the villi at 96 h (20.3 ± 3.8, d = − 0.9, large effect) and in the crypts at 120 h (7.3 ± 1.2, d = − 0.8, medium effect). The mean ranks of Muc4 expression in the crypts deviated significantly across groups (p = 0.0134) (Fig. 5).
Immunohistochemistry of Muc4 positively stained goblet cells. a Jejunum, villus, and crypt sections. b Jejunum, counts of Muc4-positive goblet cells per villus and crypt. c Colon, crypt sections. d Colon, counts of Muc4-positive goblet cells per crypt. (Original magnification 20×) (*denotes statistical significance, where p < 0.05)
In the colon, the number of Muc4-stained goblet cells was 8.9 ± 2.5 per crypt in vehicle control rats. In irinotecan-treated rats, the number of Muc4-stained goblet cells decreased significantly at 72 and 96 h (1.8 ± 0.45 and 1.3 ± 0.5, respectively, p < 0.05 for both, d = 2.3, large effect). The mean ranks of Muc4-stained goblet cells in the colon deviated significantly across all groups (p = 0.0055) (Fig. 5).
Real-time PCR was used to compare the relative gene expression of Muc4 between vehicle control and irinotecan-treated rats in the jejunum. Muc4 gene expression did not change following treatment with irinotecan (Fig. 4).
Discussion
GI mucositis is a severe, dose-limiting, toxic side effect of cytotoxic chemotherapy and radiotherapy [7, 8], often leading to reductions in treatment and reduced survival [7, 35]. Mucin composition and secretion is believed to be involved in the pathophysiology of mucositis, based on previous studies [6, 30, 31, 39, 40, 43]. This study has, for the first time, investigated mucin expression, at both a protein and genetic level, during irinotecan-induced mucositis in a tumour-bearing rat model and also demonstrated enterocyte loss during mucositis.
Paired goblet cells (expressed as a percentage of total goblet cells) significantly increased in the villi of the jejunum at 72 and 96 h, which suggests increased enterocyte cell death as goblet cells are not usually found adjacent in the intestine. This is likely to affect the ability of the intestine to carry out functions usually undertaken by enterocytes, such as secretion and absorption, and mucosal barrier function. Downstream effects of impaired secretion and absorption include malnutrition, a known complication of cancer treatments [18, 24].
Previous studies in non-tumour-bearing rat models for chemotherapy-induced mucositis have demonstrated goblet cells and mucins to be affected by chemotherapy agents, 5-FU and irinotecan [39, 40]. However, the model used in this study includes tumours, which may add an additional immunological load, and has investigated genetic expression of Muc2 and Muc4 in addition to protein expression in these earlier studies. The findings of this study are consistent with previous studies in non-tumour-bearing rat models for chemotherapy-induced mucositis [39, 40]. Whilst no solid conclusions regarding the impact of the tumour can be made from this study, the similarity of findings may suggest that this mammary adenocarcinoma may not affect intestinal mucins. However, the model used in this study includes tumours, which may add an additional immunological load, and has investigated genetic expression of Muc2 and Muc4 in addition to protein expression in these earlier studies. Specifically, Muc2 and Muc4 expression tended to decrease in the crypts of the jejunum, and Muc2 expression was shown to be significantly decreased at 72 h in the colon following irinotecan administration in both this study and the previous non-tumour-bearing study using irinotecan [42]. Sulphated mucins in the jejunum tended to increase (not significant) following irinotecan administration, similar to findings in the above-mentioned previous study by Stringer and colleagues, which also demonstrated an increase in sulphated mucins in an irinotecan-induced DA rat model of mucositis [42]. Sulphated mucins increase the viscosity and decrease bacterial digestion of the mucin barrier [29]. This increase may be in response to the damage, in an effort to restore mucin barrier integrity and function during mucositis, potentially preventing further mucosal damage and bacterial translocation from the lumen.
The number of Muc2-positive cells significantly decreased in the crypts of the colon at 72 h, and the number of Muc4-positive cells also declined. Gene expression of Muc2 and Muc4 using real-time PCR has not been previously examined in a mucositis setting. This study has shown for the first time that gene expression of Muc2 and Muc4 did not alter significantly during irinotecan-induced mucositis. The most likely explanation of the decrease in Muc2-positive cells without corresponding genetic changes following irinotecan is the chemotherapy-induced damage to goblet cells and mucins resulting in less goblet cells being present. This suggests a rapid exocytosis of mucins from goblet cells. This rapid secretion of mucin may also contribute to the decreased levels of Muc2 and Muc4 in goblet cells. This decrease in Muc2 may lead to a subsequent loss of mucus barrier integrity, affecting mucosal protection and increasing susceptibility to luminal insults.
Nitric oxide (NO) has been previously shown to stimulate mucin exocytosis, decreasing mucin stores [3, 27]. NO is created by three isoforms: inducible (iNOS), epithelial (eNOS), and neuronal nitric oxide synthase (nNOS). iNOS has been shown to be up-regulated in response to inflammation, generating a sustained increase in NO [23]. Inflammatory activity has also been shown to increase during mucositis, demonstrated through increased expression of pro-inflammatory cytokines [20, 41]. nNOS is an enteric neurotransmitter. This might suggest an increase in inflammation or dysfunction of the ENS may be contributing to the rapid mucus exocytosis.
The decrease in Muc2 and Muc4 expression in goblet cells in this study supports the suggestion that depletion of mucin stores may result in loss of integrity to the mucus barrier during mucositis. This may mean maintaining mucin levels may be a clinically viable way of reducing the pathophysiology of mucositis. An intervention study by Yamamoto et al. [45] supports this; lafutidine, which stimulates mucin accumulation, inhibited cisplatin-induced body weight loss in male Wistar rats and had significantly more mucin content in the ileal mucosa (P < 0.05) [45]. While this might be the result of reduced mucin loss from lafutidine reducing the severity of mucositis, it is unlikely as famotidine was less effective in inhibiting weight loss compared to lafutidine in the same study [45]. This suggests that maintaining mucin content might be of clinical use in reducing mucositis. Furthermore, as there is no increase in gene expression for Muc2 or Muc4, the production is not up-regulated to offset mucin loss, and therefore the effects of mucin depletion may persist long term in the intestine, meaning maintenance of mucin content may be important after mucositis symptoms have ceased.
Further long-term studies should be carried out to investigate when mucin stores return following chemotherapy-induced mucositis. This information could be valuable when predicting how susceptible patients will be to chemotherapy-induced intestinal mucositis in subsequent cycles, based on the level of integrity of the mucus barrier, how it changes over time, and when mucin stores may return.
In conclusion, this study has, for the first time, demonstrated preliminary evidence of enterocyte cell death, suggesting secretory and absorptive enterocyte functions may be reduced and mucosal barrier integrity lost. Furthermore, this study has determined irinotecan-induced mucositis is associated with changes in Muc2 and Muc4 expression in goblet cells. These phenomena may be the result of up-regulated inflammatory signalling or altered ENS signalling, affecting mucus secretion and therefore resulting in systemic effects from the subsequent loss of mucosal barrier integrity.
References
Bowen JM, Gibson RJ, Cummins AG, Tyskin A, Keefe DM (2007) “Irinotecan changes gene expression in the small intestine of the rat with breast cancer”. Cancer Chemother Pharmacol 59(3):337–348
Bowen JM, Gibson RJ, Keefe DM, Cummins AG (2005) Cytotoxic chemotherapy upregulates pro-apoptotic Bax and Bak in the small intestine of rats and humans. Pathology 37(1):56–62
Branka JE, Vallette G, Jarry A, Laboisse CL (1997) Stimulation of mucin exocytosis from human epithelial cells by nitric oxide: evidence for a cGMP-dependent and a cGMP-independent pathway. Biochem J 323(Pt 2):521–524
Carraway KL, Perez A, Idris N, Jepson S, Arango M, Komatsu M, Haq B, Price-Schiavi SA, Zhang J, Carraway CA (2002) Muc4/sialomucin complex, the intramembrane ErbB2 ligand, in cancer and epithelia: to protect and to survive. Prog Nucleic Acid Res Mol Biol 71:149–185
Cohen J (2013) Statistical power analysis for the behavioral sciences. Taylor and Francis, London
de Koning BA, Sluis M, Lindenbergh-Kortleve DJ, Velcich A, Pieters R, Buller HA, Einerhand AW, Renes IB (2007) Methotrexate-induced mucositis in mucin 2-deficient mice. J Cell Physiol 210(1):144–152
Elting LS, Cooksley C, Chambers M, Cantor SB, Manzullo E, Rubenstein EB (2003) The burdens of cancer therapy. Clinical economic outcomes of chemotherapy-induced mucositis. Cancer 98(7):1531–1539
Elting LS, Cooksley CD, Chambers MS, Garden AS (2007) Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys 68(4):1110–1120
Gibson RJ, Bowen JM, Alvarez E, Finnie J, Keefe DM (2007) Establishment of a single-dose irinotecan model of gastrointestinal mucositis. Chemotherapy 53(5):360–369
Gibson RJ, Bowen JM, Keefe DM (2005) Palifermin reduces diarrhea and increases survival following irinotecan treatment in tumor-bearing DA rats. Int J Cancer 116(3):464–470
Gibson RJ, Keefe DM, Clarke JM, Regester GO, Thompson FM, Goland GJ, Edwards BG, Cummins AG (2002) The effect of keratinocyte growth factor on tumour growth and small intestinal mucositis after chemotherapy in the rat with breast cancer. Cancer Chemother Pharmacol 50(1):53–58
Gibson RJ, Keefe DM, Thompson FM, Clarke JM, Goland GJ, Cummins AG (2002) Effect of interleukin-11 on ameliorating intestinal damage after methotrexate treatment of breast cancer in rats. Dig Dis Sci 47(12):2751–2757
Jass JR, Walsh MD (2001) Altered mucin expression in the gastrointestinal tract: a review. J Cell Mol Med 5(3):327–351
Keefe DM (2004) Gastrointestinal mucositis: a new biological model. Support Care Cancer 12(1):6–9
Keefe DM, Brealey J, Goland GJ, Cummins AG (2000) Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut 47(5):632–637
Keefe DM, Cummins AG, Dale BM, Kotasek D, Robb TA, Sage RE (1997) Effect of high-dose chemotherapy on intestinal permeability in humans. Clin Sci (Lond) 92(4):385–389
Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, Migliorati CA, McGuire DB, Hutchins RD, Peterson DE (2007) Updated clinical practice guidelines for the prevention treatment of mucositis. Cancer 109(5):820–831
Kern KA, Norton JA (1988) Cancer cachexia. JPEN J Parenter Enteral Nutr 12(3):286–298
Li YY, Hsieh LL, Tang RP, Liao SK, Yeh KY (2009) Macrophage-derived interleukin-6 up-regulates MUC1, but down-regulates MUC2 expression in the human colon cancer HT-29 cell line. Cell Immunol 256(1–2):19–26
Lima-Junior RC, Figueiredo AA, Freitas HC, Melo ML, Wong DV, Leite CA, Medeiros RP, Marques-Neto RD, Vale ML, Brito GA, Oria RB, Souza MH, Cunha FQ, Ribeiro RA (2012) Involvement of nitric oxide on the pathogenesis of irinotecan-induced intestinal mucositis: role of cytokines on inducible nitric oxide synthase activation. Cancer Chemother Pharmacol 69(4):931–942
Logan RM, Stringer AM, Bowen JM, Gibson RJ, Sonis ST, Keefe DM (2008) Serum levels of NFkappaB and pro-inflammatory cytokines following administration of mucotoxic drugs. Cancer Biol Ther 7(7):1139–1145
Logan RM, Stringer AM, Bowen JM, Gibson RJ, Sonis ST, Keefe DM (2009) Is the pathobiology of chemotherapy-induced alimentary tract mucositis influenced by the type of mucotoxic drug administered? Cancer Chemother Pharmacol 63(2):239–251
Nathan C (1997) Inducible nitric oxide synthase: what difference does it make?. J Clin Invest 100(10):2417–2423
Ollenschlager G, Thomas W, Konkol K, Diehl V, Roth E (1992) Nutritional behaviour and quality of life during oncological polychemotherapy: results of a prospective study on the efficacy of oral nutrition therapy in patients with acute leukaemia. Eur J Clin Invest 22(8):546–553
Pfaffl MW (2004) Quantification strategies in real-time PCR. In: Bustin S (ed) A-Z of quantitative PCR. International University Line, La Jolla, pp 87–112
Pico JL, Avila-Garavito A, Naccache P (1998) Mucositis: its occurrence, consequences, and treatment in the oncology setting. Oncologist 3(6):446–451
Plaisancie P, Barcelo A, Moro F, Claustre J, Chayvialle JA, Cuber JC (1998) Effects of neurotransmitters, gut hormones, and inflammatory mediators on mucus discharge in rat colon. Am J Physiol 275(5 Pt 1):G1073–G1084
Robbe C, Capon C, Coddeville B, Michalski JC (2004) Structural diversity and specific distribution of O-glycans in normal human mucins along the intestinal tract. Biochem J 384(Pt 2):307–316
Robertson AM, Wright DP (1997) Bacterial glycosulphatases and sulphomucin degradation. Can J Gastroenterol 11(4):361–366
Saegusa Y, Ichikawa T, Iwai T, Goso Y, Ikezawa T, Nakano M, Shikama N, Saigenji K, Ishihara K (2008) Effects of acid antisecretory drugs on mucus barrier of the rat against 5-fluorouracil-induced gastrointestinal mucositis. Scand J Gastroenterol 43(5):531–537
Saegusa Y, Ichikawa T, Iwai T, Goso Y, Okayasu I, Ikezawa T, Shikama N, Saigenji K, Ishihara K (2008) Changes in the mucus barrier of the rat during 5-fluorouracil-induced gastrointestinal mucositis. Scand J Gastroenterol 43(1):59–65
Saltz L, Shimada Y, Khayat D (1996) CPT-11 (irinotecan) and 5-fluorouracil: a promising combination for therapy of colorectal cancer. Eur J Cancer 32A(Suppl 3):S24–S31
Saltz LB (2003) Understanding and managing chemotherapy-induced diarrhea. J Support Oncol 1(1):35–46 (discussion 38–41, 45–36)
Sanguinetti A, Santini D, Bonafe M, Taffurelli M, Avenia N (2015) “Interleukin-6 and pro inflammatory status in the breast tumor microenvironment”. World J Surg Oncol 13:129
Savarese DM, Hsieh C, Stewart FM (1997) Clinical impact of chemotherapy dose escalation in patients with hematologic malignancies and solid tumors. J Clin Oncol 15(8):2981–2995
Sonis ST, Costa JW Jr, Evitts SM, Lindquist LE, Nicolson M (1992) Effect of epidermal growth factor on ulcerative mucositis in hamsters that receive cancer chemotherapy. Oral Surg Oral Med Oral Pathol 74(6):749–755
Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, Bekele BN, Raber-Durlacher J, Donnelly JP, Rubenstein EB (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100(9 Suppl):1995–2025
Specian RD, Oliver MG (1991) Functional biology of intestinal goblet cells. Am J Physiol 260(2 Pt 1):C183–C193
Stringer AM, Gibson RJ, Bowen JM, Logan RM, Ashton K, Yeoh AS, Al-Dasooqi N, Keefe DM (2009) Irinotecan-induced mucositis manifesting as diarrhoea corresponds with an amended intestinal flora and mucin profile. Int J Exp Pathol 90(5):489–499
Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh AS, Hamilton J, Keefe DM (2009) Gastrointestinal microflora and mucins may play a critical role in the development of 5-Fluorouracil-induced gastrointestinal mucositis. Exp Biol Med (Maywood) 234(4):430–441
Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh AS, Keefe DM (2008) Faecal microflora and beta-glucuronidase expression are altered in an irinotecan-induced diarrhea model in rats. Cancer Biol Ther 7(12):1919–1925
Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh AS, Laurence J, Keefe DM (2009) Irinotecan-induced mucositis is associated with changes in intestinal mucins. Cancer Chemother Pharmacol 64(1):123–132
Verburg M, Renes IB, Meijer HP, Taminiau JA, Buller HA, Einerhand AW, Dekker J (2000) Selective sparing of goblet cells and paneth cells in the intestine of methotrexate-treated rats. Am J Physiol Gastrointest Liver Physiol 279(5):G1037–G1047
Wadler S, Benson AB 3rd, Engelking C, Catalano R, Field M, Kornblau SM, Mitchell E, Rubin J, Trotta P, Vokes E (1998) Recommended guidelines for the treatment of chemotherapy-induced diarrhea. J Clin Oncol 16(9):3169–3178
Yamamoto H, Ishihara K, Takeda Y, Koizumi W, Ichikawa T (2013) Changes in the mucus barrier during cisplatin-induced intestinal mucositis in rats. Biomed Res Int 2013:276186
Funding
This study was funded by NHMRC funding (1016696).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Daniel Thorpe declares that he has no conflict of interest, Ms. Masooma Sultani declares that she has no conflict of interest, and Dr. Andrea Stringer declares that she has no conflict of interest. Furthermore, all applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thorpe, D., Sultani, M. & Stringer, A. Irinotecan induces enterocyte cell death and changes to muc2 and muc4 composition during mucositis in a tumour-bearing DA rat model. Cancer Chemother Pharmacol 83, 893–904 (2019). https://doi.org/10.1007/s00280-019-03787-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03787-5