Abstract
Purpose
Anthracyclines are a mainstay of the treatment of several childhood malignancies, but their utility is limited by dose-related cardiotoxicity. This study is aimed to explore the link between exposure of paediatric cancer patients to doxorubicin and its metabolite doxorubicinol, and cardiac troponin I (cTnI).
Methods
In a prospective pilot study plasma doxorubicin, doxorubicinol, and cTnI concentrations were measured in samples from children undergoing cancer chemotherapy. A mixed-effects population pharmacokinetic model for doxorubicin and doxorubicinol and in combination with a turn-over model for cTnI were developed.
Results
Seventeen patients, aged 3.4–14.7 year, treated for a variety of cancers had 99 doxorubicin and 119 doxorubicinol concentrations analysed from samples drawn between 0.5 and 336 h after the start of the infusion. Eleven patients had received previous doses of anthracyclines, with a median cumulative prior dose of 90 mg/m2 (range 0–225 mg/m2). The median administered doxorubicin dose was 30 mg/m2 (range 25–75 mg/m2). Doxorubicin disposition was described by a three-compartment model with first-order elimination and metabolism to doxorubicinol. Body surface area was related to all clearance and distribution parameters and age further influenced clearance (CL, 58.7 L/h/1.8 m2 for an average 8.4-year-old patient). Combined doxorubicin and metabolite exposure stimulated a temporary increase in cTnI in plasma, with a concentration of 11.8 µg/L required to achieve half-maximal effect. Prior cumulative anthracycline dosage received by patients was predictive of an increased cTnI baseline prior to a new doxorubicin dose.
Conclusion
Prior anthracycline exposure increased baseline cTnI in a dose-dependent manner, consistent with the known cumulative risk of anthracycline exposure-induced cardiotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthracyclines play a vital role in the treatment of several childhood malignancies, but their utility is clouded by dose-related cardiotoxicity [1,2,3]. These drugs, especially doxorubicin and daunorubicin, are used in up to about two-thirds of paediatric oncology patients, and have a significant impact on therapeutic success [3]. However, a significant adverse-effect of doxorubicin therapy is dose-dependent late cardiotoxicity, with long-term survivors of paediatric malignancy having a five-fold to six-fold increased likelihood of cardiac malfunction compared to healthy controls [4]. It has been reported that more than one-half of treated children will develop asymptomatic cardiac dysfunction, whilst one-sixth will develop significant heart failure secondary to cardiomyopathy [5]. Although not fully understood, such cardiotoxicity may be due to the generation of free radicals, oxidative stress, reduced antioxidant enzyme activity and decreased thiol groups [1, 6]. Importantly, Olson et al. [7] provided evidence that doxorubicinol (the C-13 alcohol metabolite of doxorubicin) may contribute to cardiotoxicity associated with doxorubicin chemotherapy. Doxorubicinol is formed in the cardiac tissues and accumulates in sufficient concentrations to compromise systolic and diastolic heart function, most likely by perturbation of calcium homeostasis. In support, Mushlin et al. [8] subsequently published data from isolated rat aorta studies indicating that the time-related development of myocardial contractile dysfunction most likely involves doxorubicinol and doxorubicin acting in concert. With improved disease outcomes and increased survival, particularly in children, strategies aimed at reducing anthracycline-induced cardiotoxicity are of importance [1].
A number of clinical risk factors for the development of doxorubicin related cardiotoxicity have been identified, including higher single and cumulative doses, younger age, and short infusion times [9]. More recently, genetic susceptibility to anthracycline-induced cardiotoxicity was recognised as a potential contributor to outcome in paediatric oncology patients, with a non-synonymous variant in the retinoic acid receptor γ (RARG) gene being highly associated with cardiotoxicity [10].
The use of doxorubicin is further challenging due to large inter-subject pharmacokinetic variability [11]. Age-related differences in body weight and surface area as well as maturation processes can result in different exposure in children given the same body-size adjusted dose of a cytotoxic drug. Kontny et al. [12] reported a population pharmacokinetic model for doxorubicin in adults and in children older than 3 years which explained much of the variability by differences in body surface area. Voller et al. [13] focused on children under 3 years, and noted an age-dependent maturation of doxorubicin clearance with significantly lower clearances in very young children.
Markers of anthracycline cardiotoxicity frequently used in human clinical trials include cardiac troponin I (cTnI). Two recent reviews suggested that cTnI may be of prognostic value in dealing with anthracycline cardiotoxicity [14, 15]. The development of biochemical markers have allowed for non-invasive measures of myocardial damage (troponin), and of the neurohumoral response that ensues in heart failure settings (natriuretic peptide) [16,17,18,19]. cTnI has been shown to be sensitive and specific for myocardial damage, and its use in the clinic has become common place in the diagnostic and prognostic evaluation of acute coronary syndromes (ACS) [14, 17, 20]. Some clinical studies demonstrate that even small increases of troponin during acute ischemic episodes are associated with increased risks of future cardiac events [17] and thus cardiac troponin monitoring during or after anthracycline therapy may be prognostically useful.
Although it has been demonstrated by others that doxorubicin exposure correlates with response to treatment in childhood acute myeloid leukaemia (AML) [21], a causative link between doxorubicin pharmacokinetics and cardiotoxicity is yet to be fully established or understood. The present study was designed to investigate the existence of a relationship, if any, between doxorubicin and doxorubicinol pharmacokinetics and biochemical markers of cardiotoxicity in children with cancer.
Methods
Patient eligibility and ethics
Paediatric oncology patients were recruited from the Royal Children’s Hospital, Brisbane, Australia (now Lady Cilento Children’s Hospital, Brisbane). Patients were eligible if younger than 18 years, were scheduled to receive at least two doses of doxorubicin for any form of childhood cancer, and they had not experienced any prior adverse events from doxorubicin. Details of previous anthracycline administration were recorded. The study was approved by the Royal Children’s Hospital Human Research Ethics Committee (HREC), with informed parental consent, and where appropriate, a patient’s assent obtained for each participant. The maximum volume of blood collected from each patient was 2 mL/kg of body weight as approved by the HREC.
Study design, treatment, blood sampling
Doxorubicin was prescribed by the oncologist for each patient based on their body surface area. The stopping time of an intravenous infusion was taken as the point at which the line used to infuse doxorubicin was clear of visible (red) drug. Blood was drawn at the following time points: pre-infusion; then (post-infusion) 5–10 min, 1–2 h, 2–12 h, 24–120 h (1–5 days), 168 h (7 days). The 24–120-h sampling period was chosen for logistical reasons and for patient convenience. Blood was collected via a venous catheter (Port-a-Cath, Hickman line), after withdrawal of a 5 mL discard of fluid/blood. The fluid/blood discards were kept for doxorubicin and doxorubicinol analysis to check for potential contamination of samples due to re-adsorption of drug to the catheter lumen during dosing and/or release of administered drug on sample collection.
Measurement of doxorubicin and doxorubicinol
Blood and discard blood/fluid for doxorubicin and doxorubicinol were collected in EDTA tubes and transported on ice for processing within 5–10 min of collection. Samples were centrifuged at 1800g for 15 min at 24 °C and the plasma supernatants were stored at −80 °C.
Plasma samples were assayed for doxorubicin and doxorubicinol [22]; presently, lower quantification limits were achieved using a Shimadzu LC-20 pump system with a RF-10AXL fluorescence detector. The lower limit of quantification (LLQ) was 4.7 ng/mL, at which the coefficients of variation (CV%) for doxorubicin and doxorubicinol were 10.6 and 20.1%, respectively, and accuracies were 102 and 92.6%, respectively. The lower limit of detection (LLD) was 0.95 ng/mL for both compounds.
Measurement of cTnI
Samples for determination of cTnI were collected, prior to the doxorubicin dose and at the same time points samples for doxorubicin concentrations were sampled, as in serum separator tubes and analysed without delay by immunoassay (UniCel DxI 800, Beckman Coulter Inc, Fullerton CA). The upper reference limit for this assay is <0.04 μg/L (=99th percentile), and concentrations ≥0.04 μg/L were taken as being positive for a cTnI leak.
Population model development
Modelling was conducted using NONMEM version 7.3 [23], in combination with the Intel FORTRAN compiler, and PsN (Perl-speaks-NONMEM) version 4.1 [24]. Structural model parameter estimates, inter-individual variability (IIV) and residual unexplained variability (RUV) were estimated using first-order conditional estimation with interaction (FOCE + I).
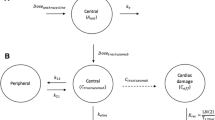
The base pharmacokinetic model for doxorubicin and doxorubicinol was adapted from published studies [12, 13]. Therefore, initially a 3-compartment model for doxorubicin, and a 1-compartment for doxorubicinol was tested with first-order metabolism to doxorubicinol (Q m) fixed to 32.1 L/h/1.8 m2 [12]. Other model structures were evaluated subsequently. Clearance of doxorubicin (CL) and doxorubicinol (CLm), volumes of distribution of the central compartment and the two peripheral compartment for doxorubicin (V1, V2, V3, respectively) and doxorubicinol (V4), and the inter-compartmental clearances for doxorubicin (Q2, Q3) were estimated. The metabolism from parent to metabolite was included as metabolic clearance of doxorubicin into a ‘metabolite’ compartment. As mass units were used to describe the concentrations of doxorubicin and metabolite, the CLm and V4 were adjusted using the molecular weight to account for the stoichiometric conversion from doxorubicin to metabolite. Estimation of IIV, including the off-diagonal elements (covariances) of the variance matrix, were tested on all parameters using an exponential error model. The RUV was estimated using proportional, additive and combined error models separately for doxorubicin, doxorubicinol and plasma cardiac cTnI. The first concentration after a given dose reported as below the LLD was assigned to half the value of LLD; any further concentration reported below the LLD within the same dosing interval were removed [25, 26]. Covariates available included weight, age, gender, height, and body surface area. These were initially screened graphically versus parameter estimates. An a priori assumption was made that all size covariates would influence all clearance and distribution covariates. Covariates were retained in the model if biologically plausible and a statistically significant decrease in the objective function value (OFV) was noted. The OFV is an internally generated metric of NONMEM used to identify potential improvements in fit of the model to the data. The difference between a pair of OFV values for nested models approximates the Chi-square (χ 2) distribution, which can be hypothesis-tested for significance (\( \chi^{ 2}_{ 1,0.0 5} \; = \; 3. 8 4 \)) [23]. The influence of continuous covariates was screened, in turn, using linear, power and exponential functions.
Pharmacokinetic parameters relating to drug transfer between peripheral compartments, and covariate relationships related to effect were fixed to the final values of the doxorubicin-metabolite pharmacokinetic model, prior to evaluating the effect of doxorubicin and doxorubicinol exposure on plasma cTnI concentrations. The cardiotoxic exposure effect (E) of stimulation of cTnI release into plasma was described using an E max function (Eq. 1):
where E max is a dimensionless parameter which describes the maximum possible effect caused by drug exposure; ConcDox+Doxol is the combined plasma concentrations of doxorubicin (Dox) and doxorubicinol (Doxol); EC50 is the combined plasma concentration producing half-maximal effect. The time-course of plasma cTnI concentration following doxorubicin and doxorubicinol exposure was described using a turn-over effect model [27], where the cardiotoxic exposure effect of doxorubicin-stimulated and doxorubicinol-stimulated cTnI was described using the following functions (Eqs. 2, 3):
where k in is the zero-order release rate constant into plasma of cTnI, and k deg is the first-order rate constant of loss from the plasms compartment. At steady state, baseline cTnI concentrations (\( C_{{{\text{base}}_{\text{cTnI}} }} ) \) is equal to the ratio of k in to k deg according to Eq. 3. After the addition of the structural models for cTnI to the doxorubicin metabolite model, potential stochastic models were evaluated followed by covariate model development and model evaluation steps. Covariates tested were infusion rates of doxorubicin on E and prior cumulative anthracyclines dose amounts (mg/m2) on \( C_{{{\text{base}}_{\text{cTnI}} }} \).
During model development goodness-of-fit (GOF) plots (if shrinkage was low), and prediction-corrected and variance-corrected visual predictive checks (pcvcVPC) were used as diagnostics [28]. The percentile bootstrap 95% confidence intervals around the final population model parameters were obtained in PsN using an automated non-parametric bootstrap with sample replacement of 500 runs [29]. The NONMEM control stream used for the final model is shown in “Appendix”.
Results
Patients and dosing
Nineteen children aged 3.42–14.67 years (median 7.50) were enrolled on the study, of which 17 had blood sampled for analysis of one or two doses of doxorubicin (median age 7.00 years, range 3.42–14.67). Blood samples after a total of 24 doses of doxorubicin were analysed. Eleven patients had received doses of anthracyclines prior to the first observed dose in this study (median 100 mg/m2, range 25–225 mg/m2). Six patients had not received any anthracyclines prior to the first dose analysed. The median dose of previous anthracycline for all doses analysed was 95 mg/m2 (range 0–225 mg/m2). Only one patient received dexrazoxane as a cardioprotectant. Patient characteristics are detailed in Table 1. Echocardiography was obtained as per individual patient treatment protocol or treating physician. No patient had any significant echocardiographic changes or abnormal fractional shortening during the course of the study.
Four of the 17 patients (23.5%) sampled had a confirmed leak of cTnI, defined as ≥0.04 μg/L, after doxorubicin dosing. All four patients had positive troponin results within 24 h of a doxorubicin dose. Two patients, a male aged 13.6 years and a female aged 14.5 years, had a respective cTnI measurement of 0.1 and 0.05 μg/L immediately prior to the observed dose of doxorubicin. Both patients received doses of doxorubicin of 180 and 120 mg/m2, for 55 and 47 days, respectively, prior to the doxorubicin dose monitored during this study. Of the six patients who had never been previously exposed to doxorubicin therapy, four had a baseline cTnI ≥ 0.01 μg/L. The maximum baseline level noted in this group was 0.03 μg/L, with an average of 0.016 μg/L. Of the 11 patients who had prior exposure to doxorubicin therapy, eight had a baseline cTnI ≥ 0.01 μg/L. The maximum baseline level noted in this group was 0.08 μg/L, with an average of 0.023 μg/L.
Population modelling
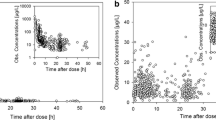
One hundred doxorubicin concentrations (of which 15% were below the LLD), 120 doxorubicinol concentrations (11% below LLD), and 104 cTnI measurements were available for modelling. The data are illustrated in Fig. 1; 33% of doxorubicin and doxorubicinol samples were taken within 12 h of the start of the infusion, 14% of samples were taken between 12–24 h and 53% after 24-h post-dose. For all patients a baseline cTnI measurement was available and 50% of data points were measured within 12 h of the start of the infusion, 12% between 12–24 h and 38% after 24-h post-dose.
The time-course of doxorubicin and doxorubicinol in plasma up to 336 h after administration was best described by the adopted compartmental model from the literature [12, 13] (Fig. 2, “Appendix”). The typical population CL for doxorubicin (IIV expressed as CV%) was 58.7 L/h/1.8 m2 (18.6%), and typical V1 was 32.2 L/1.8 m2. The typical CLm was 19.3 L/h/1.8 m2 (29.4%), and V4 was 508 L/1.8 m2. The typical Q m was fixed; the estimated IIV of Q m was 33.2%. A combined additive-logarithmic error model best described the RUV for both the parent and metabolite. As previously reported [13], age influenced CL significantly and improved the fit (ΔOFV = −39.9). Furthermore, the addition of BSA on all clearance and distribution parameters further improved the fit (ΔOFV = −33.3). No other factor further significantly explained the variability in doxorubicin pharmacokinetics, or improved the fit of the model.
The maximum effect (E max) on release of cTnI after combined exposure was estimated as 0.15, the first-order rate of cTnI degradation (k deg) was 0.6 h−1 (representative of a turn-over half-life of 1.2 h), and the summed doxorubicin and doxorubicinol concentration required to achieve half-maximal effect (EC50) was estimated as 11.8 µg/L. With pre-dose cTnI concentrations available for all patients, the baseline cTnI was estimated as 20.5 pg/mL (BSV 24.7%). Inclusion of prior cumulative anthracyclines doses received by the patients improved the model fit (ΔOFV = −4.9), and resulted in an estimated cTnI baseline increase by 0.31% with every 1 mg/m2 increase in prior to cumulative anthracyclines doses.
Standard GOF graphics supported the model building. Conditional weighted residuals versus time after dose (hours) for the final model are illustrated in Fig. 1. The pcvcVPC of the final model is shown in Fig. 3. The final model was evaluated using a non-parametric bootstrap which produced similar parameter estimates similar to those from the final model (Table 2), indicating that the estimates for the population PK parameters in the final model are robust and stable.
Prediction-corrected and variance-corrected VPCs for doxorubicin (a), doxorubicinol (b) and cardiac troponin I (c). The raw data are represented as black circles and lines. Grey shaded areas are the 90% confidence interval for the 5th, 50th, and 95th percentile of the simulated data. The length of the confidence interval areas illustrates bin size
Discussion
The primary aim of this pilot study was to assess the relationship, if any, between doxorubicin pharmacokinetics and markers of cardiotoxicity.
cTnI concentration data were obtained for each set of doxorubicin and doxorubicinol concentrations, which facilitated description of the time-course of cTnI concentrations arising after doxorubicin administration. Increased doxorubicin infusion rate and prior anthracycline dosing showed linear relationships with baseline cTnI concentration; however, only prior anthracycline dosing could be shown to significantly increase baseline cTnI. For every 1 mg/m2 increase in prior anthracycline therapy, there was a 0.31% increase in baseline cTnI, a result supported by a previous study which showed induction of cTnI soon after doxorubicin administration [30]. The relationship between cumulative anthracycline dose and baseline cTnI in the present study, and the extension of the pharmacokinetic model for doxorubicin to include cTnI, provided a novel aspect which contributes towards increasing an understanding of the dose-concentration-toxicity relationship in children with cancer. Presently, we showed evidence of a trend towards cTnI elevation shortly after doxorubicin administration in this population, with a subsequent resolution close to population average cTnI baseline measurements within 100 h.
An estimated turn-over half-life of 1.2 h was calculated, which is in keeping with the range that has been noted in rats [27], and in humans, with an observed a cTnI half-life of 2 h [31]. It is unclear if earlier and more frequent troponin sampling, perhaps during or shortly after doxorubicin dosing, would have made a difference to the results. Although increased cTnI measurements are useful prognostically in terms of doxorubicin-cardiotoxicity in patients [14], we caution that the relatively low levels of cTnI presently recorded are reflected in the parameter estimates of E max and EC50, and should be applied with some caution with respect to wider extrapolation. It is also important to note that the prior cumulative doses of doxorubicin ranged from 0 to 225 mg/m2, but in clinical practice doses much higher than 225 mg/m2 are more typically associated with significant myocardial injury [32]. Recent work by Nathan et al. indicated that the incidence of congestive heart failure approached 10% in children exposed to cumulative doses above 300 mg/m2 at 20-year follow-up after treatment [32]. Thus, the relatively low cumulative doses of doxorubicin presently prescribed may also have contributed to the relatively low doxorubicin-induced troponin (cTnI) concentrations. Nonetheless, there was a significant relationship between prior cumulative anthracycline dose and baseline cTnI. Looking for trends in troponin and relative increases from previous baseline values may reveal evidence of early cardiotoxicity, and thereby assist in the monitoring and avoidance of progression of cardiotoxic effects.
The importance of monitoring doxorubicin cardiotoxicity has been recognised previously, especially given the increased survival rates in modern paediatric oncology practice, but it still unclear what the long-term outcomes of doxorubicin treatment in childhood are later in life. There is some evidence that in high-risk paediatric acute lymphoblastic leukaemia, elevated levels of cTnI and N-terminal pro-brain natriuretic peptide (NT-proBNP), which is a marker of ventricular wall stress, are detected within 90 days of anthracycline therapy, and are associated with impaired left ventricular function cardiac function up to 4 years after therapy is completed [33]. However, it is unclear if this impaired cardiac function persists later in life, and whether elevated troponin concentrations immediately after doxorubicin dosing correlate with persistent impaired cardiac function up to decades after chemotherapy.
Clearly, in extending the conclusions of this study, further investigation and validation with a larger independent dataset is warranted, and also with other chemotherapeutic and novel targeted therapies to determine whether cardiotoxic effects can be monitored by TnI levels, and whether these relationships with other medications are similar to those presently observed.
Conclusion
This pilot study identified that prior anthracycline exposure and infusion rates correlated with baseline cTnI levels, which may be useful in the assessment of long-term cardiotoxicity risk prediction. Such a relationship was noted despite the relatively low cumulative doses of doxorubicin observed. The present study contributes to an improved understanding of the factors underscoring dose-concentration-toxicity relationships in children with cancer.
References
Gianni L, Herman EH, Lipshultz SE, Minotti G, Sarvazyan N, Sawyer DB (2008) Anthracycline cardiotoxicity: from bench to bedside. J Clin Oncol 26(22):3777–3784. doi:10.1200/JCO.2007.14.9401
Hori H, Kudoh T, Nishimura S, Oda M, Yoshida M, Hara J, Tawa A, Usami I, Tanizawa A, Yumura-Yagi K, Kato K, Kobayashi R, Komada Y, Matsuo K, Horibe K, Japan Associationof Childhood Leukemia S (2016) Acute and late toxicities of pirarubicin in the treatment of childhood acute lymphoblastic leukemia: results from a clinical trial by the Japan Association of Childhood Leukemia Study. Int J Clin Oncol. doi:10.1007/s10147-016-1062-1
van Dalen EC, Raphael MF, Caron HN, Kremer LC (2009) Treatment including anthracyclines versus treatment not including anthracyclines for childhood cancer. Cochrane Database Syst Rev (1):CD006647. doi:10.1002/14651858.CD006647.pub2
Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, Donaldson SS, Green DM, Sklar CA, Robison LL, Leisenring WM (2009) Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 339:b4606. doi:10.1136/bmj.b4606
van der Pal HJ, van Dalen EC, Hauptmann M, Kok WE, Caron HN, van den Bos C, Oldenburger F, Koning CC, van Leeuwen FE, Kremer LC (2010) Cardiac function in 5-year survivors of childhood cancer: a long-term follow-up study. Arch Intern Med 170(14):1247–1255. doi:10.1001/archinternmed.2010.233
Scully RE, Lipshultz SE (2007) Anthracycline cardiotoxicity in long-term survivors of childhood cancer. Cardiovasc Toxicol 7(2):122–128. doi:10.1007/s12012-007-0006-4
Olson RD, Mushlin PS, Brenner DE, Fleischer S, Cusack BJ, Chang BK, Boucek RJ Jr (1988) Doxorubicin cardiotoxicity may be caused by its metabolite, doxorubicinol. Proc Natl Acad Sci USA 85(10):3585–3589
Mushlin PS, Cusack BJ, Boucek RJ Jr, Andrejuk T, Li X, Olson RD (1993) Time-related increases in cardiac concentrations of doxorubicinol could interact with doxorubicin to depress myocardial contractile function. Br J Pharmacol 110(3):975–982
Tukenova M, Guibout C, Oberlin O, Doyon F, Mousannif A, Haddy N, Guerin S, Pacquement H, Aouba A, Hawkins M, Winter D, Bourhis J, Lefkopoulos D, Diallo I, de Vathaire F (2010) Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol 28(8):1308–1315. doi:10.1200/JCO.2008.20.2267
van den Anker JN (2015) How to improve the safe and effective use of doxorubicin in children with cancer. Clin Pharmacokinet 54(11):1091–1093. doi:10.1007/s40262-015-0300-4
Frost BM, Eksborg S, Bjork O, Abrahamsson J, Behrendtz M, Castor A, Forestier E, Lonnerholm G (2002) Pharmacokinetics of doxorubicin in children with acute lymphoblastic leukemia: multi-institutional collaborative study. Med Pediatr Oncol 38(5):329–337. doi:10.1002/mpo.10052
Kontny NE, Wurthwein G, Joachim B, Boddy AV, Krischke M, Fuhr U, Thompson PA, Jorger M, Schellens JH, Hempel G (2013) Population pharmacokinetics of doxorubicin: establishment of a NONMEM model for adults and children older than 3 years. Cancer Chemother Pharmacol 71(3):749–763. doi:10.1007/s00280-013-2069-1
Voller S, Boos J, Krischke M, Wurthwein G, Kontny NE, Boddy AV, Hempel G (2015) Age-dependent pharmacokinetics of doxorubicin in children with cancer. Clin Pharmacokinet. doi:10.1007/s40262-015-0272-4
Germanakis I, Anagnostatou N, Kalmanti M (2008) Troponins and natriuretic peptides in the monitoring of anthracycline cardiotoxicity. Pediatr Blood Cancer 51(3):327–333. doi:10.1002/pbc.21633
Mavinkurve-Groothuis AM, Kapusta L, Nir A, Groot-Loonen J (2008) The role of biomarkers in the early detection of anthracycline-induced cardiotoxicity in children: a review of the literature. Pediatr Hematol Oncol 25(7):655–664. doi:10.1080/08880010802244001
Tang WH, Francis GS, Morrow DA, Newby LK, Cannon CP, Jesse RL, Storrow AB, Christenson RH, Apple FS, Ravkilde J, Wu AH, National Academy of Clinical Biochemistry Laboratory M (2007) National Academy of Clinical Biochemistry Laboratory Medicine practice guidelines: clinical utilization of cardiac biomarker testing in heart failure. Circulation 116(5):e99–e109. doi:10.1161/CIRCULATIONAHA.107.185267
Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, Storrow AB, Wu AH, Christenson RH, National Academyof Clinical B (2007) National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation 115(13):e356–e375. doi:10.1161/CIRCULATIONAHA.107.182882
Apple FS, Wu AH, Jaffe AS, Panteghini M, Christenson RH, Cannon CP, Francis G, Jesse RL, Morrow DA, Newby LK, Storrow AB, Tang WH, Pagani F, Tate J, Ordonez-Llanos J, Mair J, National Academyof Clinical B, Medicine ICfSoMoCDL (2007) National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine practice guidelines: analytical issues for biomarkers of heart failure. Circulation 116(5):e95–e98. doi:10.1161/CIRCULATIONAHA.107.185266
Apple FS, Jesse RL, Newby LK, Wu AH, Christenson RH, NationalAcademy of Clinical B, Damage ICfSoMoC (2007) National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine Practice Guidelines: analytical issues for biochemical markers of acute coronary syndromes. Circulation 115(13):e352–e355. doi:10.1161/CIRCULATIONAHA.107.182881
Gupta S, de Lemos JA (2007) Use and misuse of cardiac troponins in clinical practice. Prog Cardiovasc Dis 50(2):151–165. doi:10.1016/j.pcad.2007.01.002
Palle J, Frost BM, Peterson C, Gustafsson G, Hellebostad M, Kanerva J, Schmiegelow K, Lonnerholm G, Nordic Society for Pediatric H, Oncology (2006) Doxorubicin pharmacokinetics is correlated to the effect of induction therapy in children with acute myeloid leukemia. Anticancer Drugs 17(4):385–392. doi:10.1097/01.cad.0000198911.98442.16
Gilbert CM, McGeary RP, Filippich LJ, Norris RL, Charles BG (2005) Simultaneous liquid chromatographic determination of doxorubicin and its major metabolite doxorubicinol in parrot plasma. J Chromatogr B Analyt Technol Biomed Life Sci 826(1–2):273–276
Beal S, Sheiner LB, Boeckmann A, Bauer RJ (2009) NONMEM User’s Guides. (1989–2009)
Lindbom L, Pihlgren P, Jonsson EN (2005) PsN-Toolkit–a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Progr Biomed 79(3):241–257
Hennig S, Waterhouse TH, Bell SC, France M, Wainwright CE, Miller H, Charles BG, Duffull SB (2007) A d-optimal designed population pharmacokinetic study of oral itraconazole in adult cystic fibrosis patients. Br J Clin Pharmacol 63(4):438–450. doi:10.1111/j.1365-2125.2006.02778.x
Beal SL (2001) Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 28(5):481–504
Mikaelian I, Dunn ME, Mould DR, Hirkaler G, Geng W, Coluccio D, Nicklaus R, Singer T, Reddy M (2013) Differential analysis of transient increases of serum cTnI in response to handling in rats. Pharmacol Res Perspect 1(2):e00011. doi:10.1002/prp2.11
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO (2011) Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13(2):143–151
Parke J, Holford NH, Charles BG (1999) A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput Methods Progr Biomed 59(1):19–29
Arola OJ, Saraste A, Pulkki K, Kallajoki M, Parvinen M, Voipio-Pulkki LM (2000) Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res 60(7):1789–1792
Hickman PE, Potter JM, Aroney C, Koerbin G, Southcott E, Wu AH, Roberts MS (2010) Cardiac troponin may be released by ischemia alone, without necrosis. Clin Chim Acta 411(5–6):318–323. doi:10.1016/j.cca.2009.12.009
Nathan PC, Amir E, Abdel-Qadir H (2016) Cardiac outcomes in survivors of pediatric and adult cancers. Can J Cardiol 32(7):871–880. doi:10.1016/j.cjca.2016.02.065
Lipshultz SE, Miller TL, Scully RE, Lipsitz SR, Rifai N, Silverman LB, Colan SD, Neuberg DS, Dahlberg SE, Henkel JM, Asselin BL, Athale UH, Clavell LA, Laverdiere C, Michon B, Schorin MA, Sallan SE (2012) Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high-risk acute lymphoblastic leukemia: associations with long-term echocardiographic outcomes. J Clin Oncol 30(10):1042–1049. doi:10.1200/JCO.2010.30.3404
Acknowledgements
This work was supported by the Children’s Hospital Foundation, Queensland (AM). The authors acknowledge the contribution of the Australian Centre of Pharmacometrics with respect to the NONMEM license and hardware.
Author contributions
KK wrote the manuscript, supported data collection and data interpretation; SH performed model development, supported data and result interpretation and manuscript writing; RN and ML performed assay development, sample analysis and manuscript writing; BC and RP supported study design and manuscript review; AM designed and performed the study, collected data and reviewed manuscript writing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Children’s Hospital Foundation, Queensland (AM). The authors acknowledge the contribution of the Australian Centre of Pharmacometrics with respect to the NONMEM license and hardware.
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research ethics committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
In the original publication of this article, there were errors in the Appendix; this errors have now been corrected.
An erratum to this article is available at http://dx.doi.org/10.1007/s00280-017-3338-1.
Appendix: NMTRAN control stream for the final model
Appendix: NMTRAN control stream for the final model


Rights and permissions
About this article
Cite this article
Kunarajah, K., Hennig, S., Norris, R.L.G. et al. Population pharmacokinetic modelling of doxorubicin and doxorubicinol in children with cancer: is there a relationship with cardiac troponin profiles?. Cancer Chemother Pharmacol 80, 15–25 (2017). https://doi.org/10.1007/s00280-017-3309-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3309-6