Abstract
Purpose
In this study, whether HOTAIR is a prognostic biomarker will be detected, and its regulative effects of chemosensitivity to doxorubicin in TCC cells will be examined.
Methods
The expression of HOTAIR was detected by quantitative real-time PCR. Overall survival rate was calculated by Kaplan–Meier method with the log-rank test for comparisons. MTT assay was used to detect cell proliferation ability and chemosensitivity. Dual-color flow cytometric method was used to detect cell apoptosis.
Results
HOTAIR was up-regulated in bladder transitional cell carcinoma (TCC) tissues and cell lines compared with normal bladder transitional cell (NBTC) tissues and bladder epithelial immortalized SV-HUC-1 cells, and its expression level had positive correlation with histological grades of TCC. Moreover, HOTAIR was an independent prognostic biomarker of overall survival for TCC patients. The expression and silence vector for HOTAIR were transfected into T24 and J82 cells to up-regulate and silence the HOTAIR expression, respectively. In T24 and J82 cells, HOTAIR over-expression promoted cell proliferation and inhibited chemosensitivity to doxorubicin and cell apoptosis induced by doxorubicin; silence of HOTAIR showed opposite regulative effects.
Conclusions
In summary, lncRNA HOTAIR was an independent prognostic biomarker of overall survival in TCC patients and could regulate chemosensitivity to doxorubicin of human TCC cells. HOTAIR might provide a new potential therapeutic target and stratagem for TCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bladder cancer is the most general malignant cancer in genitourinary system; the main classification is bladder transitional cell carcinoma (TCC) which has high morbidity and mortality [1]. The prognosis of TCC is poor, because TCC shows significant malignant relapse, multiple invasion and metastasis. The therapy strategy for TCC includes surgical operation, intravesical chemotherapy and biological therapy. Surgical operation is the key preference, and chemotherapy following surgical excision is considered as an effective supplementary therapy to prevent recurrence and metastasis of TCC [2]. Therefore, new therapeutic strategies and targets are exigent, necessary and helpful to basic research and clinical therapy for TCC.
Long noncoding RNA (lncRNA) is a kind of endogenous RNAs, which cannot be coded to protein. Over the past several years, the studies about lncRNAs were rising rapidly. LncRNAs are involved in the regulation of some very important cell biological characteristics, such as cell cycle, autophage and apoptosis [3–5]. Some lncRNAs showed anomalous expression and functions in some malignant tumors, which act as oncogenes or tumor suppress genes [6, 7].
HOTAIR (HOX transcript antisense RNA, Gene ID: 33510) gene is a lncRNA located at 12q13.13. Previous reports had suggested its oncogenesis role in many kinds of malignant cancer [8–10]. In our previous microarray assays, the expression of HOTAIR shows over tenfold up-regulation in TCC samples than that in normal bladder transitional cell (NBTC) samples. The following small-sample expression detection confirmed HOTAIR over-expression in TCC. Moreover, our study found HOTAIR also up-regulated in human brain glioma, and knockdown of HOTAIR could inhibit malignant biological behaviors of human glioma cells [11]. Those discoveries suggested that HOTAIR might act as an oncogene in TCC. However, little is known about HOTAIR which acts as a prognostic biomarker of TCC and its influence on chemosensitivity in TCC cells. In this study, whether HOTAIR is a prognostic biomarker will be detected, and its regulative effects of chemosensitivity to doxorubicin in TCC cells will be examined, and further penetrating researches might be helpful to develop the effective clinical treatments to TCCs.
Materials and methods
Clinical specimens
A total of 35 TCC and 16 NBTC specimens were from Shengjing Hospital of China Medical University from December 2010 to January 2012. The Ethics Committees of China Medical University approved this study, and then the patient’s permission was obtained before operation. The patients had not experienced radiation or chemotherapy before surgery. All patients included 24 men and 11 women (mean age: 58.7 ± 3.3 years, age range: 51–65 years); included 12 cases of Grade I (low grade tendency of urinary tract epithelial papilloma), 13 cases of Grade II (papillary thyroid carcinoma, low grade) and ten cases of Grade III (papillary thyroid carcinoma, high grade); and included five cases of lymphatic metastasis and two cases distant metastasis. The pathological data were affirmed after operation, and the tissue samples were stored at −80 °C.
Cell culture
Human TCC T24 and J82 cell lines and human bladder epithelial immortalized SV-HUC-1 cell line were obtained from China Academy of Chinese Medical Sciences. Those cells were cultured in DMEM medium which contained 10 % fetal bovine serum (Life Technologies, USA), and culture condition was 5 % CO2 at 37 °C.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted with Trizol reagent. cDNA was synthesized with High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA). Then HOTAIR expression level was detected with SYBR (Applied Biosystems, USA), whose primers were 5′-GGTAGAAAAAGCAACCACGAAGC-3′ (sense) and 5′-ACATAAACCTCTGTCTGTGAGTGCC-3′ (antisense). GAPDH was selected as endogenous controls, whose primers were 5′-GTCAACGGATTTGGTCTGTATT-3′ (sense) and 5′-AGTCTTCTGGGTGGCAGTGAT-3′ (antisense). The C t value about detected gene between 18 and 30 was selected and quantified with the 2−ΔΔCt method.
Vector construction
The full-length HOTAIR was cloned into pcDNA3.1 (Invitrogen, USA) to construct the HOTAIR expression vector pc-HOTAIR by Sangon Company (Shanghai, China). The empty pcDNA3.1 vector was used as control (pc-NC). The specific silence oligonucleotides for HOTAIR were cloned into pSilencer (ThermoFisher, USA) to construct the HOTAIR silence vector sh-HOTAIR by Sangon Company (Shanghai, China), and the sequences were 5′-GATCCGCCACATGAACGCCCAGAGATTTTCAAGAGAAATCTCTGGGCGTTCATGTGGTTTTTTG-3′ (sense) and 5′-AATTCAAAAAACCACATGAACGCCCAGAGATTTCTCTTGAAAATCTCTGGGCGTTCATGTGGCG-3′ (antisense). The empty pSilencer vector was used as control (sh-NC).
Transfection
Cells at 70–80 % concentration were seeded into six-well plates before transfection. Cells were transfected with vectors using Lipofectamine™ 3000 reagent (Invitrogen, USA) following the manufacturer’s protocol. After 4 h, cells were cultured with normal media and harvested 48 h later.
Biological behavior assay in TCC cells
Cell proliferation ability detected by MTT was assessed as previously described [12]. Apoptosis rate was examined with dual-color flow cytometric method. Cells were harvested, and their apoptosis level was detected with Annexin V-FITC apoptosis detection kit (KeyGEN, China) following the manufacturer’s protocol. The flow cytometry (BD, USA) and CELLQuest 3.0 software (BD, USA) were applied to obtain and analyze apoptosis data.
Chemosensitivity assay
Cells were seeded into 96-well plates (3000 cells/well) and treated with doxorubicin (sigma, USA) at various concentrations 24 h later. After 48 h, MTT method was used to detect the cell viability. The calculative formula for suppression rate was: cell suppression rate = (1 − ODtreated/ODcontrol) × 100 %. The dose–response curve at different concentrations was charted to calculate the IC50 using a probit regression model.
Western blot analysis
A total of 20 mg of protein was used for Western blotting. After SDS-PAGE, samples were transferred to PVDF membranes. After blocking the membranes, they were incubated with primary polyclonal against cleaved PARP (9541, Cell Signaling, USA) and secondary anti-rabbit antibody by turns. Proteins were enhanced by chemiluminescence detection kit. The protein quantification was performed using ImageJ software.
Statistical analysis
Statistical analysis was completed with software SPSS 13.0 (IBM, IL). The data were presented as mean ± SD of three independent experiments and compared using Student’s t test and one-way ANOVA. The Kaplan–Meier method with the log-rank test for comparisons was used to calculate the overall survival rate. Variables with a value of p < 0.05 in the univariate analysis were included in the subsequent multivariate analysis based on the Cox proportional hazards model. p < 0.05 means significant difference.
Results
Up-regulation of HOTAIR expression in TCC specimens
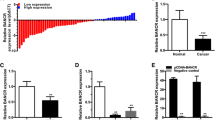
The results showed that the HOTAIR expression in TCC samples was much higher than that in NBTC samples (Fig. 1a), and the HOTAIR expression in T24 and J82 cells was also much higher than that in control SV-SUC-1 cells (p < 0.05) (Fig. 1b), which provide us initial evidence that HOTAIR may play a role in TCC tumorgenesis.
a Relative HOTAIR expression in normal bladder transitional cell (NBTC) tissues (n = 16) and different grades of bladder transitional cell carcinoma (TCC) tissues (n = 35). b Relative HOTAIR expression in SV-HUC-1 cells, T24 and J82 cell lines (n = 5). c Kaplan–Meier analysis indicated that higher HOTAIR expression had a significantly shorter survival time than lower HOTAIR expression. Data are presented as mean ± SD. *p < 0.05 versus NBTCs or SV-HUC-1 group
Meantime, the correlation between HOTAIR expression and specific clinical pathological characteristics of TCC was investigated. TCC patients with higher HOTAIR expression tended to have disease with a worse histological grade (p < 0.05). Nevertheless, HOTAIR expression has no significant correlation with other pathological characteristics, such as tumor size, gender, lymphatic and distant metastasis (p > 0.05).
HOTAIR was an independent prognostic biomarker for TCC patients
Interestingly, we found that HOTAIR was an independent prognostic biomarker of overall survival for TCC patients. The Kaplan–Meier analysis showed that patients with higher HOTAIR expression had a significantly shorter survival time than patients with lower HOTAIR expression (Fig. 1c). Meanwhile, the univariate Cox proportional hazards regression analysis for overall survival demonstrated that, compared with patients with lower HOTAIR expression, TCC patients with higher HOTAIR expression had a significantly worse prognosis (p < 0.05). Furthermore, the multivariate analysis proved that higher HOTAIR expression was an independent biomarker of overall survival (p < 0.05) in TCC patients.
HOTAIR promotes cell proliferation in TCC cells
To estimate the biological influence of HOTAIR on cell proliferation in TCC cells, the expression vector and silence vector for HOTAIR were transfected into T24 and J82 cells to up-regulate and down-regulate HOTAIR expression, respectively (Fig. 2a); 72 h after transfection, compared to negative control (transfection with empty vector, NC), cell proliferation was advanced remarkably by over-expression of HOTAIR and was significantly inhibited by HOTAIR silence (both p < 0.05). Thus, HOTAIR acted as an oncogene in TCC cells and advanced TCC cell malignant growth (Fig. 2b, c).
HOTAIR inhibits chemosensitivity of TCC cells to doxorubicin
Doxorubicin is a common cytotoxic drug used in intravesical chemotherapy and systemic chemotherapy in TCC patients. Compared with NC, the dose–response curve shifted outward for HOTAIR silence group which indicates sensitization to the doxorubicin, and HOTAIR over-expression showed opposite tendency (Fig. 3a–d). Compared with NC, half-maximal inhibitory concentration (IC50) of doxorubicin in HOTAIR over-expression group increased significantly, whereas IC50 for HOTAIR silence group decreased remarkably (p < 0.05, Fig. 3e). These results demonstrate that HOTAIR inhibits chemosensitivity of TCC T24 and J82 cells to doxorubicin.
a, b Effects of HOTAIR overexpression or silence on chemosensitivity of T24 cells to doxorubicin (n = 5). c, d The effects of HOTAIR overexpression or silence on chemosensitivity of J82 cells to doxorubicin (n = 5). e The doxorubicin concentration of 50 % inhibition of cell growth (IC50) for HOTAIR expression changes of T24 and J82 cells (n = 5). f The effects of HOTAIR expression changes on the cell apoptosis of T24 and J82 cells induced by doxorubicin (n = 5). g The expression changes of cleaved-PARP protein in T24 and J82 cells induced by doxorubicin (n = 5). h The relative quantitative analysis of cleaved-PARP protein normalized to GAPDH protein. IDV is the abbreviation for “integrated density values.” *p < 0.05 versus blank and NC group
HOTAIR inhibits cell apoptosis of TCC cells induced by doxorubicin
Flow cytometry was applied to assess the impact of HOTAIR expression changes on apoptosis rate of TCC cells induced by 500 ng/ml doxorubicin. In T24 and J82 cells induced by doxorubicin HOTAIR, over-expression significantly decreased cell apoptosis and the expression of cleaved-PARP protein, whereas HOTAIR silence advanced cell apoptosis and the expression of cleaved-PARP protein inversely (both p < 0.05, Fig. 3f–h). These results demonstrated that chemosensitivity changes induced by HOTAIR might be mediated by the cell apoptosis pathway.
Discussion
The recent studies find that more than 75 % of the human genome can be transcribed to RNA, but only about 1.2 % of those RNAs can be translated to proteins; a large portion of those noncoding RNAs might have important and complicate roles in all kinds of physiological and pathological functions [13]. Noncoding RNAs include lncRNA and short noncoding RNAs, such as miRNA, siRNA and piRNA. Some recent reports describe lncRNAs have functional roles in almost all cellular functions, such as proliferation, apoptosis, autophage, invasion and metastasis [14–17]. However, minority lncRNAs role and their relationship with diseases had been quite understood although many thus genes were screened and identified recently. Intriguingly, there is increasing evidence that lncRNAs have moved into the cancer research filed, and lncRNA-mediated regulation is also important in tumourigenesis [18, 19].
Our research discovered HOTAIR was highly expressed in TCC tissues and cells, and its expression level had positive correlation with TCC histological grades. Moreover, HOTAIR was an independent prognostic biomarker of TCC, and patients with higher HOTAIR expression had a remarkably shorter overall survival time than patients with lower HOTAIR expression. Some lncRNAs had been reported as prognostic biomarkers of TCC [20–22]. Previous reports have demonstrated that HOTAIR could be a prognostic biomarker for several malignant tumors, such as gastric adenocarcinoma and glioma [23, 24], although there were no reports about HOTAIR which serves as a prognostic factor for TCC patient. Thus, HOTAIR plays an important role in the genesis and development of TCC and is a good biomarker of worse prognosis in TCC.
HOTAIR is a well-studied lncRNA which is transcribed from the mammalian HOXC gene cluster in the antisense direction [25]. Previous reports had suggested its oncogenesis role in many kinds of malignant cancer. In gastric cancer, anomalous high expression of HOTAIR advanced proliferation, invasion and metastases and prognosticated the poor outcomes [26]. Our results showed that cell proliferation of TCC cells was promoted remarkably by over-expression of HOTAIR and inhibited significantly by HOTAIR silence. Our findings indicated HOTAIR modulate cell growth and provided solid evidence for the HOTAIR functional role in TCC.
Up to date, the therapeutic approaches for patients are tendency to individualize treatment according to the characteristic of tumor and the status of patients. Chemotherapy is a common assistant manner to reduce the recurrence and metastasis risk. But, the dose of chemotherapy was difficult to balance between the toxicity and efficacy. Chemotherapy sensitivity is a perfect guidance for the measurement of maximized effective and minimized side effects customized [27]. Till now, there is no report about HOTAIR influence on chemosensitivity of TCC, especially to doxorubicin.
In our study, HOTAIR over-expression depressed chemosensitivity to doxorubicin, IC50 for doxorubicin concentration presented a conspicuous accrescence, and HOTAIR silence showed opposite regulative effects. Doxorubicin is a drug used widely in intravesical chemotherapy and systemic chemotherapy for TCC. It is an anthracycline antibiotic, which made cell growth arrest and apoptosis by integrating with nucleus DNA and damaging its structure. At present, drug resistance had been critical for the chemotherapy failure, although several approaches associated with chemo-resistance had been made to repair the DNA damage and apoptosis. In this study, flow cytometry confirmed that HOTAIR over-expression inhibits apoptosis induced by doxorubicin; silence of HOTAIR advanced it. In this context, silence of HOTAIR could enhance the chemosensitivity of TCC cells to doxorubicin, and HOTAIR might be a new potential target and provide guidance for TCC chemotherapy.
In conclusion, HOTAIR, which up-regulated in TCC, was an independent prognostic biomarker of overall survival in TCC patients. HOTAIR functions as an oncogene to regulate chemosensitivity to doxorubicin in TCC. Those achievements are helpful to the mechanism research of TCC carcinogenesis and might provide a new potential therapeutic target and stratagem for TCC.
References
Murta-Nascimento C, Schmitz-Dräger BJ, Zeegers MP, Steineck G, Kogevinas M, Real FX, Malats N (2007) Epidemiology of urinary bladder cancer: from tumor development to patient’s death. World J Urol 25:285–295
Hassen W, Droller MJ (2000) Current concepts in assessment and treatment of bladder cancer. Curr Opin Urol 10:291–299
Xu XD, Li KR, Li XM, Yao J, Qin J, Yan B (2014) Long non-coding RNAs: new players in ocular neovascularization. Mol Biol Rep 41(7):4493–4505
Zhao Y, Feng G, Wang Y, Yue Y, Zhao W (2014) Regulation of apoptosis by long non-coding RNA HIF1A-AS1 in VSMCs: implications for TAA pathogenesis. Int J Clin Exp Pathol 7(11):7643–7652
Song J, Ahn C, Chun CH, Jin EJ (2014) A long non-coding RNA, GAS5, plays a critical role in the regulation of miR-21 during osteoarthritis. J Orthop Res 32(12):1628–1635
Modali SD, Parekh VI, Kebebew E, Agarwal SK (2015) Epigenetic regulation of the lncRNA MEG3 and its target c-MET in pancreatic neuroendocrine tumors. Mol Endocrinol 29(2):224–237
Wang X, Li M, Wang Z, Han S, Tang X, Ge Y, Zhou L, Zhou C, Yuan Q, Yang M (2015) Silencing of long noncoding RNA MALAT1 by miR-101 and miR-217inhibits proliferation, migration and invasion of esophageal squamous cell carcinoma cells. J Biol Chem 290(7):3925–3935
Song B, Guan Z, Liu F, Sun D, Wang K, Qu H (2015) Long non-coding RNA HOTAIR promotes HLA-G expression via inhibiting miR-152 in gastric cancer cells. Biochem Biophys Res Commun 464(3):807–813
Li H, An J, Wu M, Zheng Q, Gui X, Li T, Pu H, Lu D (2015) LncRNA HOTAIR promotes human liver cancer stem cell malignant growth through downregulation of SETD2. Oncotarget 6(29):27847–27864
Yan TH, Lu SW, Huang YQ, Que GB, Chen JH, Chen YP, Zhang HB, Liang XL, Jiang JH (2014) Upregulation of the long noncoding RNA HOTAIR predicts recurrence in stage Ta/T1 bladdercancer. Tumour Biol 35(10):10249–10257
Ke J, Yao YL, Zheng J, Wang P, Liu YH, Ma J, Li Z, Liu XB, Li ZQ, Wang ZH, Xue YX (2015) Knockdown of long non-coding RNA HOTAIR inhibits malignant biological behaviors of human glioma cells via modulation of miR-326. Oncotarget 6(26):21934–21949
Shang C, Guo Y, Hong Y, Liu YH, Xue YX (2015) MiR-21 up-regulation mediates glioblastoma cancer stem cells apoptosis and proliferation by targeting FASLG. Mol Biol Rep 42(3):721–727
Alaimo S, Giugno R, Pulvirenti A (2014) ncPred: ncRNA-disease association prediction through tripartite network-based inference. Front Bioeng Biotechnol 2:71
Fang TT, Sun XJ, Chen J, Zhao Y, Sun RX, Ren N, Liu BB (2014) Long non-coding RNAs are differentially expressed in hepatocellular carcinoma cell lines with differing metastatic potential. Asian Pac J Cancer Prev 15(23):10513–10524
Nie F, Sun M, Yang J, Xie M, Xu TP, Xia R, Liu YW, Liu XH, Zhang EB, Lu KH, Shu YQ (2015) Long noncoding RNA ANRIL promotes non-small cell lung cancer cell proliferation and inhibits apoptosis by silencing KLF2 and P21 expression. Mol Cancer Ther 14(1):268–277
Wang Y, Liu X, Zhang H, Sun L, Zhou Y, Jin H, Zhang H, Zhang H, Liu J, Guo H, Nie Y, Wu K, Fan D, Zhang H, Liu L (2014) Hypoxia-inducible lncRNA-AK058003 promotes gastric cancer metastasis by targeting γ-synuclein. Neoplasia 16(12):1094–1106
Wang Y, Guo Q, Zhao Y, Chen J, Wang S, Hu J, Sun Y (2014) BRAF-activated long non-coding RNA contributes to cell proliferation and activates autophagy in papillary thyroid carcinoma. Oncol Lett 8(5):1947–1952
Banet G, Bibi O, Matouk I, Ayesh S, Laster M, Kimber KM, Tykocinski M, de Groot N, Hochberg A, Ohana P (2000) Characterization of human and mouse H19 regulatory sequences. Mol Biol Rep 27(3):157–165
Kraus TF, Greiner A, Guibourt V, Lisec K, Kretzschmar HA (2015) Identification of stably expressed lncRNAs as valid endogenous controls for profiling of human glioma. J Cancer 6(2):111–119
Wang HM, Lu JH, Chen WY, Gu AQ (2015) Upregulated lncRNA-UCA1 contributes to progression of lung cancer and is closely related to clinical diagnosis as a predictive biomarker in plasma. Int J Clin Exp Med 8(7):11824–11830
Zhi F, Wang Q, Xue L, Shao N, Wang R, Deng D, Wang S, Xia X, Yang Y (2015) The use of three long non-coding RNAs as potential prognostic biomarkers of astrocytoma. PLoS One 10(8):e0135242
Zhao XL, Zhao ZH, Xu WC, Hou JQ, Du XY (2015) Increased expression of SPRY4-IT1 predicts poor prognosis and promotes tumor growth and metastasis in bladder cancer. Int J Clin Exp Pathol 8(2):1954–1960
Zhao W, Dong S, Duan B, Chen P, Shi L, Gao H, Qi H (2015) HOTAIR is a predictive and prognostic biomarker for patients with advanced gastric adenocarcinoma receiving fluorouracil and platinum combination chemotherapy. Am J Transl Res 7(7):1295–1302
Zhang JX, Han L, Bao ZS, Wang YY, Chen LY, Yan W, Yu SZ, Pu PY, Liu N, You YP, Jiang T, Kang CS, Chinese Glioma Cooperative Group (2013) HOTAIR, a cell cycle-associated long noncoding RNA and a strong predictor of survival, is preferentially expressed in classical and mesenchymal glioma. Neuro Oncol 15(12):1595–1603
Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY (2007) Functional demarcation of active and silent chromatin domains in human HOX loci by noncodingRNAs. Cell 129(7):1311–1323
Endo H, Shiroki T, Nakagawa T, Yokoyama M, Tamai K, Yamanami H, Fujiya T, Sato I, Yamaguchi K, Tanaka N, Iijima K, Shimosegawa T, Sugamura K, Satoh K (2013) Enhanced expression of long non-coding RNA HOTAIR is associated with the development of gastric cancer. PLoS One 8(10):e77070
Hong Y, Shang C, Xue YX, Liu YH (2015) Silencing of Bmi-1 gene enhances chemotherapy sensitivity in human glioblastoma cells. Med Sci Monit 21:1002–1007
Acknowledgments
This work was supported by the National Nature Science Foundation of China (81301834, 81172408, 30901480, 81272716).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Rights and permissions
About this article
Cite this article
Shang, C., Guo, Y., Zhang, H. et al. Long noncoding RNA HOTAIR is a prognostic biomarker and inhibits chemosensitivity to doxorubicin in bladder transitional cell carcinoma. Cancer Chemother Pharmacol 77, 507–513 (2016). https://doi.org/10.1007/s00280-016-2964-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-2964-3