Abstract
Purpose
The options for improving the chemotherapeutic regimen consisting of bolus plus infusion of 5-fluorouracil (5-FU) include omitting the 5-FU bolus injection. We examined the effects of a 5-FU bolus injection on the activity of dihydropyrimidine dehydrogenase (DPD), which is the first and rate-limiting enzyme of 5-FU catabolism, in rats.
Methods
The rats were divided into three groups, and then continuous infusion (50 mg/m2/h) for 4 h was started with a bolus injection of saline, 20 mg/kg 5-FU, or 60 mg/kg 5-FU. Plasma 5-FU, uracil (Ura), dihydrouracil (UH2) levels, and hepatic DPD activity were determined after administration of 5-FU.
Results
The half-life after the end of the infusion (t 1/2, 4–8 h) of 5-FU in the rats given the bolus injection was significantly longer than in those that had been given saline, and it increased with increasing 5-FU bolus injection dosage (r = 0.801, p < 0.01). The plasma UH2/Ura ratio, an indirect biomarker of hepatic DPD activity, tended to be lower in the rats that had received a 5-FU bolus injection than in those that had not, and it remained low after infusion ended. The hepatic DPD activity in rats that had received a 5-FU bolus injection was significantly lower than in those that had not. Negative correlation was observed between DPD activity and bolus injection dosage (r = −0.691, p < 0.05).
Conclusions
A bolus injection suppresses hepatic DPD activity and its effects are dependent on dosage, resulting in slower elimination of 5-FU from the blood and contributing to long-term systemic exposure to 5-FU.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
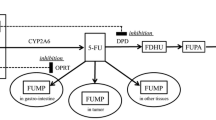
The anti-cancer agent 5-fluorouracil (5-FU) is an analog of the endogenous pyrimidine uracil (Ura). It is widely used and is the mainstay of modern treatment regimens for patients with colorectal cancer and other cancer types [1–3]. The folinic acid, 5-FU, and oxaliplatin (FOLFOX) and the folinic acid, 5-FU, and irinotecan (FOLFIRI) regimens, which involve long-term infusion following a bolus injection of 5-FU, are accepted as standard treatments for colorectal cancers [1, 2]. Recently, the following therapy options have been examined from the perspective of patient management: (1) replacement of the long-term infusion of 5-FU with the repetitive oral administration of 5-FU derivatives such as capecitabine and tegafur; and (2) omission of a bolus injection of 5-FU from chemotherapy comprising long-term infusion of 5-FU [4–8]. Most studies on the development of chemotherapeutic regimens for improving pharmacological effects in colorectal cancer patients focus on an additional administration of targeted monoclonal antibodies, or an alteration of the long-term infusion of 5-FU with repetitive oral administration of 5-FU derivatives. Although critical evaluations have been performed with regard to the necessity of a bolus injection of 5-FU [5–8], few studies have examined the effects of a bolus injection of 5-FU on the pharmacokinetics (PK) and pharmacodynamics of 5-FU.
Our research group previously examined the effect of a 5-FU bolus injection on the steady-state PK in colorectal cancer patients treated with the irinotecan (CPT-11) + 5-FU/leucovorin (LV) + UFT/LV chemotherapy regimen [4]. In the study, we concluded that a bolus injection of 5-FU provided unexpectedly long-term exposure to 5-FU, which can hardly be explained by the PK profile of 5-FU including an apparent half-life of about 10 min [3]. This study has led to speculation that a bolus injection of 5-FU might inhibit and depress the activity of dihydropyrimidine dehydrogenase (DPD), which is the first and rate-limiting enzyme of endogenous pyrimidine and 5-FU catabolism, in the liver. However, the detailed mechanism remains unknown, and the results of the study suggest that non-clinical animal experiments might be needed to shed more light on the issue [4].
In the current study, to further ascertain the effects of a bolus injection of 5-FU, we carried out a PK study using rats and focused particularly on DPD activity in the liver. To the best of our knowledge, this is the first study on the effects of a bolus injection of 5-FU on its PK and hepatic DPD activity.
Materials and methods
Materials
5-FU, Ura, dihydrouracil (UH2), the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH), and ethylenediaminetetraacetic acid (EDTA) were obtained from Wako Pure Chemical Industries Ltd. (Osaka, Japan). 5-bromouracil (5-BU), which was used as an internal standard (IS) in the liquid chromatography–tandem mass spectrometry (LC–MS/MS) analysis, was purchased from Sigma-Aldrich Co. (Steinheim, Germany). We obtained aminoethylisothiouronium bromide from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Potassium phosphate, magnesium chloride, benzamidine, and sucrose were supplied by Nacalai Tesque Inc. (Kyoto, Japan). 2-Mercaptoethanol was obtained from Kanto Chemical Co., Inc. (Tokyo, Japan). All other reagents were of analytical grade and were used without further purification.
PK study of 5-FU in rats
All animal protocols were approved by an institutional review board prior to performing the research and were in accordance with the Kyoto Pharmaceutical University Guidelines for Animal Experimentation. Male Wistar rats (10 weeks of age and weighing 321 ± 23 g) were purchased from Nippon SLC Co., Ltd. (SLC, Hamamatsu, Japan). All rats were housed in a temperature-controlled facility with a 12-h light/dark cycle, and the animals were fed standard rodent chow. Free access to food and water was permitted prior to the experiments.
Figure 1 shows the experimental protocols used for PK study of 5-FU in the rats. After intraperitoneal administration of 50 mg/kg sodium pentobarbital anesthetic, the rats were placed supine under a surgical lamp to maintain their body temperature during the experiment. To perform continuous infusion of 5-FU, the femoral vein of each rat was catheterized with vinyl tubes (SV-31; Natsume Corp., Tokyo, Japan), and 5-FU solution (10 mg/mL in saline) was infused at a rate of 50 mg/m2/h (28.4 mg/kg in 300 g body weight rat) using an infusion pump (CXF 1020; ISIS Co., Ltd, Osaka, Japan). Body surface area (BSA) (m2) was determined using the following formula: BSA = k × w 2/3/104, where k is constant (m2/g2/3) and chosen to be 9.5, and w is the weight of each rat (g) [9]. The rats were divided into three groups (n = 6 in each group), and then continuous infusion was started with a bolus injection of saline, 20 mg/kg 5-FU, or 60 mg/kg 5-FU (10 mg/mL in saline) administration into the external right jugular vein at 13:00 h of the day. The 5-FU dosage was determined based on the clinical dose and blood concentration profiles observed in human patients. Blood samples (230 μL) were collected from the external left jugular vein at 0.08, 0.25, 0.5, 1, 1.5, 2, 3, and 4 h after the start of infusion and transferred to heparinized centrifuge tubes. To investigate the plasma concentration–time profile of 5-FU after stopping infusion, blood samples (230 μL) were also collected from the external left jugular vein at 0.08, 0.25, 0.5, 1, 1.5, 2, 3, and 4 h after stopping infusion and transferred to heparinized centrifuge tubes. The blood sampling time and volume were selected based on the short elimination half-life of 5-FU, and the guidelines provided by the European Federation of Pharmaceutical Industries Associations (EFPIA) and the European Centre for the Validation of Alternative Methods (ECVAM) [10]. After centrifugation of the blood samples at 12,000 rpm for 15 min, the obtained plasma samples were stored at −80 °C until required for analysis. After final blood sampling, all the rats were euthanized by cervical dislocation. After perfusion with phosphate-buffered saline, the liver was harvested for the evaluation of DPD activity.
DPD activity assay
The activity of DPD in the liver of rats treated with or without a bolus injection of 5-FU was evaluated. The experimental method used was similar to a previously published method [11, 12]. Briefly, the harvested livers were homogenized in three volumes of 35 mmol/L potassium phosphate (pH 7.4), 2.5 mmol/L magnesium chloride, 10 mmol/L 2-mercaptoethanol, 0.25 mol/L sucrose, 1.0 mmol/L benzamidine, 1.0 mmol/L aminoethylisothiouronium bromide, and 5.0 mmol/L EDTA, using a homogenizer (PT 10-35 GT; KINEMATICA AG, Switzerland). The homogenate was centrifuged at 9000×g for 20 min at 4 °C. Subsequently, the supernatant was centrifuged at 100,000×g for 60 min at 4 °C and the resulting supernatant was used as the cytosolic fraction. The protein concentration of the cytosolic fraction was measured using the method described by Lowry et al. [13]. Cytosolic incubation was carried out in a final volume of 1.0 mL with 35 mmol/L potassium phosphate (pH 7.4), 2.5 mmol/L magnesium chloride, 10 mmol/L 2-mercaptoethanol, 200 µmol/L NADPH, and 500 µg of cytosolic protein, and each sample was pre-incubated at 37 °C for 20 min. In preliminary experiments, we measured the concentration of 5-FU in each sample and confirmed the absence or lower limit of detection of 5-FU. The metabolic reaction was started by the addition of a 5-FU solution at a final concentration of 40 µmol/L. Twenty minutes after starting the reaction, 500 µL of ice-cold methanol was added to stop the reaction. DPD activity was evaluated by the recovery of 5-FU.
LC–MS/MS assay for 5-FU, Ura, and UH2
To indirectly estimate hepatic DPD activities, the plasma ratio of dihydrouracil/uracil (UH2/Ura), which is reported as a surrogate biomarker of hepatic DPD activity, was determined with the plasma concentration of 5-FU. The assay for 5-FU, Ura, and UH2 in rat plasma was performed using a previously reported method with minor modifications [14]. The LC–MS/MS system consisted of an API 3200 triple-quadrupole mass spectrometer equipped with a turbo ion spray sample inlet as an interface for electrospray ionization (ESI), an LC-10AD micropump (Shimadzu Corp., Kyoto, Japan), and an AS8020 automatic sample injector (Toso, Tokyo, Japan). The instrument was controlled by an Analyst Workstation (Applied Biosystems, CA, USA). The mobile phase was acetonitrile/0.1 % formic acid (20:80, v/v), and the flow rate was 0.2 mL/min. Chromatographic separations were accomplished using a COSMOSIL® HILIC Packed column (2.0 × 150 mm, 5 μm; Nacalai Tesque, Inc., Kyoto, Japan) maintained at 50 °C. The mass spectrometer used a selected ion monitoring (SRM) method and operated in the positive ion mode. The following instrument-dependent parameters were applied: ion spray voltage, 5500 V; source temperature, 500 °C; curtain gas, ten arbitrary units; collision gas, three arbitrary units; ion source gas, one of 70 arbitrary units; and gas, two of 50 arbitrary units. The declustering potential, the entrance potential, the collision energy, and the collision cell exit potential were set at 36.0, 7.8, 23.0, and 3.2 V for 5-FU; 32.6, 11.5, 23.0, and 4.0 V for Ura; 18.2, 6.2, 20.0, and 2.3 V for UH2; and 34.9, 10.9, 25.0, and 4.0 V for the IS, respectively. SRM analyses were performed with transition m/z 131.0 → 114.0 for 5-FU; 113.0 → 70.0 for Ura; 115.0 → 55.0 for UH2; and 191.0 → 174.0 for the IS. Saturated ammonium acetate (150 μL) was added to standard and unknown plasma samples (100 μL) containing 10 μL IS (5-BU, 25 μg/mL in 50 % methanol), and mixed vigorously for 15 s. Acetic acid/isopropyl alcohol (1 mL; 1:10, v/v) was added, vortexed for over 30 s, and centrifuged for 15 min at 12,000 rpm. After centrifugation, the supernatant was transferred to a fresh centrifuge tube and evaporated to dryness under a stream of nitrogen at 60 °C. The mobile phase (100 μL) was added to the resulting residue, vortexed for over 30 s, and transferred to HPLC sample vials. The reconstituted solution (30 µL) was injected into the LC–MS/MS system. The lower limits of quantification (LLOQ) for 5-FU, Ura, and UH2 were less than 0.01 μg/mL from 100 μL of plasma sample. Each calibration curve was linear over LLOQ with a correlation coefficient (r 2) exceeding 0.99.
PK analysis
Non-compartmental PK analysis (NCA) was performed to obtain PK parameters for 5-FU by using the NCA analysis program of the WinNonlin® Version 6.3 software (Pharsight Co., Mountain View, CA, USA). The area under the plasma concentration–time curve from 4 h after starting continuous infusion of 5-FU to infinity (AUC4 h–∞) and from 0 to infinity (AUC0–∞) were calculated using the linear trapezoidal rule. The terminal slope (λ z ) was determined by the linear regression of at least three data points from the terminal portion of the plasma concentration–time curve using Best Fit program of the WinNonlin®. The elimination half-life after the end of the infusion (t 1/2, 4–8 h) was calculated using formula t 1/2, 4–8 h = ln 2/λ z . The area under the first moment curve from time of dosing to infinity (AUMC0–∞) was also calculated by the linear trapezoidal rule. The mean residence time (MRT) was calculated using the formula AUMC0–∞/AUC0–∞. Total plasma clearance (CLtot) was calculated using the formula D/AUC0–∞, where D means the total administered dose of 5-FU. The distribution volume (Vd) was calculated by multiplying CLtot by MRT. In the group not subjected to the 5-FU bolus injection, the plasma concentration of 5-FU at the steady state was calculated as the average plasma concentration of 5-FU after the time taken to reach the steady state (T ss) during infusion, in which T ss was determined by the time required for the plasma concentration of 5-FU to reach ±15 % of the plasma concentration at 4 h (the end of the continuous infusion).
Statistical analysis
All values are expressed as mean ± standard deviation (SD). Two-group comparisons were made using the Student’s unpaired t test. Comparisons across multiple groups were performed with one-way analysis of variance (one-way ANOVA) followed by the Bonferroni test. The differences between the means were considered statistically significant when p < 0.05. Pearson correlation analysis was performed to assess correlations between each PK parameter or DPD activity and bolus injection dosage.
Results
PK analysis of 5-FU
Two rats in the group treated with bolus injection of 60 mg/kg 5-FU died within the experimental period. Figure 2 depicts the mean plasma 5-FU concentration–time profile after rats were administered continuous infusion of 5-FU, with or without a bolus injection of 5-FU. In rats treated with a continuous infusion of 5-FU without a bolus injection, the plasma concentration of 5-FU rapidly reached a plateau at around 1 h and reached steady-state concentrations at 2.66 ± 0.16 µg/mL. The plasma concentrations of 5-FU 4 h after starting continuous infusion with 20 and 60 mg/kg 5-FU bolus injections were 3.17 ± 1.45 and 3.09 ± 0.27 µg/mL, respectively; in all groups, the plasma concentrations at the end of infusion reached steady-state levels. Although there was no difference in the plasma concentration at the steady state between the three groups, in rats treated with a bolus injection, the plasma concentration of 5-FU 7 and 8 h after starting infusion (3 and 4 h after stopping infusion) was significantly higher than in rats that did not receive a bolus injection. Table 1 shows the PK parameters of 5-FU observed for each group. The t 1/2, 4–8 h was significantly longer in the rats treated with a bolus injection than in the rats injected with saline, and it increased with the increasing dosage of the 5-FU bolus injection (r = 0.801, p < 0.01). Although the plasma concentrations at 4 h were the same in all groups, the AUC4 h–∞ increased along with the dosage of the 5-FU bolus injection (r = 0.553, p < 0.05). The CLtot and Vd levels also decreased with increasing dosage of 5-FU bolus injection (r = −0.683, p < 0.01 for CLtot; r = −0.887, p < 0.05 for Vd).
Mean plasma concentration profiles of 5-fluorouracil (5-FU) after continuous infusion of 5-FU (50 mg/m2/h) with or without a bolus injection of 5-FU (20 or 60 mg/kg). (Filled circle) continuous infusion of 5-FU without a bolus injection of 5-FU; (square) with a bolus injection of 20 mg/kg 5-FU; (open circle) with a bolus injection of 60 mg/kg 5-FU. *p < 0.05 and # p < 0.05 statistically significant difference compared with rats not treated with a bolus injection of 5-FU (control), which was evaluated using the Student’s unpaired t test. Results are presented as the mean ± SD for 4–6 rats
Plasma UH2/Ura ratio and hepatic DPD activity
Figure 3 shows the mean plasma concentration profiles of the UH2/Ura ratio after continuous infusion of 5-FU, with or without a 5-FU bolus injection. After starting continuous infusion of 5-FU, the UH2/Ura plasma ratio in the rats not given a 5-FU bolus injection decreased and reached a plateau for the remaining duration of infusion. The UH2/Ura plasma ratio gradually recovered to its initial level after stopping continuous infusion. However, in the rats treated with a bolus injection of 5-FU, the UH2/Ura plasma ratio tended to be lower than in those not given a 5-FU bolus injection, and the recovery of the UH2/Ura plasma ratio to its initial levels was not observed after stopping the continuous infusion. Figure 4 shows DPD activity in rats after continuous infusion of 5-FU, with or without a bolus injection of 5-FU. The hepatic DPD activities in the rats given a bolus injection of 20 or 60 mg/kg 5-FU were 2.61 ± 0.5 and 1.93 ± 0.88 nmol/min/mg protein, respectively, which were significantly lower than in the rats not given a bolus injection (3.44 ± 0.21 nmol/min/mg protein). There was a significant negative correlation between DPD activity and bolus injection dosage (r = −0.691, p < 0.05).
Mean plasma concentration profiles of the dihydrouracil/uracil (UH2/Ura) ratio after continuous infusion of 5-fluorouracil (5-FU) (50 mg/m2/h) with or without a bolus injection of 5-FU (20 or 60 mg/kg). (Filled circle) Continuous infusion of 5-FU without a bolus injection of 5-FU; (square) with a bolus injection of 20 mg/kg 5-FU; (open circle) with a bolus injection of 60 mg/kg 5-FU. Results are presented as the mean ± SD for 4–6 rats
Hepatic dihydropyrimidine dehydrogenase (DPD) activity after continuous infusion of 5-fluorouracil (5-FU) (50 mg/m2/h) with or without bolus injection of 5-FU (20 or 60 mg/kg). The hepatic DPD activity was determined after pharmacokinetic experiments. *p < 0.05 statistically significant difference compared with rats not treated with a bolus injection of 5-FU (control), which was evaluated using the Student’s unpaired t test. Values are the mean ± SD of four experiments
Discussion
In the field of oncology, researchers are focusing on the development of cancer chemotherapeutic strategies to improve the efficacy of 5-FU and decrease its toxicity. Recently, two options have been proposed to improve patient management and clinical responses to 5-FU-based chemotherapeutic regimens: replacement of the long-term infusion of 5-FU with the repetitive oral administration of 5-FU derivatives or omission of the 5-FU bolus injection [4–8]. In fact, the FOLFIRI and FOLFOX regimens consist of a bolus injection and continuous infusion of 5-FU. However, the capecitabine and oxaliplatin (XELOX) regimen does not include a 5-FU bolus injection. Unfortunately, the pharmacokinetic and pharmacological effects of a bolus injection before continuous infusion of 5-FU, or the repetitive oral administration of 5-FU derivatives, are still unknown. In the current study, to examine the effects of a bolus injection of 5-FU, a PK study was performed and the hepatic DPD activity was determined in rats continuously infused with 5-FU, with or without a bolus injection of 5-FU.
Interestingly, in the rats treated with a bolus injection of 5-FU, the elimination of 5-FU after the plasma concentration reached steady-state levels was slower than in the rats that did not receive a bolus injection; the t 1/2, 4–8 h was prolonged, resulting in higher plasma levels of 5-FU and AUC4 h–∞, and lower CLtot with increasing 5-FU bolus injection dosage. This observation raises at least two possibilities: (1) the inhibition of the metabolism of 5-FU and (2) the buildup of an intracellular pool of 5-FU by bolus injection. In the current study, to estimate the time course of the hepatic DPD activity after 5-FU bolus injection, the plasma UH2/Ura ratio, which is a possible surrogate biomarker of hepatic DPD activity [15–17], was determined in rats. After administration of 5-FU, a decrease in the plasma UH2/Ura ratio was observed, which can be explained by the inhibition by 5-FU of the catabolism of Ura to UH2 by DPD. Moreover, a bolus injection of 5-FU kept the plasma UH2/Ura ratio low, and the recovery of plasma UH2/Ura ratio was delayed. These results suggest that a 5-FU bolus injection could lead to qualitative and/or quantitative changes of DPD with the long-term inhibition of the catabolism of 5-FU and contribute to higher plasma levels of 5-FU. In our previous clinical study, we assessed long-term exposure to 5-FU in Japanese patients with advanced colorectal cancer and concluded that a bolus injection of 5-FU provided unexpectedly long-term exposure to 5-FU [4]. These findings suggest that a 5-FU bolus injection induces the long-term suppression of hepatic DPD activity, resulting in a prolongation of t 1/2, 4–8 h and a long-term exposure to 5-FU. Regarding to the possibility of the buildup of an intracellular pool, the pooled 5-FU might be re-absorbed into systemic circulation, which may also contribute to the prolongation of t 1/2, 4–8 h. However, it is hard to evaluate the distribution alteration of 5-FU with the results of the current study because the Vd parameters were determined based on AUC0–∞ values and it is an inadequate parameter to evaluate the PK after the end of infusion. Further studies are needed to investigate this possibility.
To directly evaluate hepatic DPD activity after treatments including 5-FU bolus injection, DPD activity was determined using a cytosolic fraction of the liver. The results of the DPD activity assay indicated that a 5-FU bolus injection induced the suppression of hepatic DPD activity in proportion to the dosage. Some previous studies have reported the suppression of hepatic DPD activity induced by administration of 5-FU [12, 17, 18]. Our previous study revealed that repeated bolus administration of 5-FU significantly decreases hepatic DPD activities, resulting in a decrease of CLtot and an increase of AUC0–∞ in colorectal cancer model rats [17]. Kuwahara et al. [19] performed a clinical study on Japanese patients with stage III/IVa esophageal squamous cell carcinoma treated with a 5-FU/cisplatin-based chemotherapy regimen and reported that significantly higher plasma levels of 5-FU were observed in the second cycle of chemotherapy, compared with the first cycle. This elevation of the plasma concentration of 5-FU after its repeated administration can be explained by the autoregulation of 5-FU catabolism and the inhibition of DPD. Considering the results of the current study, these findings suggest that not only repeated administration but also a bolus injection of 5-FU causes suppression of hepatic DPD activity, and this contributes to the maintenance of high plasma levels of 5-FU.
The findings of the present study provide evidence that a bolus injection of 5-FU suppresses hepatic DPD activity, resulting in slower elimination and higher plasma levels of 5-FU. Moreover, the effect is dose-dependent. However, this study has at least one limitation: the effect of a bolus injection on the pharmacological efficacy and toxicity was not investigated. To obtain optimal clinical efficacy and lower toxicity in patients on 5-FU/LV infusion-based chemotherapy, a consistent target range of AUC, i.e., 20–25 mg h/L, has been established, regardless of the dosing schedule [2, 20]. There are conflicting reports on the necessity of a bolus injection of 5-FU. Some critical evaluations have suggested that a bolus injection is not always necessary to ensure the pharmacological efficacy because it causes very high plasma concentrations of the chemotherapeutic agents [5–8]. However, our research group has recently highlighted the use of bolus injections for the treatment of metastatic cancer to ensure clinical efficacy in 5-FU-based combination chemotherapy [4]. Further studies are needed to confirm the necessity of a bolus injection of 5-FU.
In conclusion, we found that a bolus injection of 5-FU suppresses hepatic DPD activity in a dose-dependent manner, resulting in slower elimination of 5-FU from the blood and contributing to long-term systemic exposure to 5-FU. These results might help provide an improved 5-FU-based combination chemotherapeutic strategy for cancer patients. Additional investigations are required into the effects of a bolus injection of 5-FU on pharmacological efficacy and toxicity.
References
Kochi M, Akiyama Y, Aoki T, Hagiwara K, Takahashi T, Hironaka K, Teranishi F, Osuka F, Takeuchi M, Fujii M, Nakajima T (2013) FOLFIRI plus bevacizumab as a first-line treatment for Japanese patients with metastatic colorectal cancer: a JACCRO CC-03 multicenter phase II study. Cancer Chemother Pharmacol 72:1097–1102
Saif MW, Choma A, Salamone SJ, Chu E (2009) Pharmacokinetically guided dose adjustment of 5-fluorouracil: a rational approach to improving therapeutic outcomes. J Natl Cancer Inst 101:1543–1552
Sakaeda T, Yamamori M, Kuwahara A, Nishiguchi K (2009) Pharmacokinetics and pharmacogenomics in esophageal cancer chemoradiotherapy. Adv Drug Deliv Rev 61:340–388
Tamura T, Kuwahara A, Kadoyama K, Yamamori M, Nishiguchi K, Inokuma T, Takemoto Y, Chayahara N, Okuno T, Miki I, Fujishima Y, Sakaeda T (2011) Effects of bolus injection of 5-fluorouracil on steady-state plasma concentrations of 5-fluorouracil in Japanese patients with advanced colorectal cancer. Int J Med Sci 8(5):406–412
Grothey A, Sargent D, Goldberg RM, Schmoll HJ (2004) Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 22:1209–1214
Venook A (2005) Critical evaluation of current treatments in meta-static colorectal cancer. Oncologist 10:250–261
Lee JJ, Chu E (2007) An update on treatment advances for the first-line therapy of metastatic colorectal cancer. Cancer J 13:276–281
Sabharwal A, Kerr D (2007) Chemotherapy for colorectal cancer in the metastatic and adjuvant setting: past, present and future. Expert Rev Anticancer Ther 7:477–487
Noguchi C, Miyata H, Sato Y, Iwaki Y, Okuyama S (2011) Evaluation of bone toxicity in various bones of aged rats. J Toxicol Pathol 24(1):41–48
Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal JM, van de Vorstenbosch C, European Federation of Pharmaceutical Industries Association and European Centre for the Validation of Alternative Methods (2001) A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol 21(1):15–23
Tateishi T, Nakura H, Watanabe M, TanakaM Kumai T, Kobayashi S (1996) Preliminary examination of the influence of incubation time or cytosolic protein concentration on dihydropyrimidine dehydrogenase activity. Clin Chim Acta 252:1–9
Kobuchi S, Kuwano S, Imoto K, Okada K, Nishimura A, Ito Y, Shibata N, Takada K (2013) A predictive biomarker for altered 5-fluorouracil pharmacokinetics following repeated administration in a rat model of colorectal cancer. Biopharm Drug Dispos 34(7):365–376
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Büchel B, Rhyn P, Schürch S, Bühr C, Amstutz U, Largiadèr CR (2013) LC-MS/MS method for simultaneous analysis of uracil, 5,6-dihydrouracil, 5-fluorouracil and 5-fluoro-5,6-dihydrouracil in human plasma for therapeutic drug monitoring and toxicity prediction in cancer patients. Biomed Chromatogr 27(1):7–16
Gamelin E, Boisdron-Celle M, Gu´erin-Meyer V, Delva R, Lortholary A, Genevieve F, Larra F, Ifrah N, Robert J (1999) Correlation between uracil and dihydrouracil plasma ratio, fluorouracil (5-FU) pharmacokinetic parameters, and tolerance in patients with advanced colorectal cancer: a potential interest for predicting 5-FU toxicity and determining optimal 5-FU dosage. J Clin Oncol 17(4):1105
Kobuchi S, Ito Y, Okada K, Imoto K, Kuwano S, Takada K (2013) Pharmacokinetic/pharmacodynamic modeling of 5-fluorouracil by using a biomarker to predict tumor growth in a rat model of colorectal cancer. J Pharm Sci 102(6):2056–2067
Kobuchi S, Ito Y, Okada K, Imoto K, Kuwano S, Takada K (2013) Pre-therapeutic assessment of plasma dihydrouracil/uracil ratio for predicting the pharmacokinetic parameters of 5-fluorouracil and tumor growth in a rat model of colorectal cancer. Biol Pharm Bull 36(6):907–916
Milano G, Chamorey AL (2002) Clinical pharmacokinetics of 5-fluorouracil with consideration of chronopharmacokinetics. Chronobiol Int 19(1):177–189
Kuwahara A, Yamamori M, Nishiguchi K, Okuno T, Chayahara N, Miki I, Tamura T, Kadoyama K, Inokuma T, Takemoto Y, Nakamura T, Kataoka K, Sakaeda T (2010) Effect of dose-escalation of 5-fluorouracil on circadian variability of its pharmacokinetics in Japanese patients with Stage III/IVa esophageal squamous cell carcinoma. Int J Med Sci. 7(1):48–54
Gamelin E, Delva R, Jacob J, Merrouche Y, Raoul JL, Pezet D, Dorval E, Piot G, Morel A, Boisdron-Celle M. Individual 5-fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: results of a multicenter randomized trial in patients with metastatic colorectal cancer. J Clin Oncol 26(13):2099–2105
Acknowledgments
This study was supported in part by a Grant-in-Aid for Young Scientists (B) (No. 15K18937) from Ministry of Education, Culture, Sports, Science, and Technology (MEXT, Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kobuchi, S., Hayashi, A., Taniguchi, M. et al. Effects of a bolus injection of 5-fluorouracil on dihydropyrimidine dehydrogenase activity in rats receiving continuous infusion of 5-fluorouracil. Cancer Chemother Pharmacol 78, 517–523 (2016). https://doi.org/10.1007/s00280-016-3105-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3105-8