Abstract
Purpose
Busulfan (Bu) exposure is critical for efficacy and safety. Body weight (BW), or adjusted ideal body weight (AIBW)-based dosing (WBD) algorithm, has been used in hematopoietic stem cell transplantation (HSCT). A recently completed phase 2 study revealed that 33.6 % of the subjects were under-, or over-exposed, with this WBD algorithm. This paper was to investigate Bu dosing algorithm in an attempt to improve the suboptimal Bu exposure.
Methods
Population PK modeling was conducted using the data from 207 patients. Dosing algorithm was developed based on derived covariate model of CL. Model-based simulation was conducted to assist test PK study design. A simplified CL estimation method was proposed based on the PK structure model for Bu.
Results
A one-compartment structure model adequately described the PK profile of Bu following an IV infusion. BSA best described the inter-individual variability of CL. The proposed dosing algorithm was dose (mg) = (31.7 × BSA − 11.6) × target AUC [µM min]/1,000. With this dosing algorithm, 14.3 % patients could be under- or over-exposed. A test PK study with reduced study duration and three PK samples can provide as nearly as good an estimate of CL compared to 12 PK samples on two different occasions.
Conclusion
The proposed dosing algorithm can significantly improve the sub-exposure of Bu. A shortened test PK study duration with reduced PK samples can provide as near as good estimate for Bu CL. A simplified CL estimation method is valid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The exposure of busulfan (Bu) is known to be critical for efficacy and safety [1–3]. Introduction of the intravenous (IV) formulation of Bu in the late 1990s resulted in significant improvements in the safety and efficacy of Bu [4–6], probably due to less variability in exposure compared with the oral formulation [7, 8]. In the hematopoietic stem cell transplantation (HSCT) community, the initial dose of Bu for the Q6h dosing schedule (e.g., 0.8 mg/kg) or for the once daily dose schedule (e.g., 3.2 mg/kg) was often calculated using the actual body weight (BW), the ideal body weight (IBW), the adjusted ideal body weight (AIBW) [9–12]. Using either BW or IBW for calculation of Bu dose has the potential of underexposure for overweight or obese subjects [10, 13].
Population pharmacokinetics (PK) modeling has demonstrated that BSA or AIBW, followed by IBW or BW, can best describe the inter-individual variability (IIV) of Bu clearance (CL) [11]. Clearance normalized by BW for normal weight subjects, and by AIBW for obese subjects (BMI > 26.9 kg/m2), appeared to be a constant, suggesting that comparable area under the curve (AUC) values can be achieved for subjects with normal weight or obese subjects if Bu dose is calculated using BW or AIBW, respectively [11]. Similar conclusions were drawn using oral doses of Bu [13].
In a prospective, multicenter, single-arm, phase 2 study investigating the safety and efficacy of an IV busulfan, cyclophosphamide, and etoposide (BuCyE) regimen in subjects with Hodgkin’s lymphoma (HL) or non-Hodgkin’s lymphoma (NHL) undergoing first autologous HSCT [14], the test PK Bu dose was calculated for subject as below: The BW, IBW, and AIBW were calculated for each subject. For subjects whose BW is ≤IBW, then the Bu dose was calculated as 0.8 mg/kg BW; for subjects with BW > IBW, the Bu dose was calculated as 0.8 mg/kg AIBW. This dosing algorithm was referred to as “weight-based dosing” [WBD] in this manuscript. Results from this trial revealed that 31.1 % of the subjects were below 80 % of the targeted AUC, and 2.5 % were above 120 % of the targeted AUC. Further examination of the data showed that the percentage of subjects who were underexposed increased with BMI: 18 % for BMI between 18 and 26.9 kg/m2, 32 % for BMI between 27 and 35 kg/m2, and 39 % for BMI > 35 kg/m2, with no obese subjects being overexposed. Using the same lower limit of AUC (900 µM min) as in the previously published study [11], the percentage of subjects with AUC <900 μM min still increased with BMI, although the percentages were lower (11.3, 14.3, and 25 %, respectively). These results suggest that using AIBW to calculate Bu dose for obese subjects resulted in underexposure.
This paper describes a model-based approach applied to the dataset described above to further investigate the WBD algorithm in an attempt to improve the suboptimal Bu exposure. Since PK-guided dose adjustment is a practical way to achieve the optimal therapeutic exposure window of Bu for subjects undergoing HSCT, optimizing PK study design such as using a low dose of Bu with a reduced infusion duration was explored. Furthermore, a simplified estimation for Bu CL (or AUC) was proposed for use when population PK analysis is not feasible.
Materials and methods
Clinical trial design
Pharmacokinetic data used for the analysis were from a prospective, multicenter, single-arm, phase 2 study in subjects with HL or NHL investigating the safety and efficacy of an IV BuCyE regimen [14]. PK-guided Bu dosing adjustment was implemented in two PK measurements: test PK, prior to initiation of the preparative regimen, and “real-time” PK, on the first day of IV Bu administration during the preparative regimen, to confirm the findings from the test PK.
Subjects received a 0.8 mg/kg test dose of IV Bu, administered as a 2-h continuous infusion, on a single day between Days −14 and −11 (Day 0 is the day of stem cell infusion). Based on the test PK results, the remaining Bu dose was calculated to achieve a total AUC of 20,000 of μM min. One-fourth of this dose was given as a 3-h infusion on Day −8, during which a second PK analysis was done. The same daily Bu dose was administered on Days −7, −6, and −5, unless the Day −8 PK results showed total AUC outside the target ±20 %. The Bu dose for each subject was calculated using either BW or AIBW. If the BW was less than or equal to the IBW, then the BW was used to calculate the Bu dose. Otherwise, the AIBW was used. The IBW was calculated for all subjects as follows:
The AIBW was calculated as IBW + 25 % of the difference between BW and IBW.
Subjects and PK samplings
A total of 207 subjects with HL (n = 66) or NHL (n = 141) were enrolled in the study and included in the analysis. The median age of the subjects was 52 years (range 19–72 years). The median BW was 83.4 kg (range 38.8–178.2 kg). Sixty percent of the subjects were overweight or obese, 39 % were normal weight, and 1 % was underweight.
Out of 207 enrolled subjects, 203 received the BuCyE preparative regimen: 200 received individualized Bu doses based on the test PK results and three received 3.2 mg/kg on Days −8 and −7, due to non-evaluable test PK results (these doses were further adjusted on Days −6 and −5, based on the second PK results obtained on Day −8).
For test PK, six serial blood samples were collected at the end of the 2-h infusion and at 2.25, 2.5, 4, 5, and 6 h after the start of the infusion. For the second PK on Day −8, samples were collected at the end of the 3-h infusion and at 3.25, 3.5, 4.5, 6, and 8 h after the start of the infusion. A blood sample prior to the infusion was also collected in the test PK, as well as the second PK. Test PK samples were collected from 207 subjects on Day −14, and second PK samples were collected from 203 subjects on Day −8 (n = 201) or Day −7 (n = 2). In one subject, second PK results were not evaluable and therefore were not reportable.
Population PK model and dose algorithm
A population PK model for IV Bu was developed using Bu plasma concentrations from test PK measurements and Day −8 or −7 PK measurements. A one- or two-compartment PK model was explored. To identify the key factors that contribute to Bu plasma exposure, covariate analysis of Bu CL and volume of distribution (V) was conducted through visual examination followed by stepwise forward inclusion and backward elimination. Significance of covariates was based on a number of factors including: reduction in IIV in the related PK parameter; good precision of the estimated parameter values; a statistically significant drop in the minimum value of objective function (MVOF) of >6.635 (p < 0.01, degrees of freedom = 1) for nested models; and clinically meaningful impact on PK parameters. During backward elimination, covariates that did not result in a MVOF increase of >10.828 (p < 0.001) when removed were excluded. The following covariates were tested: body size including BW/IBW/AIBW/body surface area (BSA)/body mass index (BMI), age, gender, and creatinine CL (CLcr).
To evaluate the WBD algorithm, dose based on either BW or AIBW, the following test was conducted, where θ BW was a parameter to be estimated. If the WBD dosing algorithm was considered to be optimal, the appropriate cutoff BW for dose calculation using BW or AIBW was to be determined.
Since CL is the PK parameter determining AUC, the dosing algorithm to achieve the target AUC could be established once the covariate model for CL was developed.
Variability in PK parameters for the test PK and preparative regimen was explored. Three different scenarios were tested: (1) the typical values of CL and V derived from test PK versus preparative regimen; (2) IIV derived from test PK versus preparative regimen; (3) residual errors from test PK versus preparative regimen.
Test PK study design modification
In a clinic setting of the hematopoietic stem cell transplantation, minimal PK samples and short study duration are important. The following simulations were conducted to evaluate if reducing PK samples and study duration would not compromise the precision of Bu CL estimation. The population PK model was used to simulate Bu plasma concentrations of 1,035 virtual subjects following a 1-h infusion of 0.4 mg/kg (dose and infusion time were reduced by half). Three to six plasma PK samples were collected following Bu administration (Table 3). The PK samples from each scenario were used in the population PK model to estimate Bu CL. The Bu CL using 12 PK samples from test PK and Day −8 or −7 PK using the observed PK data from the trial served as a reference for comparison.
Bu CL estimation using a simplified method
As population PK modeling is not often feasible in a clinic setting, a simplified PK analysis without compromising the precision of Bu CL was proposed based on the population PK model for Bu. Comparison between the Bu CL derived using population PK modeling and this simplified PK analysis method was conducted to demonstrate the validity of the proposed method.
Software
The population PK model was developed using NONMEM (nonlinear mixed-effect model) version 7.2.0 and the FOCE INTER (first-order conditional estimation with interaction method in NONMEM) estimation method. Pre- and post-analysis data processing were performed using R version 2.15.1.
Results
Population PK model
A one-compartment population PK model adequately describes the Bu PK profile following IV infusion. Age or CLcr (serum creatinine CL > 2.0 mg/dL) across the range of the study did not have any significant impact on Bu PK parameters (data not shown). Through visual examination, body size (BW/IBW/AIBW/BSA/BMI) appeared to be a significant covariate for both CL and V (data not shown). Those plots also suggested that BMI and IBW were the two least appropriate parameters for body size to be correlated to CL, since they resulted in the largest random variability. For other three covariates, BW, BSA, and AIBW, the random variability appeared to be similar; however, different correlations with CL were observed. For example, CL appeared to increase with BW to the power <1; CL increases with BSA following a linear relationship; CL increases with AIBW in a different patterns when AIBW less than 70–75 kg (which corresponding to the actual BW around 85–90 kg), and greater than 75 kg, respectively.
Covariate analysis using stepwise regression revealed that BSA was the most statistically significant covariate responsible for IIV of CL and V, followed by BW, AIBW, IBW, and BMI, which agreed with what was observed from the plots. Key covariate models with the MVOF and IIV % are shown in Table 1. For example, introducing BSA as a covariate for CL, and BSA and SEX on V reduced the MVOF from the base model by 378.499 points. The IIV was reduced from 23.4 to 13.7 % for CL and from 25.4 to 9.49 % for V. Switching between BW and AIBW as given in Eq. (1) resulted in worse model fitting (data not shown). All other tested covariate models that did not result in a reduction in MVOF of >6.635 were excluded.
Typical values of V and CL for test PK and preparative regimen were similar (47.5 vs. 51.0 L for V, and 12.9 vs. 12.8 h/L for CL). The values of MVOF, IIV %, and residual error in two occasions of test PK and preparative regimen are also shown in Table 1. There was no noticeable inter-occasional variability (test PK vs. preparative regimen PK) for CL, suggesting that CL derived from one occasion can be used to guide the subsequent dose adjustment to achieve the target AUC.
In the final covariate model, BSA correlated linearly with both CL and V, and gender correlated with V.
Diagnostic plots suggest that the final model well described the PK profiles of Bu following IV infusion (Fig. 1). The 50th percentiles of the predicted and observed values were in agreement; the dose corrective visual predictive check also demonstrated that the model well described the PK profiles of Bu following two different doses (Fig. 2).
The final covariate models are shown below:
BSA is calculated as: BSA (m2) = ([height (cm) × weight (kg)]/3,600)½. The value of 2.0 in the parenthesis is the median BSA of our study. For a subject with BSA of 2.0 m2, CL is 12.7 L/h for both male and female subjects; and V is 50.3 L for male and 46.3 L for female, respectively. With the same BSA, the V for males is approximately 10 % greater than females; with the same BSA, gender does not have any statistically significant impact on CL. Equations 2 and 3 only apply to the BSA ranging from 1.27 to 3.01 m2.
As an additional validation of the population PK model, AUC derived via noncompartment anlaysis (NCA) on the observed plasma concentration from test PK was compared with the values calculated from Dose/CL, where CL is the Bayesian estimate of CL from population PK modeling. A good agreement between these two methods was observed (Table 2).
Recommended dosing algorithm
Based on Eq. (3), the dose to achieve the target AUC after unit conversion was given in Eq. 4:
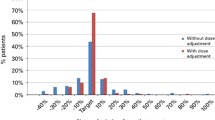
To test whether the dose algorithm given in Eq. (4) can improve the percentage of subjects with AUC values within the targeted range (±20 % of the target AUC), it was assumed that the same subjects in “test PK” were administered a Bu dose based on their BSA using Eq. 4. The targeted AUC was 1,250 µM min. Using the Bayesian individual estimation of CL from the population PK model, each AUC value was calculated as AUCindividual = Doseindividual/CLindividual. Results showed that only 14.3 % of the subjects (instead of 33.6 % using the WBD algorithm) would have been exposed to IV Bu outside the target AUC range, with 6.9 % of the subjects being underexposed and 7.4 % overexposed.
Test PK study design modification
Though the suboptimal exposure was significantly improved following the proposed dosing algorithm, 14.3 % of the subjects could still fail to achieve the target AUC, as the 13.7 % IIV in CL cannot be identified based on the information collected from subjects. Thus, PK-directed dose adjustment is a practical way to achieve the optimal therapeutic AUC window of Bu exposure for the subjects undergoing HSC.
The modification of the test PK study design was focused on reducing PK samples and study duration. Bu plasma concentrations of 1,035 virtual subjects following a 1-h infusion of 0.4 mg/kg were simulated using the final population PK model. Bu CL was estimated using the simulated Bu plasma concentrations under different scenarios, as shown in Table 3. Bu CL derived using 12 PK samples from test PK and preparative regimen served as the reference value. As presented in Table 3, the deviation from the reference values for all scenarios was <0.9 %. This exercise demonstrated that no compromise in CL estimation would be expected with shortened test PK study duration (1 h infusion and 3 h sampling time post-infusion) and three PK samples.
For a clinic facility without population PK analysis capacity, a simplified PK analysis method to estimate CL, which can subsequently calculate AUC, was proposed as follows.
-
To use three PK samples post the end of infusion to estimate the terminal slope k on the log-transformed concentration–time curve: Log C obs = intercept − kt
-
To use the infusion time, T inf, to estimate C max:
$$ C_{\hbox{max} } = e^{{\left( {{\text{intercept}} - kT_{\inf } } \right)}} $$(5) -
To calculated Bu CL for each individual: \( {\text{CL}} = \frac{\text{Dose}}{{C_{\hbox{max} } T_{\inf } }}(1 - e^{{ - kT_{\inf } }} ) \)
The comparison of derived Bu CL using population PK modeling versus the simplified method proposed above is presented in Fig. 3. A good agreement of these two methods was observed, as the points were uniformly distributed along the unit line. The relative difference in CL using these two methods was 3 %.
In summary, the PK-guided dose for Bu can follow the steps below:
-
To select a low target AUC for test PK, e.g., AUCtar = 1,250/2 (µM min);
-
To calculate dose for test PK: Dose (mg) = (31.7 × BSA − 11.6) AUCtar/1,000;
-
To set infusion time as 1 h;
-
To take three PK samples starting from 15 or 30 min post the end of infusion;
-
To conduct population PK analysis to estimate individual CL; or to estimate CL using the simplified method proposed in Eq. 5;
-
Calculate AUCobs = Dose/CL. Subsequent dose adjustment modification coefficient for the condition regimen is AUCobs/AUCtar;
The suggested method can also be applied to “real-time” PK setting.
Discussion
Covariate analysis demonstrated that BSA was the appropriate covariate describing Bu CL. The linear equation for CL with BSA holds the physiological/biological grounds. BSA is widely used as the biometric unit for normalizing physiological parameters related to flow, such as cardiac output and renal clearance. For many drugs especially for anticancer therapy, the dose selection is often determined based on BSA, as CL is correlated to BSA. This conclusion agrees with what was concluded by Nguyen et al. [11]. The derived linear equation for CL should only be applied to the range covered in our study ranging from 1.27 to 3.01 m2. For example, if we apply this equation to pediatric patients (e.g., BSA is <0.37 m2), a negative CL will be derived. A value of BSA of 0.37 m2 represents a pediatric subject with 9 kg and 60 cm height. This also suggested that we should expect the difference between pediatric patients and adults. A recent publication by McCune et al. [15] has demonstrated the difference in the covariates that contributing CL for infants, pediatric patients, as well as adults. Since there still exists 14 % of random IIV in CL, instead of quantitatively identifying the sources that contribute to the variability in CL to guide individualized dosing algorithm for Bu, PK-guided individual dose adjustment appears to be a practical way. Whether prior PK information can guide the subsequent dose adjustment depends on if CL of Bu remains the same from time to time. In our analysis, the inter-occasional variability (test PK vs. preparative regimen PK) for CL was negligible (CL for test PK was 12.9 vs. 12.8 L/h for preparative regimen, and the IIV % for CL of test PK and preparative regimen was 15.3 and 13.7 %, respectively). Therefore, prior PK information (e.g., pre-preparative test PK) can accurately direct the dose for preparative regimen.
A simple PK study with short study duration is important in a clinic setting. Our analysis suggested that three post-infusion PK samples with a reduced dose and shortened infusion duration (total 4-h study duration) can provide as near as good estimates of Bu CL compared to that of using 12 PK samples with longer infusion durations at two different occasions. Although only two PK samples are theoretically necessary to identify inter- and intra-subject variability, we propose to have three PK time points to ensure the accuracy in Bu CL estimation.
Population PK analysis is often not feasible in a clinic setting. Therefore, a simplified dose modification using three PK samples was provided in the paper. In addition, this method does not require a PK sample immediately after the end of infusion. As a sample immediately after the end of infusion tends to result in more frequent errors because of drug infusion contamination, a PK sample 15 or 30 min after end of infusion was suggested.
Our population PK modeling derived the same conclusion as reported by Nguyen et al. [11] that BSA is the most appropriate covariate for CL. Our analysis further confirmed the benefit of the BSA-based dosing algorithm compared with the WBD algorithm. The observed Bu exposure following 0.8 mg/kg using AIBW for severely obese subjects, and the population PK analysis suggested that dosing adjustment based on AIBW for severely obese subjects cannot ensure the Bu exposure to be within the target range. To explore the disagreement between our analysis with given by Nguyen et al., we examined the subject demographics data. The body weight distributions in the two analyses were different. In our analysis, 59 % of the subjects had BMI > 27 kg/m2 with a maximum BMI of 54 kg/m2, whereas in Nguyen et al., 39 % of the subjects had BMI > 27 kg/m2 with a maximum BMI of 46.9 kg/m2. The discrepancy is in the higher BMI population.
In a recent publication by McCune et al. [15], a two-compartment PK model using fat content as a covariate well describes the PK data following BU IV infusion primarily for neonates to pediatric subjects, with limited data for adults). Our analysis suggested that a one-compartment model with BSA as a covariate for CL best described the adult PK of Bu from underweight to severely obese subjects.
In conclusion, the BSA-based dosing algorithm derived from population PK modeling is recommended to achieve targeted Bu exposure. Given the clinical setting of Bu administration under HSCT, CL estimates using simulated PK data also suggested that using three post-infusion PK samples with a shortened duration of infusion in a test PK design provides estimation of Bu CL with similar accuracy to using 12 PK samples. A simplified PK analysis to estimate CL can provide good estimate compared to population PK modeling.
References
Geddes M, Kangarloo SB, Naveed F, Quinlan D, Chaudhry MA, Stewart D et al (2008) High busulfan exposure is associated with worse outcomes in a daily i.v. busulfan and fludarabine allogeneic transplant regimen. Biol Blood Marrow Transplant 14:220–228
Andersson BS, de Lima MJ, Saliba RM, Shpall EJ, Popat U, Roy Jones R et al (2011) Pharmacokinetic dose guidance of IV busulfan with fludarabine with allogeneic stem cell transplantation improves progression free survival in patients with AML and MDS; Results of a randomized phase III study. Blood 118:892
Andersson BS, Thall PF, Madden T, Couriel D, Wang X, Tran HT et al (2002) Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant 8:477–485
Dean RM, Pohlman B, Sweetenham JW, Sobecks RM, Kalaycio ME, Smith SD et al (2010) Superior survival after replacing oral with intravenous busulfan in autologous stem cell transplantation for non-Hodgkin lymphoma with busulfan, cyclophosphamide and etoposide. Br J Haematol 148:226–234
Lee JH, Choi SJ, Lee JH, Kim SE, Park CJ, Chi HS et al (2005) Decreased incidence of hepatic veno-occlusive disease and fewer hemostatic derangements associated with intravenous busulfan vs oral busulfan in adults conditioned with busulfan + cyclophosphamide for allogeneic bone marrow transplantation. Ann Hematol 84:321–330
Kashyap A, Wingard J, Cagnoni P, Roy J, Tarantolo S, Hu W et al (2002) Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant 8:493–500
Andersson BS, Madden T, Tran HT, Hu WW, Blume KG, Chow DS et al (2000) Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as pretransplantation conditioning therapy: a phase I study. Biol Blood Marrow Transplant 6:548–554
Andersson BS, Kashyap A, Gian V, Wingard JR, Fernandez H, Cagnoni PJ et al (2002) Conditioning therapy with intravenous busulfan and cyclophosphamide (IV BuCy2) for hematologic malignancies prior to allogeneic stem cell transplantation: a phase II study. Biol Blood Marrow Transplant 8:145–154
Russell JA, Kangarloo SB (2008) Therapeutic drug monitoring of busulfan in transplantation. Curr Pharm Des 14:1936–1949
de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R et al (2004) Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood 104:857–864
Nguyen L, Leger F, Lennon S, Puozzo C (2006) Intravenous busulfan in adults prior to haematopoietic stem cell transplantation: a population pharmacokinetic study. Cancer Chemother Pharmacol 57:191–198
Russell JA, Kangarloo SB, Williamson T, Chaudhry MA, Savoie ML, Turner AR et al (2013) Establishing a target exposure for once-daily intravenous busulfan given with fludarabine and thymoglobulin before allogeneic transplantation. Biol Blood Marrow Transplant 19:1381–1386
Gibbs P, Gooley T, Corneau B, Murray G, Stewart P, Appelbaum FR et al (1999) The impact of obesity and disease on busulfan oral clearance in adults. Blood 93:4436–4440
Lill M, Costa LJ, Yeh RF, Lim S, Stuart R, Waller EK et al (2013) Pharmacokinetic-directed dose adjustment is essential for intravenous busulfan exposure optimization. Biol Blood Marrow Transplant 19(2 Supplement):S132
McCune JS, Bemer MJ, Barrett JS, Scott Baker K, Gamis AS, Holford NH (2014) Busulfan in infant to adult hematopoietic cell transplant recipients: a population pharmacokinetic model for initial and Bayesian dose personalization. Clin Cancer Res 20:754–763
Acknowledgments
The authors thank Seattle Cancer Care Alliance that conducted the PK Study. The authors also thank Ms. Paula Kaptur for her assistance during the manuscript preparation.
Conflict of interest
The study was sponsored by Otsuka Pharmaceutical Development and Commercialization, Inc. All authors were Otsuka full time employers when this work was completed. X. Wang. K. Kato, Y. Wang, C. Gallo conducted the analysis, interpreted the results, and prepared the manuscript. K. Kato, E. Rock, and E. Armstrong designed and supervised the clinical study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Kato, K., Le Gallo, C. et al. Dosing algorithm revisit for busulfan following IV infusion. Cancer Chemother Pharmacol 75, 505–512 (2015). https://doi.org/10.1007/s00280-014-2660-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2660-0