Abstract
Purpose
Multidrug resistance mediated by ABCB1 has been perceived to be one of the obstacles for cancer chemotherapy. This meta-analysis was performed to verify the effect of the ABCB1 rs1045642 and rs1128503 polymorphisms on the response to Taxane-containing chemotherapy.
Methods
Pooled odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were employed to evaluate the impact of these two ABCB1 polymorphisms. R scripts were developed to perform the meta-analysis.
Results
A total of nine articles (including nine studies for rs1045642 and five for rs1128503) were collected in our systematic review. However, our meta-analysis showed no significant effect of these two ABCB1 polymorphisms on the response to Taxane-containing regimens.
Conclusions
This study highlights the unsuitability of relying on the ABCB1 rs1045642 and rs1128503 polymorphisms as therapeutic response biomarkers of Taxane-containing chemotherapy. Further polycentric studies in larger and multiracial populations are needed to validate the conclusions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As one of the cornerstones of systemic treatment, Taxanes are widely used in chemotherapy for different types of cancers. The platinum-based doublet regimen with Taxane is regarded as a standard combinational therapeutic approach. However, the response to Taxane-containing chemotherapy varies greatly between individuals. Together with the external environmental influence and clinical factors, inherited genetic variations, such as single nucleotide polymorphisms (SNPs), can lead to inter-individual variability. For this reason, identifying biomarkers that indicate the response to Taxane-containing regimens is increasingly understood to be an important way to optimize the survival of cancer patients.
ATP-binding cassette subfamily B member 1 (ABCB1), also known as multiple drug resistance protein 1 (MDR1), functions as a transmembrane active efflux pump for many types of drugs [1]. ABCB1 regulates the transport of a vast spectrum of drugs and mediates the elimination of xenobiotics. Upregulation of ABCB1 has been regarded as one of the major obstacles for chemotherapy and correlates with undesirable treatment response [2,3,4]. The rs3213619 polymorphism of the ABCB1 gene significantly impacts the risk of Paclitaxel-induced peripheral neuropathy [5]. The pharmacokinetic changes caused by genetic variations, such as SNPs, involved in some drug transporter proteins may directly and adversely impact the efficacy of many therapeutic agents [6]. Although it is a synonymous variant, several studies showed that the T allele of the ABCB1 rs1045642 (C3435T) polymorphism leads to both decreased expression level and diminished activity [7, 8]. No consensus has yet been reached on the clinical significance of the ABCB1 rs1128503 (C1236T) variant. Both the T allele [9] and C/C homozygote [10] of this synonymous polymorphism have been reported to be significantly associated with better outcomes after chemotherapy. Given the importance of the ABCB1 gene, it is necessary to assess the impact of these two polymorphisms on the response to Taxane-containing regimens.
Although several case–control studies have tried to evaluate the impact of the ABCB1 rs1045642 and rs1128503 polymorphisms, those scattered evidence remained inconclusive. Not only different criteria for sample selection used in the previous studies, but also some confounding factors, such as ethnicity, sample size, and chemotherapy strategies, may have led to the incommensurability between the results. We performed this meta-analysis to draw more credible evidence by systematically integrating eligible data sets. We sought to clarify the effects of the ABCB1 rs1045642 and rs1128503 polymorphisms on the response to Taxane-containing chemotherapy.
Materials and methods
Literature search
We queried the Web of Science, PubMed, and Cochrane Library databases on August 2, 2017. Keyword combinations for Taxane drugs (Paclitaxel, Docetaxel, Taxol, Taxane, and Cabazitaxel), polymorphism (polymorphism, SNP, and variant), gene symbols, and synonyms for the ABCB1 gene (ABCB1, MDR1, CLCS, P-GP, PGY1, ABC20, CD243, and GP170), and cancer (epithelioma, adenocarcinoma, osteosarcoma, carcinoma, and cancer) were used to form a Boolean query formula. Both the query text and search results were reviewed independently by three authors (M.X., Y.L., and Q.J.). Inconsistencies in the numbers of the yielded papers were discussed to reach consensus.
Eligibility criteria
Studies were included on the following grounds: (1) manuscripts from peer-reviewed journals; (2) case–control studies assessing the association between the ABCB1 single nucleotide polymorphisms (rs1045642 and rs1128503) and response to Taxane-containing chemotherapy regimens; (3) studies with all included samples receiving Taxane-containing regimens; (4) no inconsistencies in genotype data for both cases and controls; and (5) studies with enough genotype data to estimate the odds ratio (OR) and 95% confidence interval (CI) in at least one genetic comparison model. Three individual authors (M.X., Y.L., and D.L.) performed the literature selection process. Another author (X.Y.) performed an investigation to reach an eventual agreement with all of the authors when any information regarding the screening results was not the same.
Data extraction
For each relevant study, the name of the first author, year of publication, country, cancer types, chemotherapy strategies, response evaluation criteria, and genotype numbers were carefully extracted independently by four authors (M.X., Y.L., Y.C., and J.F.) using a unified table with a pre-defined data format. All disagreements were resolved by an internal discussion and deliberation until a consensus was reached. A proofread was performed by two authors (Q.J. and X.Y.) for error reduction.
Statistics analysis
All statistical analyses were conducted in the R environment (version: 3.3.3, https://cran.r-project.org/) with the built-in functions of the “meta” package (version: 4.7-1, http://cran.r-project.org/web/packages/meta/) [11] as well as our customized analysis widgets (developed by M.X. and Y.L.). Four authors (M.X., Y.L., Q.J., and X.Y.) independently participated in the analysis, and any disagreement regarding the results was resolved by collective confirmatory calculation. The aggregated estimate of the OR and corresponding 95% CI were calculated for the dominant model (CT + TT vs CC, C stands for the cytosine and T for the thymine), the recessive model (TT vs CT + CC), the heterozygote model (CT vs CC), and the homozygote model (TT vs CC). Heterogeneity assessment was conducted using the Cochran’s Chi-square-based Q-test. A P value less than 0.10 indicated that the between-study heterogeneity was significant, suggesting that the DerSimonian and Laird method (random-effects model) should be applied for the aggregation of data [12]. Otherwise, when no evidence for high heterogeneity was found (P value no less than 0.10), the pooled ORs and 95% CIs were measured using a fixed-effect model employing the Mantel–Haenszel algorithm [13]. The estimated OR and 95% CI were graphically presented by forest plots. Implementation of subgroup analysis according to the region (Asian or European), cancer types (breast cancer, non-small cell lung cancer, or others), and chemotherapy strategies (Platinum-based or not) was performed by a module in our customized R scripts. The existence of publication bias was detected using a funnel plot via visual inspection. Funnel asymmetry may indicate a publication bias in the meta-analysis. Leave-one-out sensitivity analysis was carried out by iteratively removing a single study from the pooled data set (n, n stands for the number of involved studies) and re-analysing the remaining studies (n-1) to confirm that our results were not statistically driven by any individual study. At the same time, if the removal of one study could significantly impact the results of the heterogeneity evaluation, that study was identified as the source of heterogeneity. A Galbraith plot was generated to visually detect the studies that caused heterogeneity [14].
All of the investigators in this study adhered to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [15].
Results
Characteristics of the eligible studies
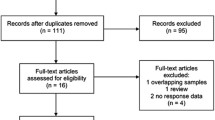
The initial literature screen from the databases and reference searches returned a total of 171 articles. Preliminarily, 11 articles met the pre-defined eligibility criteria after layers of screening [16,17,18,19,20,21,22,23,24,25,26]. After the full-text level review, one study was found to involve patients without Taxane treatment [20]. Another used a definition of disease control [complete response (CR), partial response (PR), or stable disease (SD)] that is not coherent with the one for chemotherapy responders (CR or PR) used in the other studies [26]. Nine studies were ultimately included [16,17,18,19, 21,22,23,24,25]. These studies covered head and neck cancer, gastric cancer, breast cancer, lung cancer, esophagus cancer, and others. A total of 701 individuals (277 responders and 424 non-responders) from nine studies were involved in our rs1045642 polymorphism study. Two studies reported data on European populations, and seven reported on Asian populations. However, one of these nine studies only provided data in the recessive model [25], and another two only used the dominant model [16, 17]. As for the rs1128503 polymorphism, 423 samples (167 responders and 256 non-responders) were enrolled. These samples came from four studies of Asian populations and one from a European population. One study only showed data for the dominant model [17]. The workflow for literature identification is illustrated in Fig. 1. The characteristics of the involved studies are shown in Table 1.
Quantitative synthesis and subgroup analysis
Overall, the summary OR and 95% CI of the combined analyses for the ABCB1 rs1128503 polymorphism revealed no significantly altered response to Taxane-containing chemotherapy (homozygote model: OR = 1.14, 95% CI 0.28–4.62; heterozygote model: OR = 1.24, 95% CI 0.67–2.32; dominant model: OR = 1.22, 95% CI 0.51–2.94, Fig. 2; recessive model: OR = 0.91, 95% CI 0.58–1.42; Table 2). Quantitative synthesis of the involved studies provided no evidence of an association between the ABCB1 rs1045642 polymorphism and chemotherapy response (homozygote model: OR = 1.31, 95% CI 0.80–2.15; heterozygote model: OR = 1.27, 95% CI 0.80–2.02; dominant model: OR = 1.05, 95% CI 0.74–1.49, Fig. 3; recessive model: OR = 0.82, 95% CI 0.39–1.76; Table 3).
Forest plot of the effect of the ABCB1 rs1128503 polymorphism on the response of Taxane-containing chemotherapy regimens according to the dominant model. Pooled ORs and 95% CIs were calculated under both the fixed and random-effects models. A stratified analysis according to the cancer types was performed. Each study is indicated according to the first author’s family name and year of publication. The area of the grey square centred on the estimated OR for an individual study is proportional to its corresponding weight under the fixed-effect model, and the horizontal line represents the matching 95% CI. The columns labelled Weight (fixed) and Weight (random) represent the percentage weight given to an individual study under the fixed and random-effects models. The meta-analysed measures for both the whole and subgroups were plotted as the grey diamonds, while the lateral points indicate the 95% CI for this estimate. The vertical dotted line was used to represent the pooled OR from the random-effect model, while the dashed one flagged the pooled OR from the fixed-effect model. The vertical solid line represents no effect (OR = 1)
Forest plot of the effect of the ABCB1 rs1045642 polymorphism on the response of Taxane-containing chemotherapy regimens according to the dominant model. Pooled ORs and 95% CIs were calculated under both the fixed and random-effects models. A stratified analysis according to the cancer types was performed. Each study is indicated according to the first author’s family name and year of publication. The area of the grey square centred on the estimated OR for an individual study is proportional to its corresponding weight under the fixed-effect model, and the horizontal line represents the matching 95% CI. The columns labelled Weight (fixed) and Weight (random) represent the percentage weight given to an individual study under the fixed and random-effects models. The meta-analysed measures for both the whole and subgroups were plotted as the grey diamonds, while the lateral points indicate the 95% CI for this estimate. The vertical dotted line was used to represent the pooled OR from the random-effect model, while the dashed one flagged the pooled OR from the fixed-effect model. The vertical solid line represents no effect (OR = 1)
For the ABCB1 rs1128503 and rs1045642 polymorphisms, no evidence of a significant association was detected when the meta-analyses were restricted to studies of patients with breast cancer or non-small cell lung cancer. Similarly, the pooled effect estimate remained insignificant for subgroups enrolling subjects with other cancer types (Figs. 2, 3). After stratifying the data according to the region, no statistically significant association between the response to Taxane-containing chemotherapy and these two ABCB1 polymorphisms was found. The differences between therapeutic strategies were evaluated based on the group assignment according to the components of chemotherapy. Neither the Platinum-based group nor non-Platinum-based group of these two ABCB1 polymorphisms showed significantly increased or decreased sensitivity to Taxane-containing chemotherapy. For the rs1045642 polymorphism, no substantial differences for the Asian and European subgroups were observed. As for the rs1128503 polymorphism, the subgroup of the European population with only one study showed increased sensitivity in three genetic models, but not the Heterozygote model.
Publication bias and sensitivity analysis
No obvious asymmetric distribution was observed in the funnel plots of all of the genetic models for the ABCB1 rs1128503 (Fig. 4) and rs1045642 (Fig. 5) polymorphisms. The leave-one-out sensitivity analysis for both rs1128503 and rs1045642 polymorphisms showed that all of the recalculated ORs and corresponding 95% CIs were materially unaltered, suggesting that our meta-analysis was stable (data not shown).
Publication bias analysis of the meta-analysis of the ABCB1 rs1128503 polymorphism. A Begg’s funnel plot with pseudo 95% confidence limits was drawn showing the OR vs the standard error (SE) for the natural logarithm of OR. ORs and 95% CIs were calculated under the dominant model. Each grey square represents a single study. The asymmetric degree of the funnel shape indicates the possibility of publication bias
Publication bias analysis of the meta-analysis of the ABCB1 rs1045642 polymorphism. A Begg’s funnel plot with pseudo 95% confidence limits was drawn showing the OR vs the standard error (SE) for the natural logarithm of OR. ORs and 95% CIs were calculated under the dominant model. Each grey square represents a single study. The asymmetric degree of the funnel shape indicates the possibility of publication bias
Heterogeneity analysis
As for the ABCB1 rs1128503 polymorphism, significant heterogeneity was observed in the homozygote model and dominant model. The source of heterogeneity was identified in the leave-one-out sensitivity analysis. When a single study was removed [18], the heterogeneity in both the homozygote model and dominant model was significantly reduced (homozygote model: heterogeneity test P value = 0.43; dominant model: heterogeneity test P value = 0.53). Although the removal of this study slightly changed the pooled ORs and 95% CIs, no significant association between this polymorphism and patient response to Taxane-containing regimens was observed (homozygote model: OR = 0.71, 95% CI 0.35–1.46; dominant model: OR = 0.87, 95% CI 0.47–1.60).
The recessive model of the rs1045642 polymorphism showed significant heterogeneity. Galbraith plots were used to elucidate the source of heterogeneity. An outlier [25] was identified (Fig. 6). Although the removal of this study from the recessive model diminished the heterogeneity, submarginal significance still existed (heterogeneity test P value = 0.09).
Discussion
Individualized chemotherapy for cancers is tailored to enhance its effectiveness, which is frequently compromised by pharmacoresponse-related genetic variation [27,28,29]. It is of great importance to find molecular biomarkers of chemotherapy drug sensitivity and resistance that may facilitate the improvement of rationally-based treatment decisions. Given the important biological effects of the ABCB1 alleles rs1045642 and rs1128503, a quantitative synthesis based on eligible data was performed. The findings of this meta-analysis suggested that neither the rs1045642 polymorphism nor the rs1128503 polymorphism could influence the effectiveness of Taxane-containing chemotherapy.
Several specific patient characteristics may significantly influence treatment effects. Subgroup analyses could be undertaken to assess these differences [30]. Many confounding factors, such as ethnicity, lifestyle, medical conditions, and medication, may contribute to the regional differences of therapeutic effects. To elucidate the variation between different regions, we stratified the pooled dataset into two subgroups. For the rs1045642 polymorphism, neither the Asian population nor the European population showed significantly altered sensitivity to Taxane-containing chemotherapy regimens. For the rs1128503 polymorphism, there was not enough evidence to associate the minor allele carriers with the increased sensitivity in European patients, even though significance was detected in the European subgroup in three genetic models. This was due to the very limited number of involved studies and samples. The response to chemotherapy may also vary between different cancer types. However, this meta-analysis indicated that these two ABCB1 polymorphisms had no obvious impact on breast cancer, non-small cell lung cancer, or the subgroup of other cancers. Chemotherapy strategies were developed depending on the circumstances that play an important role in the advancement of treatment efficiency. The pooled effect estimates showed that the variant alleles of these two ABCB1 polymorphisms could not significantly affect the sensitivity of the treatment. This was true whether the chemotherapy was Platinum-based or Platinum-free.
Heterogeneity may misdirect the interpretation of this meta-analysis. After filtering out the identified sources of heterogeneity for the rs1128503 polymorphism, the heterogeneity was significantly diminished and the estimate of the pooled ORs and 95% CIs remained stable. As for the rs1045642 polymorphism, the outlier detected in the Galbraith plot could not significantly relieve the heterogeneity. This suggested that there are hidden confounding factors that could lead to the heterogeneity.
This study was conducted to reach comprehensive conclusions about the impact of the ABCB1 polymorphisms in response to Taxane-containing chemotherapy regimens. However, several possible limitations should be considered. First, the ethnical impact was not fully discussed in this study, because all of the involved studies originated from Asian and European nations. Furthermore, the composite effect with other clinical factors and gene variants was not evaluated due to the present data status. Moreover, the sample sizes in the meta-analysis for these two ABCB1 polymorphisms were small. In addition, in meta-analyses of rare events, small variances in the involved data may lead to dramatic changes in the results. The use of relative measures of effects (e.g., OR) could further exaggerate this instability [31, 32]. Finally, the ABCB1 polymorphism rs2032582 (2677G > T/A) was not included in this meta-analysis because of incomplete genotype frequency information and a lack of comparability. Despite these limitations, our meta-analysis was still shown to be useful. On the one hand, the precision of the estimation was improved by integrating multiple data sets and enlarging the sample size. On the other hand, the stability revealed by sensitivity analysis and the uncovering of no publication bias reinforced our confidence in the cogency of our meta-analysis.
Conclusions
In conclusion, this meta-analysis did not provide convincing evidence for a significant association between the ABCB1 rs1045642 and rs1128503 polymorphisms and the response to Taxane-containing regimens based on the published literature. Future research in larger populations with explicit corresponding information is required to evaluate the discrepancies among different Taxane drugs and chemotherapy strategies as well as to elucidate the potential synergistic effect of polymorphisms in the ABCB1 gene and possible impact of ethnicity, gender, and environmental exposure.
References
Leschziner GD, Andrew T, Pirmohamed M, Johnson MR (2006) ABCB1 genotype and PGP expression, function and therapeutic drug response: a critical review and recommendations for future research. Pharmacogenomics J 7(3):154–179
Wu C-P, Hsieh C-H, Wu Y-S (2011) The emergence of drug transporter-mediated multidrug resistance to cancer chemotherapy. Mol Pharm 8(6):1996–2011
Wang R, Sumarpo A, Saiki Y, Chen N, Sunamura M, Horii A (2016) ABCB1 is upregulated in acquisition of taxane resistance: lessons from esophageal squamous cell carcinoma cell lines. Tohoku J Exp Med 240(4):295–301
Hansen SN, Westergaard D, Thomsen MBH et al (2015) Acquisition of docetaxel resistance in breast cancer cells reveals upregulation of ABCB1 expression as a key mediator of resistance accompanied by discrete upregulation of other specific genes and pathways. Tumor Biol 36(6):4327–4338
Boora GK, Kanwar R, Kulkarni AA et al (2016) Testing of candidate single nucleotide variants associated with paclitaxel neuropathy in the trial NCCTG N08C1 (Alliance). Cancer Med 5(4):631–639. https://doi.org/10.1002/cam4.625
Mizuno N, Niwa T, Yotsumoto Y, Sugiyama Y (2003) Impact of drug transporter studies on drug discovery and development. Pharmacol Rev 55(3):425–461
Hitzl M, Drescher S, van der Kuip H et al (2001) The C3435T mutation in the human MDR1 gene is associated with altered efflux of the P-glycoprotein substrate rhodamine 123 from CD56 + natural killer cells. Pharmacogenetics 11(4):293–298
Hoffmeyer S, Burk O, von Richter O et al (2000) Functional polymorphisms of the human multidrug-resistance gene: Multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA 97(7):3473–3478
Caronia D, Patiño-Garcia A, Peréz-Martínez A et al (2011) Effect of ABCB1 and ABCC3 polymorphisms on osteosarcoma survival after chemotherapy: a Pharmacogenetic Study. PLOS One 6(10):e26091. https://doi.org/10.1371/journal.pone.0026091
Schaich M, Kestel L, Pfirrmann M et al (2009) A MDR1 (ABCB1) gene single nucleotide polymorphism predicts outcome of temozolomide treatment in glioblastoma patients. Ann Oncol 20(1):175–181. https://doi.org/10.1093/annonc/mdn548
Schwarzer G (2007) Meta: an R package for meta-analysis. R News 7(3):40–45
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22(4):719–748
Galbraith R (1988) A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med 7(8):889–894
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Chang H, Rha SY, Jeung HC et al (2009) Association of the ABCB1 gene polymorphisms 2677G > T/A and 3435C > T with clinical outcomes of paclitaxel monotherapy in metastatic breast cancer patients. Ann Oncol 20(2):272–277
Choi JR, Kim J-O, Kang DR et al (2015) Genetic variations of drug transporters can influence on drug response in patients treated with docetaxel chemotherapy. Cancer Res Treat 47(3):509–517
Grau JJ, Caballero M, Campayo M et al (2009) Gene single nucleotide polymorphism accumulation improves survival in advanced head and neck cancer patients treated with weekly paclitaxel. Laryngoscope 119(8):1484–1490
Isla D, Sarries C, Rosell R et al (2004) Single nucleotide polymorphisms and outcome in docetaxel-cisplatin-treated advanced non-small-cell lung cancer. Ann Oncol 15(8):1194–1203
Lévy P, Gligorov J, Antoine M et al (2013) Influence of ABCB1 polymorphisms and docetaxel pharmacokinetics on pathological response to neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res Treat 139(2):421–428
Pan J-h, Han J-x, Wu J-m, Huang H-n Yu Q-Z, Sheng L-J (2009) MDR1 single nucleotide polymorphism G2677T/A and haplotype are correlated with response to docetaxel-cisplatin chemotherapy in patients with non-small-cell lung cancer. Respiration 78(1):49–55
Qiao R, Wu W, Lu D, Han B (2016) Influence of single nucleotide polymorphisms in ABCB1, ABCG2 and ABCC2 on clinical outcomes to paclitaxel-platinum chemotherapy in patients with non-small-cell lung cancer. Int J Clin Exp Med 9(1):298–307
Shim HJ, Yun JY, Hwang JE et al (2010) BRCA1 and XRCC1 polymorphisms associated with survival in advanced gastric cancer treated with taxane and cisplatin. Cancer Sci 101(5):1247–1254
Tulsyan S, Chaturvedi P, Singh AK et al (2014) Assessment of clinical outcomes in breast cancer patients treated with taxanes: multi-analytical approach. Gene 543(1):69–75
Wang J, Tang J, Zhong S et al (2011) Association between MDR1 gene polymorphisms and curative effect of taxane-anthracycline chemotherapy in breast cancer. Chin J Clin Oncol 38(1):15–19
Zhou J, Deng W, Gao J, Yuan J, Li Y, Shen L (2015) Association between ABCB1 G2677T/A polymorphisms and chemosensitivity of paclitaxel in advanced gastric cancer. Chin J Gastrointest Surg 18(2):123–126
Hertz DL, Rae J (2015) Pharmacogenetics of cancer drugs. Annu Rev Med 66:65–81
Ulrich CM, Robien K, McLeod HL (2003) Cancer pharmacogenetics: polymorphisms, pathways and beyond. Nat Rev Cancer 3(12):912–920
Rodriguez-Antona C, Taron M (2015) Pharmacogenomic biomarkers for personalized cancer treatment. J Intern Med 277(2):201–217
Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM (2007) Statistics in Medicine—reporting of subgroup analyses in clinical trials. N Engl J Med 357(21):2189–2194
Greco T, Zangrillo A, Biondi-Zoccai G, Landoni G (2013) Meta-analysis: pitfalls and hints. Heart Lung Vessels 5(4):219–225
Walker E, Hernandez AV, Kattan MW (2008) Meta-analysis: Its strengths and limitations. Clevel Clinic J Med 75(6):431–439
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, the research start-up fund of Soochow University (Q413400116), and the special fund of Soochow University for medical college students’ extracurricular scientific research.
Conflict of interest
Qi Jiang declares that she has no conflict of interest. Meizhen Xu declares that she has no conflict of interest. Yina Liu declares that she has no conflict of interest. Yudi Chen declares that she has no conflict of interest. Jiarong Feng declares that she has no conflict of interest. Xuelin Wang declares that he has no conflict of interest. Shuang Liang declares that he has no conflict of interest. Dan Li declares that she has no conflict of interest. Xiaoqin Yang declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Jiang, Q., Xu, M., Liu, Y. et al. Influence of the ABCB1 polymorphisms on the response to Taxane-containing chemotherapy: a systematic review and meta-analysis. Cancer Chemother Pharmacol 81, 315–323 (2018). https://doi.org/10.1007/s00280-017-3496-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3496-1