Abstract
Purpose
Children with high-risk neuroblastoma have poor survival rates, and novel therapies are needed. We hypothesized that cabozantinib would be effective against neuroblastoma tumor cells and tumors in preclinical models via inhibition of receptor tyrosine kinase signaling pathways.
Methods
We determined neuroblastoma cell viability after treatment with cabozantinib alone and in combination with 13-cis-retinoic acid, topotecan, and temozolomide using MTT assays. Inhibition of RET and intracellular signaling was measured by Western blot analysis of treated and untreated cells. To investigate the efficacy of cabozantinib against neuroblastoma tumors in vivo, neuroblastoma cells were injected orthotopically into immunocompromised mice, and mice were treated with oral cabozantinib. Tumors were evaluated for growth by determination of in vivo luminescence and final tumor weights.
Results
All neuroblastoma cell lines were sensitive to cabozantinib, and IC50 values ranged from 1.6 to 16.2 μM. Cabozantinib treatment was synergistic with 13-cis-retinoic acid and chemotherapy agents topotecan and temozolomide. Cabozantinib treatment inhibited RET phosphorylation in all cell lines and ERK phosphorylation in more sensitive neuroblastoma cell lines. In mice with neuroblastoma xenograft tumors, cabozantinib treatment significantly reduced tumor growth.
Conclusions
Treatment of neuroblastoma tumor cells with cabozantinib inhibits RET and ERK phosphorylation and is effective against neuroblastoma tumor cell lines alone and in combination with 13-cis-retinoic acid, topotecan, and temozolomide. Cabozantinib treatment is also effective in reducing tumor growth in vivo. Cabozantinib therefore represents a novel therapeutic agent for neuroblastoma, and further preclinical and clinical studies are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Children with high-risk neuroblastoma have very poor outcomes, with 5-year disease-free survival rates between 25 and 35 % [1–3], despite treatment regimens that include intensive chemotherapy, autologous stem cell transplantation, surgery, radiation therapy, and 13-cis-retinoic acid. Cases of high-risk neuroblastoma are associated with frequent relapses and tumors that are resistant to treatment. Unfortunately, children with recurrent or refractory neuroblastoma have a <50 % response rate to alternative chemotherapy regimens [4, 5], and novel therapies are sorely needed for children with high-risk and recurrent neuroblastoma.
Neuroblastoma tumorigenesis has been shown to be regulated by several growth factors, which can each induce proliferation, block differentiation of neuroblastoma cells, or increase local angiogenesis through activation of receptor protein tyrosine kinases. Neuroblastoma cell lines and primary tumors have been shown to express varying levels of these growth factors and their cognate receptors [6–15], and increased expression of many of these growth factors is associated with advanced stage high-risk neuroblastoma tumors and poor overall patient outcomes [9, 15, 16].
Cabozantinib (XL184) is a novel small molecule inhibitor of the RET, c-Met, and vascular endothelial growth factor receptor-2 (VEGFR-2, KDR) tyrosine kinases [17–19] with the potential for tumor growth inhibition via multiple independent pathways. Cabozantinib has demonstrated significant antitumor activity in a range of preclinical models and has been shown to reduce tumor cell proliferation and viability in addition to inhibition of tumor cell migration, invasion, and tumor angiogenesis [19, 20]. Promising early clinical trial results with cabozantinib in the treatment of patients with thyroid cancer, prostate cancer, non-small cell lung cancer, and renal cell carcinoma [21–25] have led to phase 2 and phase 3 evaluations for many tumor types, and cabozantinib was recently FDA approved for treatment of progressive metastatic medullary thyroid cancer (MTC) [26].
The RET kinase is expressed in neural crest-derived cells and is required for peripheral nervous system maturation. RET inhibition has been shown to be effective against neuroblastoma in preclinical models and to be synergistic with 13-cis-retinoic acid [27]. HGF/c-Met signaling has been demonstrated to promote malignant progression of neuroblastoma [11]. Furthermore, elevated levels of c-Met expression and MET gene amplification have been identified in patients with advanced stage neuroblastoma [16, 28], and c-Met inhibitors have been shown to be effective against neuroblastoma [29]. Increased expression of the vascular endothelial growth factor (VEGF) and VEGF receptor (VEGFR) family members is associated with advanced stage, high-risk neuroblastoma tumors [15]. Based on the evidence for the role of RET, c-Met, and VEGFR signaling in neuroblastoma pathogenesis, we hypothesized that cabozantinib would have significant antitumor activity in in vitro and in vivo models of neuroblastoma and would be synergistic with 13-cis-retinoic acid and chemotherapy.
Materials and methods
Cells and culture conditions
The neuroblastoma cell lines used in this study have been previously utilized by our laboratory [27, 29], and were purchased from American Type Culture Collection (ATCC, www.atcc.org) or were generously provided by Shahab Asgharzadeh (Children’s Hospital Los Angeles, Los Angeles, CA, USA), Susan Cohn (The University of Chicago Children’s Hospital, Chicago, IL, USA), John Maris (Children’s Hospital of Philadelphia, Philadelphia, PA, USA), or the Children’s Oncology Group (COG) Cell Culture and Xenograft Repository (www.cogcell.org) [30–39]. Cell lines were grown at 37 °C in 5 % CO2 in appropriate media (Invitrogen, Carlsbad, CA, USA) supplemented with 10 % heat-inactivated fetal bovine serum (FBS) (Life Technologies, Grand Island, NY, USA), l-glutamine, sodium pyruvate, and nonessential amino acids (Sigma-Aldrich, St. Louis, MO, USA). All cell lines were authenticated by DNA profiling prior to use.
Therapeutic agents
Cabozantinib was generously provided by Exelixis, Inc. (San Francisco, CA, USA). A 10 mM stock solution was generated in DMSO (Sigma) and stored at −20 °C. Cabozantinib was diluted in phosphate-buffered saline (PBS) immediately before use. For in vivo studies, cabozantinib was diluted to a final concentration of 15 mg/mL in 0.5 % HCl. 13-cis-retinoic acid (Sigma), topotecan (Sigma), and temozolomide (Sigma) were diluted directly into media prior to use. Glial-derived neurotrophic factor (GDNF) (Prospec, Ness Ziona, Israel) was diluted in PBS containing 0.25 mg/mL BSA as a carrier. GDNF was then diluted to 50 ng/mL directly in serum-free media.
Cell viability assays
In order to determine the efficacy of cabozantinib against neuroblastoma tumor cells, a panel of human neuroblastoma tumor cell lines was tested for sensitivity in vitro to cabozantinib using a modified methyl tetrazolium (MTT; Sigma) assay [29]. 0.5–1.0 × 104 exponentially growing cells in 135 μL media were plated in individual wells in 96-well plates using an automated drug delivery system (Biomek Automated Laboratory Workstation, Beckman Coulter, Inc., Fuller, CA, USA). Twenty-four hours later, cabozantinib was added to each well at specified concentrations. After 72 h of continuous drug exposure, 15 µL of 5 mg/ml MTT was added to each well and the plates were incubated for 4 h at 37 °C. Medium was replaced with 150 µL of DMSO, and the optical density (OD) was measured at 550 nm using a microplate spectrophotometer (Anthos Labtec Instruments, Wals, Austria). Relative cell viability was calculated by subtracting the background OD of media alone and then dividing by the OD of control wells. Replicates of three wells were used for each drug concentration, and assays were duplicated on separate days. Concentration that inhibits 50 % (IC50) values was derived using best-fit trendlines and values calculated using the relevant curve-fit equations.
For cabozantinib and 13-cis-retinoic acid combination studies, cells were plated as above and treated with either cabozantinib alone, 5 μM 13-cis-retinoic acid alone, or combinations of 5 μM 13-cis-retinoic acid and increasing concentrations of cabozantinib for 72 h. Cell viability was determined as above, and combination indices (CI’s) were calculated using results from experiments using 5 μM cabozantinib and 5 μM 13-cis-retinoic acid doses and CalcuSyn v2.11 software (Biosoft, Cambridge, UK).
For combination studies using cabozantinib and topotecan or temozolomide, average cell viability for each drug alone was calculated and plotted against individual drug concentrations. The estimated concentrations at which cell viability was reduced by 10, 25, 50, 75, and 90 % (i.e., the IC10, IC25, etc.) from single-drug experiments were used to choose the concentrations of each drug to be used for the combination experiments. Cells were then plated as above, treated with the specified concentrations of cabozantinib with either topotecan or temozolomide (with separate 96-well plates for each combination) for 72 h, with cell viability determined as above and combination indices calculated using results at IC25 drug concentrations or as indicated.
Western blots
4 × 105 neuroblastoma cells were plated in 60 mm plates, allowed to adhere overnight, and then cultured for 16 h in serum-free media. Cells were then treated with either cabozantinib or vehicle for 3 h, followed by the addition of 50 ng/mL GDNF for 15 min. Cells were harvested, washed with PBS, and lysed with lysis buffer [50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 % Triton X-100, 0.1 % SDS, 2 mM EDTA, and 1X protease inhibitor (Sigma)].
Protein concentration in each sample was measured using a protein assay dye reagent (Bio-Rad, Hercules, CA, USA). From each cell line and tumor sample lysate, 30–50 μg total denatured protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Invitrogen, Carlsbad, CA, USA) using a dry blot transfer system (Invitrogen). Membranes were blocked in Odyssey blocking buffer (Li-Cor, Lincoln, NE, USA) for 1 h at room temperature and then incubated overnight at 4 °C with primary antibodies to total RET (SC-167, 1:500; Santa Cruz, Dallas, TX, USA), phosphorylated RET (SC-20252, 1:500; Santa Cruz), and vinculin (EPR8185, 1:5000; Abcam, Cambridge, MA, USA). All antibodies were diluted in Odyssey blocking buffer (Li-Cor) with 0.1 % Tween-20. Membranes were then washed three times with PBS-T (PBS plus 0.1 % Tween-20) and incubated for 1 h at room temperature with IRDye800-conjugated affinity purified anti-rabbit or anti-mouse secondary antibodies (1:2000; Rockland, Gilbertsville, PA, USA). Membranes were then visualized on an Odyssey infrared imaging system (Li-Cor).
To measure the effect of cabozantinib on intracellular signaling pathways, neuroblastoma cell lines were plated at approximately 80 % confluency and allowed to adhere overnight. Plates were washed twice with PBS and incubated in cabozantinib or solvent alone for three additional hours. Cells were lysed as above, and proteins were separated by SDS-PAGE, transferred onto nitrocellulose membranes, and blocked as described above. Immunoblots were incubated overnight at 4 °C with antibodies to total ERK (#4695, 1:2000; Cell Signaling, Danvers, MA, USA), phosphorylated ERK (#4375, 1:1000; Cell Signaling), and β-actin (#A3853, 1:5000; Sigma). Secondary antibody incubation and imaging were performed as above. Immunoblot band densities were determined with ImageJ (v1.42, NIH) as previously described (www.lukemiller.org/ImageJ_gel_analysis.pdf). Relative intensity levels were determined by dividing the band intensity of the phosphorylated protein by the band intensity of the total protein.
Animal experiments
To evaluate the efficacy of cabozantinib against neuroblastoma tumors in vivo, neuroblastoma tumor cells were injected into the exposed adrenal glands of immunocompromised mice, and mice were then treated with either vehicle alone or cabozantinib. Four- to six-week-old NCR-Mu (Taconic Farms, Hudson, NY, USA) mice were anesthetized, the left flanks were prepared in sterile fashion, and transverse incisions were performed to expose the left kidneys and adrenal glands. 106 SK-N-SH neuroblastoma tumor cells engineered to constitutively express firefly luciferase were suspended in 0.1 mL of PBS and injected into adrenal glands using a 27-gauge needle, which results in >90 % of mice developing tumors [29]. Tumor development and growth were monitored twice per week using the Xenogen Lumina system (Caliper Life Sciences, Hopkinton, MA, USA) 10 min after intraperitoneal injections of 150 mg/kg d-luciferin (Caliper Life Sciences), and tumor volume was estimated using signal intensity (in p/sec/cm2/sr). Five days after surgery, mice were randomly separated into two groups. Mice in group one were gavage fed once daily with vehicle alone (0.5 % HCl), while the other group of mice were fed with cabozantinib at 75 mg/kg. Both groups of mice were treated 5 days per week for 6 weeks and then sacrificed. Tumors were harvested, weighed, and photographed. P values for tumor growth were calculated using Student’s t tests on log values of tumor volumes as measured by Lumina signal intensity. All mice were treated according to protocols approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine.
Results
Neuroblastoma cell line sensitivity to cabozantinib alone and in combination with chemotherapy
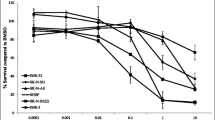
In order to determine the efficacy of cabozantinib against neuroblastoma tumor cells, a panel of twelve established human neuroblastoma tumor cell lines representing a range of biological phenotypes (Online Resource 1) was tested for sensitivity in vitro to cabozantinib using MTT assays. IC50 values were calculated and ranged from 1.6 to 16.2 μM, with 7 of the 12 cell lines having IC50 values below 5 μM (Fig. 1).
Effects of cabozantinib on neuroblastoma cell lines. a Neuroblastoma cells were treated with increasing concentrations of cabozantinib for 72 h, and cell viability was determined by MTT assays and plotted against cabozantinib dose levels. b IC50 values were calculated and plotted for each tested neuroblastoma cell line
In order to determine the efficacy of cabozantinib in combination with other anticancer therapy, neuroblastoma tumor cell lines were tested for sensitivity to cabozantinib combined with 13-cis-retinoic acid, a vitamin A analog currently used for maintenance therapy in children with neuroblastoma [2], and with topotecan and temozolomide, two chemotherapy agents commonly used in children with neuroblastoma [40, 41]. In tested cell lines known to be sensitive to retinoic acid, the combination of cabozantinib with 13-cis-retinoic acid was more effective than cabozantinib alone over a range of cabozantinib concentrations (Fig. 2a). In order to determine whether the combination of cabozantinib and 13-cis-retinoic acid demonstrated synergistic efficacy, neuroblastoma tumor cell lines were treated with cabozantinib, 13-cis-retinoic acid, or both, and viability was determined using MTT assays. Combination indices were found to be <1 for all sensitive cell lines but not in the retinoic acid-resistant cell line SK-N-AS [Fig. 2b; combination indices: LAN5 0.66; SK-N-AS 2.22; CHP-212 0.12; SK-N-BE(2) 0.48], suggesting synergistic efficacy of the combination in cell lines sensitive to retinoic acid. Furthermore, the combination of cabozantinib with either topotecan or temozolomide also resulted in synergistic efficacy against CHP-212 neuroblastoma tumor cells and with temozolomide against SK-N-SH neuroblastoma tumor cells (Fig. 2c–f), with combination indices as follows: SK-N-SH/topotecan >1; SK-N-SH/temozolomide 0.56; CHP-212/topotecan 0.27; CHP-212/temozolomide 0.12.
Efficacy of cabozantinib combined with 13-cis-retinoic acid and chemotherapy agents topotecan and temozolomide. a Neuroblastoma tumor cell lines were treated with cabozantinib (XL184) or with 5 μM 13-cis-retinoic acid combined with increasing doses of cabozantinib (CRA + XL184), and cell viability was determined at 72 h by MTT assays. b Neuroblastoma cell lines were treated with either 5 μM cabozantinib (XL184), 5 μM 13-cis-retinoic acid (CRA), or the combination of 5 μM cabozantinib plus 5 μM 13-cis-retinoic acid (CRA + XL184) for 72 h, and cell viability was determined by MTT assays. Combination indices were calculated from these results as follows: LAN5 0.66; SK-N-AS 2.22; CHP-212 0.12; SK-N-BE2(C) 0.48 (c–f). SK-N-SH (c, e) and CHP-212 (d, f) neuroblastoma tumor cell lines were treated with the combinations of cabozantinib and topotecan (c, d) or with cabozantinib and temozolomide (e, f) for 72 h at the specified concentrations, and cell viability was determined by MTT assays. Combination indices were calculated using IC25 concentrations as follows: SK-N-SH/topotecan >1; SK-N-SH/temozolomide 0.56; CHP-212/topotecan 0.27; CHP-212/temozolomide 0.12
Inhibition of RET and intracellular signaling by cabozantinib
Most neuroblastoma tumor cell lines have minimal or no c-Met expression [29]. Therefore, to evaluate whether RET inhibition was responsible for differences in neuroblastoma tumor cell sensitivity to cabozantinib, two cell lines with IC50 values <5 μM (LAN-5 and CHLA-20) and two cell lines with IC50 values >5 μM [SK-N-BE(2) and SK-N-AS] were evaluated for inhibition of RET and intracellular signaling. Neuroblastoma cells were treated with the RET ligand GDNF and then either vehicle or increasing doses of cabozantinib. GDNF treatment induced RET phosphorylation in all cell lines, which could be inhibited by treatment with cabozantinib (Fig. 3). These results demonstrate that cabozantinib is effective at inhibiting GDNF-induced RET phosphorylation in all tested neuroblastoma tumor cell lines, and therefore, differences in RET inhibition do not explain the different sensitivities of neuroblastoma tumor cells to cabozantinib therapy.
RET inhibition by cabozantinib. a Neuroblastoma tumor cells were lysed after treatment with 50 ng/mL GDNF for 15 min (“control”) or after both GDNF and cabozantinib (XL184) at specified doses. Western blots were performed for total (“RET”) and phosphorylated (“p-RET”) RET. Western blots for vinculin were used as protein loading controls. b Relative p-RET western blot band intensity (pRET/RET) was determined and plotted for each tested cell line
To determine whether cabozantinib treatment resulted in inhibition of downstream signaling pathways, neuroblastoma cell lines were treated with cabozantinib and then were harvested and lysed. Western blots for total and phosphorylated ERK were performed (Fig. 4). Cabozantinib treatment resulted in inhibition of ERK phosphorylation in LAN5 and CHLA-20 cell lines, with minimal to no effect seen in SK-N-BE(2) and SK-N-AS cell lines, suggesting that RAS/MAPK pathway inhibition is a potential mechanism underlying the relative sensitivity of neuroblastoma tumor cells to cabozantinib.
Inhibition of intracellular signaling in neuroblastoma tumor cells by cabozantinib. a Neuroblastoma cells were plated and treated with increasing concentrations of cabozantinib (XL184). Cells were lysed at baseline (“control”) and after treatment and Western blots for total (“Erk”) and phosphorylated (“p-Erk”) Erk were performed. β-actin was used as a protein loading control. b Relative western blot band intensity (pERK/ERK) was determined and plotted for each tested cell line
Inhibition of neuroblastoma xenograft tumor growth with cabozantinib
To evaluate the efficacy of cabozantinib against neuroblastoma tumors in vivo, neuroblastoma tumor cells were injected into the exposed adrenal glands of immunocompromised mice, and mice were then treated with either vehicle alone or cabozantinib. Cabozantinib treatment was well tolerated and associated with significantly reduced xenograft tumor growth rates compared to vehicle-treated controls (p < 0.05; Fig. 5). Cabozantinib treatment also resulted in significant reduction in final tumor weights and size compared to vehicle-treated control tumors (Fig. 6), with untreated tumors reaching an average final tumor weight of 2.06 g (±0.85 g) and cabozantinib-treated tumors reaching an average final tumor weight of 0.14 g (±0.04 g) (p < 0.05).
Xenograft neuroblastoma tumor growth after treatment with cabozantinib. Mice with orthotopic adrenal neuroblastoma xenograft tumors were treated with vehicle or cabozantinib. Tumor development and growth were monitored twice per week, and tumor volume was estimated using signal intensity (in p/sec/cm2/sr). Changes in signal intensity were plotted over time (a), and representative final images for untreated control and cabozantinib-treated mice are shown (b)
Final xenograft neuroblastoma tumor size after treatment with cabozantinib. Mice with orthotopic adrenal neuroblastoma xenograft tumors were treated with vehicle or cabozantinib, and tumors were harvested at the end of the experiment. a Final tumor weights were obtained and average values calculated, and b the individual final tumor weight values were plotted for the untreated control and cabozantinib-treated mice. c Representative pictures of harvested tumors are shown
Discussion
New treatment strategies are clearly needed for children with recurrent or refractory neuroblastoma. We have demonstrated that the tyrosine kinase inhibitor cabozantinib is effective against neuroblastoma in in vitro and in vivo model systems. Cabozantinib inhibits RET phosphorylation and intracellular signaling and reduces xenograft tumor growth, suggesting that cabozantinib may be an effective treatment for children with neuroblastoma.
Cabozantinib is an inhibitor of several tyrosine kinases, including RET, c-Met, and vascular endothelial growth factor receptor-2 (VEGFR-2, KDR) [17–19]. Most neuroblastoma tumor cell lines and patient tumor samples have very low or undetectable levels of c-Met gene and protein expression [29]. The efficacy of cabozantinib against neuroblastoma tumor cell lines is therefore most likely due to RET inhibition. However, the efficacy of cabozantinib in neuroblastoma tumor cells may be due to inhibition of other, unidentified kinase targets. Our data also suggest that neuroblastoma tumor cell sensitivity to cabozantinib is mediated by inhibition of downstream signaling pathways, suggesting that cabozantinib could be acting via multiple mechanisms in neuroblastoma tumor cells and tumors, leading to enhanced efficacy.
We demonstrated synergistic efficacy of the combinations of cabozantinib with 13-cis-retinoic acid and with chemotherapy agents topotecan and temozolomide. Retinoids are vitamin A analogs that induce tumor cell differentiation [42]. Retinoic acid reduces neuroblastoma cell proliferation, decreases MYCN oncogene expression, induces neuroblastoma cell differentiation, and is currently used for maintenance therapy in neuroblastoma treatment regimens [2, 43–45]. Recent studies have documented an increase in RET expression in response to retinoic acid treatment and have shown that RET inhibition interferes with retinoic acid-induced differentiation [46–49]. Other recent studies have demonstrated that retinoic acid-induced differentiation of neuroblastoma cells creates dependence on neurotrophin and glial-derived neurotrophic factor signaling, suggesting that retinoic acid treatment may sensitize neuroblastoma cells to inhibition of these pathways [50, 51]. Prior studies have identified synergistic efficacy of 13-cis-retinoic acid and the RET inhibitor vandetanib [27], suggesting that combined effects on RET may be responsible for the synergism seen with cabozantinib.
Topotecan and temozolomide are chemotherapy agents shown to be effective against neuroblastoma tumors in preclinical models [52, 53], and both agents are components of regimens commonly employed to treat children with relapsed neuroblastoma [36, 54–57]. The efficacy of cabozantinib in combination with topotecan and temozolomide provides an opportunity for testing in early-phase clinical trials for children with relapsed neuroblastoma to determine the safety and tolerability of these combinations.
In adult patients with advanced solid tumors, cabozantinib therapy was reasonably well tolerated. Commonly reported toxicities included nausea, anorexia, fatigue, diarrhea, and palmar-plantar erythrodysesthesia, and the dose approved by the US Food and Drug Administration was determined to be 140 mg (freebase weight) once daily [18]. Pharmacokinetic analyses demonstrated that the steady-state drug level in adult patients taking 140 mg once per day exceeded 2.8 μM [21], within the range of our in vitro IC50 values and suggesting that effective drug levels may be attainable in children as well. Cabozantinib therefore represents a good candidate for further investigation in children with relapsed neuroblastoma.
We have demonstrated that the novel tyrosine kinase inhibitor cabozantinib is effective against neuroblastoma tumor cells in vitro and in vivo. The evidence for the roles of VEGFR, c-Met, and RET signaling in neuroblastoma tumor cell growth and the demonstrated in vitro and in vivo efficacy of cabozantinib in these studies provide strong biological and clinical rationale for additional preclinical and clinical testing of this agent in children with relapsed or refractory neuroblastoma. Cabozantinib in combination with 13-cis-retinoic acid may prove to be an effective treatment for these children. Although the role of this combination in future up-front neuroblastoma treatment is unclear, the addition of cabozantinib to 13-cis-retinoic acid during maintenance therapy for high-risk neuroblastoma patients may further improve relapse-free survival rates.
Abbreviations
- VEGFR-2:
-
Vascular endothelial growth factor receptor-2
- ATCC:
-
American Type Culture Collection
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- FBS:
-
Fetal bovine serum
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- MTT:
-
3-(4,5-Dimethylthiazolyl-2-yl)-2,5-diphenyltetrazolium bromide
- GDNF:
-
Glial-derived neurotrophic factor
- MTC:
-
Medullary thyroid cancer
- PBS:
-
Phosphate-buffered saline
- CI:
-
Combination index
References
Ladenstein R, Philip T, Lasset C, Hartmann O, Garaventa A, Pinkerton R et al (2009) Multivariate analysis of risk factors in stage 4 neuroblastoma patients over the age of one year treated with megatherapy and stem-cell transplantation: a report from the European Bone Marrow Transplantation Solid Tumor Registry. J Clin Oncol 16:953–965
Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D et al (2009) Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a Children’s Oncology Group study. J Clin Oncol 27:1007–1013
Zage PE, Kletzel M, Murray K, Marcus R, Castleberry R, Zhang Y et al (2008) Outcomes of the POG 9340/9341/9342 trials for children with high-risk neuroblastoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer 51:747–753
Lau L, Tai D, Weitzman S, Grant R, Baruchel S, Malkin D (2004) Factors influencing survival in children with recurrent neuroblastoma. J Pediatr Hematol Oncol 26:227–232
London WB, Castel V, Monclair T, Ambros PF, Pearson ADJ, Cohn SL et al (2011) Clinical and biologic features predictive of survival after relapse of neuroblastoma: a report from the International Neuroblastoma Risk Group project. J Clin Oncol 29:3286–3292
Matsui T, Sano K, Tsukamoto T, Ito M, Takaishi T, Nakata H et al (1993) Human neuroblastoma cells express alpha and beta platelet-derived growth factor receptors coupling with neurotrophic and chemotactic signaling. J Clin Invest 92:1153–1160
Cohen PS, Chan JP, Lipkunskaya M, Biedler JL, Seeger RC (1994) Expression of stem cell factor and c-kit in human neuroblastoma. The Children’s Cancer Group. Blood 84:3465–3472
Janet T, Ludecke G, Otten U, Unsicker K (1995) Heterogeneity of human neuroblastoma cell lines in their proliferative responses to basic FGF, NGF, and EGF: correlation with expression of growth factors and growth factor receptors. J Neurosci Res 40:707–715
Eggert A, Ikegaki N, Kwiatkowski J, Zhao H, Brodeur GM, Himelstein BP (2000) High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin Cancer Res 6:1900–1908
Langer I, Vertongen P, Perret J, Fontaine J, Atassi G, Robberecht P (2000) Expression of vascular endothelial growth factor (VEGF) and VEGF receptors in human neuroblastomas. Med Pediatr Oncol 34:386–393
Hecht M, Papoutsi M, Tran HD, Wilting J, Schweigerer L (2004) Hepatocyte growth factor/c-Met signaling promotes the progression of experimental human neuroblastomas. Cancer Res 64:6109–6118
Meyers MB, Shen WP, Spengler BA, Ciccarone V, O’Brien JP, Donner DB et al (1998) Increased epidermal growth factor receptor in multidrug-resistant human neuroblastoma cells. J Cell Biochem 38:87–97
Ho R, Minturn JE, Hishiki T, Zhao H, Wang Q, Cnaan A et al (2005) Proliferation of human neuroblastomas mediated by the epidermal growth factor receptor. Cancer Res 65:9868–9875
Meister B, Grunebach F, Bautz F, Brugger W, Fink FM, Kanz L, Mohle R (1999) Expression of vascular endothelial growth factor (VEGF) and its receptors in human neuroblastoma. Eur J Cancer 35:445–449
Fakhari M, Pullirsch D, Paya K, Abraham D, Hofbauer R, Aharinejad S (2002) Upregulation of vascular endothelial growth factor receptors is associated with advanced neuroblastoma. J Pediatr Surg 37:582–587
Crosswell HE, Dasgupta A, Alvarado CS, Watt T, Christensen JG, De P et al (2009) PHA665752, a small-molecule inhibitor of c-Met, inhibits hepatocyte growth factor-stimulated migration and proliferation of c-Met-positive neuroblastoma cells. BMC Cancer 9:411
Grüllich C (2014) Cabozantinib: a MET, RET, and VEGFR2 tyrosine kinase inhibitor. Recent Results Cancer Res 201:207–214
Viola D, Cappagli V, Elisei R (2013) Cabozantinib (XL184) for the treatment of locally advanced or metastatic progressive medullary thyroid cancer. Future Oncol 9:1083–1092
Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P et al (2011) Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 10:2298–2308
Sennino B, Ishiguro-Oonuma T, Wei Y, Naylor RM, Williamson CW, Bhagwandin V et al (2012) Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov 2:270–287
Kurzrock R, Sherman SI, Ball DW, Forastiere AA, Cohen RB, Mehra R et al (2011) Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol 29:2660–2666
Gordon MS, Vogelzang NJ, Schoffski P, Daud A, Spira AI, O’Keeffe BA et al (2011) Cabozantinib (XL184) has activity in both soft tissue and bone: results of a phase II randomized discontinuation trial in patients with advanced solid tumors. J Clin Oncol 29:3010
Drilon A, Wang L, Hasanovic A, Suehara Y, Lipson D, Stephens P et al (2013) Response to Cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov 3:630–635
Smith MR, Sweeney CJ, Corn PG, Rathkopf DE, Smith DC, Hussain M et al (2014) Cabozantinib in chemotherapy-pretreated metastatic castration-resistant prostate cancer: results of a phase II nonrandomized expansion study. J Clin Oncol 32:3391–3399
Choueiri TK, Pal SK, McDermott DF, Morrissey S, Ferguson KC, Holland J et al (2014) A phase I study of cabozantinib (XL184) in patients with renal cell cancer. Ann Oncol 25:1603–1608
Elisei R, Schlumberger MJ, Müller SP, Schöffski P, Brose MS, Shah MH et al (2013) Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 31:3639–3646
Zage PE, Zeng L, Palla S, Fang W, Nilsson MB, Heymach JV et al (2010) A novel therapeutic combination for neuroblastoma: the VEGFR/EGFR/RET inhibitor vandetanib with 13-cis-retinoic acid. Cancer 116:2465–2475
Yan B, Lim M, Zhou L, Kuick CH, Leong MY, Yong KJ et al (2013) Identification of MET genomic amplification, protein expression, and alternative splice isoforms in neuroblastomas. J Clin Pathol 66:985–991
Scorsone K, Zhang L, Woodfield SE, Hicks J, Zage PE (2014) The novel kinase inhibitor EMD1214063 is effective against neuroblastoma. Invest New Drugs 32:815–824
Biedler JL, Helson L, Spengler BA (1973) Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res 33:2643–2652
Brodeur GM, Green AA, Hayes FA, Williams KJ, Williams DL, Tsiatis AA (1981) Cytogenetic features of human neuroblastomas and cell lines. Cancer Res 41:4678–4686
Reynolds CP, Tomayko MM, Donner L, Helson L, Seeger RC, Triche TJ et al (1988) Biological classification of cell lines derived from human extra-cranial neural tumors. Prog Clin Biol Res 271:291–306
Schlesinger HR, Gerson JM, Moorhead PS, Maguire H, Hummeler K (1976) Establishment and characterization of human neuroblastoma cell line. Cancer Res 36:3094–3100
Keshelava N, Seeger RC, Groshen S, Reynolds CP (1998) Drug resistance patterns of human neuroblastoma cell lines derived from patients at different phases of therapy. Cancer Res 58:5396–5405
Tumilowicz JJ, Nichols WW, Cholon JJ, Greene AE (1970) Definition of a continuous human cell line derived from neuroblastoma. Cancer Res 30:2110–2118
Ciccarone V, Spengler BA, Meyers MB, Biedler JL, Ross RA (1989) Phenotypic diversification in human neuroblastoma cells: expression of distinct neural crest lineages. Cancer Res 49:219–225
Sugimoto T, Tatsumi E, Kemshed JT, Helson L, Green AA, Minowada J (1984) Determination of cell surface membrane antigens common to both human neuroblastoma and leukemia–lymphoma cell lines by a panel of 38 monoclonal antibodies. J Natl Cancer Inst 73:51–57
Helson L, Helson C (1985) Human neuroblastoma cells and 13-cis-retinoic acid. J Neurooncol 3:39–41
Gilbert F, Feder M, Balaban G, Brangman D, Lurie DK, Podolsky R, Rinaldt V, Vinikoor N, Weisband J (1984) Human neuroblastoma and abnormalities of chromosomes 1 and 17. Cancer Res 44:5444–5449
Santana VM, Furman WL, Billups CA, Hoffer F, Davidoff AM, Houghton PJ et al (2005) Improved response in high-risk neuroblastoma with protracted topotecan administration using a pharmacokinetically guided dosing approach. J Clin Oncol 23:4039–4047
Rubie H, Chisholm J, Defachelles AS, Morland B, Munzer C, Valteau-Couanet D et al (2006) Phase II study of temozolomide in relapsed or refractory high-risk neuroblastoma: a joint Societe Francaise des Cancers de l’Enfant and United Kingdom Children Cancer Study Group-New Agents Group Study. J Clin Oncol 24:5259–5264
Miller WH (1998) The emerging role of retinoids and retinoic acid metabolism blocking agents in the treatment of cancer. Cancer 83:1471–1482
Sidell N (1982) Retinoic acid-induced growth inhibition and morphologic differentiation of human neuroblastoma cells in vitro. J Natl Cancer Inst 68:589–596
Sidell N, Altman A, Haussler MR, Seeger RC (1983) Effects of retinoic acid (RA) on the growth and phenotypic expression of several human neuroblastoma cell lines. Exp Cell Res 148:21–30
Thiele CJ, Reynolds CP, Israel MA (1985) Decreased expression of N-myc precedes retinoic acid-induced morphological differentiation of human neuroblastoma. Nature 313:404–406
Hishiki T, Nimura Y, Isogai E, Kondo K, Ichimiya S, Nakamura Y et al (1998) Glial cell line-derived neurotrophic factor/neurturin-induced differentiation and its enhancement by retinoic acid in primary human neuroblastomas expressing c-Ret, GFR alpha-1, and GFR alpha-2. Cancer Res 58:2158–2165
Oppenheimer O, Cheung N-K, Gerald WL (2007) The RET oncogene is a critical component of transcriptional programs associated with retinoic acid-induced differentiation in neuroblastoma. Mol Cancer Ther 6:1300–1309
Bunone G, Borrello MG, Picetti R, Bongarzone I, Peverali FA, de Franciscis V et al (1995) Induction of RET proto-oncogene expression in neuroblastoma cells precedes neuronal differentiation and is not mediated by protein synthesis. Exp Cell Res 217:92–99
D’Aleesio A, De Vita G, Cali G, Nitsch L, Fusco A, Vecchio G et al (1995) Expression of the RET oncogene induces differentiation of SK-N-BE neuroblastoma cells. Cell Growth Differ 6:1387–1394
Encinas M, Iglesias M, Liu Y, Wang H, Muhaisen A, Cena V et al (2000) Sequential treatment of SH-SY5Y Cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J Neurochem 75:991–1003
Takada N, Isogai E, Kawamoto Nakanishi H, Todo S, Nakagawara A (2001) Retinoic acid-induced apoptosis of the CHP134 neuroblastoma cell line is associated with nuclear accumulation of p53 and is rescued by the GDNF/Ret signal. Med Pediatr Oncol 36:122–126
Carol H, Houghton PJ, Morton CL, Kolb EA, Gorlick R, Reynolds CP, Kang MH, Maris JM, Keir ST, Watkins A, Smith MA, Lock RB (2010) Initial testing of topotecan by the pediatric preclinical testing program. Pediatr Blood Cancer 54:707–715
Keir ST, Maris JM, Reynolds CP, Kang MH, Kolb EA, Gorlick R, Lock R, Carol H, Morton CL, Wu J, Kurmasheva RT, Houghton PJ, Smith MA (2013) Initial testing (stage 1) of temozolomide by the pediatric preclinical testing program. Pediatr Blood Cancer 60:783–790
Donfrancesco A, Jenkner A, Castellano A, Ilari I, Milano GM, De Sio L, Cozza R, Fifani P, Deb G, De Laurentis C, Inserra A, Dominici C (2004) Ifosfamide/carboplatin/etoposide (ICE) as front-line, topotecan/cyclophosphamide as second-line and oral temozolomide as third-line treatment for advanced neuroblastoma over one year of age. Acta Pediatr 445:6–11
London WB, Frantz CN, Campbell LA, Seeger RC, Brumback BA, Cohn SL, Matthay KK, Castleberry RP, Diller L (2010) Phase II randomized comparison of topotecan plus cyclophosphamide versus topotecan alone in children with recurrent or refractory neuroblastoma: a children’s oncology group study. J Clin Oncol 28:3808–3815
Kushner BH, Kramer K, Modak S, Cheung N-KV (2006) Irinotecan plus temozolomide for relapsed or refractory neuroblastoma. J Clin Oncol 24:5271–5276
Bagatell R, London WB, Wagner LM, Voss SD, Stewart CF, Maris JM, Kretschmar C, Cohn SL (2011) Phase II study of irinotecan and temozolomide in children with relapsed or refractory neuroblastoma: a children’s oncology group study. J Clin Oncol 29:208–213
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
Characteristics of Neuroblastoma Tumor Cell Lines (XLS 25 kb)
Rights and permissions
About this article
Cite this article
Zhang, L., Scorsone, K., Woodfield, S.E. et al. Sensitivity of neuroblastoma to the novel kinase inhibitor cabozantinib is mediated by ERK inhibition. Cancer Chemother Pharmacol 76, 977–987 (2015). https://doi.org/10.1007/s00280-015-2871-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2871-z