Abstract
Purpose
This phase II study evaluated the response rate (RR) and safety of combination therapy with carboplatin (CBDCA) and pemetrexed (PEM) in Japanese patients with non-squamous non-small cell lung cancer (non-sq NSCLC). Further, the relationship between therapy efficacy/toxicity and genetic polymorphisms associated with PEM metabolism was analyzed.
Methods
Forty-one patients received CBDCA at a dose targeting an area under the concentration–time curve of 5 mg/mL × min and PEM of 500 mg/m2 on day 1 every 3 weeks. Single-nucleotide polymorphisms of the thymidylate synthase (TYMS) coding gene, the variable number of tandem repeat (VNTR) in the TYMS, and the methylenetetrahydrofolate reductase (MTHFR) coding gene were analyzed.
Results
The overall RR was 36.6 %. Median progression-free survival and median survival time were 4.7 months [95 % confidence interval (CI) 3.9–5.6 months] and 16.2 months (95 % CI 6.1–26.2 months), respectively. Epidermal growth factor receptor gene mutations were detected in 6 patients (14.6 %). The VNTR in the TYMS significantly correlated with anemia (p = 0.047) and thrombocytopenia (p = 0.038).
Conclusions
This combination therapy was effective and tolerable in patients with advanced non-sq NSCLC. The VNTR in the TYMS appears to be a predictive factor for anemia and thrombocytopenia in patients treated with this regimen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of patients with non-small cell lung cancer (NSCLC) are diagnosed at inoperable stages, and platinum-based chemotherapy remains a key strategy for the management of patients with advanced NSCLC [1–3]. Overall survival of front-line chemotherapy using pemetrexed (PEM) in combination with cisplatin (CDDP) was superior to that with other platinum doublets, particularly for patients with advanced non-squamous (non-sq) NSCLC [4–7].
PEM-based regimens have a mild toxicity profile and can improve patients’ quality of life (QOL) [4, 6, 8, 9]. Further, carboplatin (CBDCA)-based regimens are also widely used and are associated with relatively mild toxicity [9–11]. However, the efficacy and safety of PEM combined with CBDCA have not been well established in Japanese patients with NSCLC.
Thymidylate synthetase (TS) is one of the main targets of PEM [12], and methylenetetrahydrofolate reductase (MTHFR) is an enzyme indispensable for folate metabolism. Both enzymes are strongly associated with cell proliferation and efficacy of pyrimidine-antagonist chemotherapies, such as the one with PEM [13, 14], and studies have demonstrated a correlation between clinical efficacy of various anticancer agents and the polymorphisms of these genes [15]. Patients with homozygous mutations for the MTHFR coding gene (MTHFR) C677T had a significantly increased progression-free survival (PFS) when compared to patients with wild-type or heterozygous mutations [16]. Similarly, TS is a critical target for various chemotherapies [17, 18], including those used for the treatment of NSCLC. One study reported that TS expression correlated with PEM sensitivity in NSCLC cell lines [19]. Tanaka et al. [20] conducted a large-scale study of the Japanese population showing that TS expression was lower in adenocarcinoma than in squamous cell carcinoma of the lung.
Thus, the goal of the present phase II study was to evaluate the response rate (RR) and safety of CBDCA and PEM in Japanese patients with non-sq NSCLC. Further, the relationship between therapy efficacy/toxicity and genetic polymorphisms of folate metabolism-associated enzyme coding genes, the TS coding gene (TYMS) and MTHFR, or the variable number of tandem repeat (VNTR) in the TYMS in peripheral blood cells was examined.
Patients and methods
Eligibility criteria
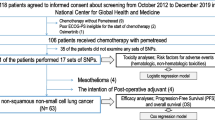
Eligibility criteria were as follows: cytologically or histologically confirmed diagnosis of non-sq NSCLC; patients without prior systemic chemotherapy, including ones with postoperative recurrence; stage IIIB or IV disease according to the 7th edition of TNM criteria; no indications for curative chemoradiotherapy; age between 20 and 74 years; Eastern Cooperative Oncology Group (ECOG) performance status 0–1; measurable disease (Response Evaluation Criteria in Solid Tumors, ver. 1.0); and normal organ function (as defined by absolute white blood cell count ≥4.0 × 109/L or neutrophil count ≥2.0 × 109/L; hemoglobin ≥9.0 g/dL; platelets ≥100 × 109/L; alanine aminotransferase [ALT] and aspartate aminotransferase [AST] ≤100 IU/L [ALT and AST ≤150 IU/L was acceptable if liver metastasis was present]; serum creatinine ≤1.2 mg/dL; calculated creatinine clearance using Cockcroft-Gault formula ≥60 ml/min; arterial partial pressure of oxygen [PaO2] ≥60 Torr or arterial hemoglobin oxygen saturation by pulse oximetry [SpO2] ≥90 % at ambient air); and projected life expectancy ≥12 weeks. The main exclusion criteria were as follows: active infection; temperature ≥38 °C; severe complications, such as heart failure, renal failure, liver dysfunction, uncontrolled diabetes mellitus, and hypertension; a concomitant malignancy within the last 5 years; central nervous system metastases with symptoms; uncontrolled pleural effusion or ascites; interstitial pneumonia or pulmonary fibrosis on chest X-ray; history of severe hypersensitivity to drug components; required concurrent treatment with systemic steroid; and pregnancy. The protocol was approved by each institutional review board. The study was performed in accordance with the ethics principles of the Declaration of Helsinki. Written informed consent was obtained from all patients. This study was registered with the University Hospital Medical Information Network (UMIN) [UMIN000002846].
Treatment
PEM at a dose of 500 mg/m2 on day 1 and CBDCA at a dose calculated to produce an area under the concentration–time curve (AUC) of 5.0 mg/mL × min on day 1 were administered intravenously every 3 weeks. The treatment was discontinued in the case of any of the following: disease progression; unacceptable toxicity; patient refusal; death during treatment; and investigator’s decision. All patients received oral folic acid (500 μg) daily and a vitamin B12 injection (1,000 μg) every 9 weeks, beginning one or more weeks before the first dose and continuing until three weeks after the last dose of study treatment. Any treatment was permitted after protocol discontinuation. Dose adjustment and cycle delay of 21 days or less were permitted to allow for resolution of toxic effects.
Assessment of toxicity and response
Toxicities or adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. Tumor responses were assessed using chest X-ray, computed tomography, or magnetic resonance imaging (when clinically indicated), before and during treatment. Assessments were repeated at least every month unless progression was detected. Responses were recorded as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) in accordance with Response Evaluation Criteria in Solid Tumors (RECIST) guidelines version 1.0. Disease control rate (DCR) was defined as the sum of the objective response (CR or PR) rate and the rate of SD. Clinical response data were all confirmed by central review.
Endpoints and statistical analysis
The primary endpoint was overall RR. Secondary endpoints were safety, PFS, and overall survival (OS). Duration of tumor response was defined as the time between the date of the first objective assessment of CR or PR and the date when PD or death was recorded from any cause. PFS was defined as the time between the date of registration and the date of PD or death from any cause. OS was defined as the time between the date of registration and the date of death from any cause. The Kaplan–Meier method was used to estimate PFS and OS.
On the assumption that threshold RR and expected RR would, respectively, be 20 and 40 %, a sample size of 36 patients was required by the Simon’s and Fleming’s designs with a one-sided α error of 0.05 and a β error of 0.20. A total of 40 patients were planned to enroll considering later exclusion of patients. All analyses were based on the intent-to-treat population.
Genetic analyses
Analyses of genetic variants were performed with the investigator blinded to patient characteristics and clinical outcome. Five milliliters of peripheral blood was taken from each patient who had enrolled in this study and who had consented to the genetic analysis. DNA was extracted from each blood sample for analysis of the MTHFR single-nucleotide polymorphisms (SNPs), C677T and A1298C. Then, each extracted DNA was used to determine the TS genotypes of the VNTR in the five prime untranslated region (5′-UTR) of TYMS, two tandem repeat (2R)/3R/4R, and the 3R-SNP, G/C, in TYMS by polymerase chain reaction (PCR) and PCR-restriction fragment length polymorphism (PCR–RFLP), using the forward primer, 5′-AAAAGGCGCGCGGAAGGGGTCCT-3′, and reverse primer, 5′-TCCGAGCCGGCCACAGGCAT-3′. DNA amplification was performed three times per sample, using the GeneAmp® PCR System 9700 (Applied Biosystems, Life Technologies Japan, Inc., Tokyo, Japan). PCR with the genomic DNA template was performed in reaction mixtures containing 1 × PCR buffer II without MgCl2, MgCl2 solution, 200 μM of deoxyribonucleoside triphosphates, 500 nM of each primer, 0.5 units of AmpliTaq® DNA Polymerase, and 100 ng of genomic DNA (all of these reagents were obtained from Applied Biosystems). The cycling conditions were one cycle of 94 °C for 5 min, 35 cycles of 94 °C for 40 s, 62 °C for 60 s, 72 °C for 40 s, with a final extension at 72 °C for 5 min. Aliquots of amplified fragments were separated on 4 % agarose gels, and the TS VNTR genotype was determined, with 2R 116 bp, 3R 144 bp, and 4R 172 bp. Samples showing the 3R genotype were analyzed further for G/C polymorphism by RFLP. PCR products were digested with HaeIII (TaKaRa Bio, Inc., Shiga, Japan) followed by electrophoresis in 4 % agarose gel and ethidium bromide stain. The 3R fragments of 66, 37, 28, and 10 bp were classified into the 3G allele, and the 3R fragments of 94, 37, and 10 bp were classified into the 3C allele, as previously reported [21]. Analysis was performed at least three times to confirm the genotype. TYMS genotype was categorized into a high-expression genotype (2R/3G, 3C/3G, 3G/3G, 3G/4R) and a low-expression genotype (2R/2R, 2R/3C, 3C/3C), depending on the 5′-UTR VNTR polymorphism and the C/G polymorphism within the third VNTR.
The association between polymorphisms and RR or chemotherapy-related toxicity was analyzed by the χ2 test or Fisher’s exact test. The correlation between polymorphisms and PFS/OS was analyzed by the log-rank test. For each test, patients were compared among each genotype, such as wild type, heterozygous, and homozygous. Statistical significance was established at p < 0.05.
Results
Patients characteristics
From November 2009 to November 2010, 41 patients were enrolled (Table 1). Twenty-four patients (58.5 %) died during the follow-up, mostly as a result of disease progression (23 of 24 patients). The median age of the enrolled patients was 63 years (range of 43–73 years), and 28 patients (68.3 %) were male. Of the 41 patients, 27 (65.9 %) had an ECOG performance status of 1, 36 (87.8 %) had stage IV disease, and 39 (95.1 %) were diagnosed with adenocarcinoma.
Epidermal growth factor receptor (EGFR) gene mutations were investigated in 40 of 41 patients. The mutation was not searched for one patient because diagnostic yield from the tissue sample for detection was not enough. The positive mutations were detected in six patients, four of whom had an exon 19 deletion mutation, while the other two had an L858R mutation in exon 21.
Treatment administered
The median number of treatment cycles delivered was four (range of 1–6 cycles), with 33 patients (80.5 %) completing more than three cycles.
The dose of agents was reduced in three patients (7.3 %) because of adverse events, including grade 3 general fatigue, grade 4 hematologic toxicity, and grade 4 anaphylaxis. Protocol treatment was terminated in eight patients (19.5 %) before completion of three cycles.
At the time of final analysis, 23 patients (56.2 %) received second- or third-line treatment, following the initial therapy. Sixteen patients (39.0 %) received cytotoxic chemotherapies as a second-line treatment and 3 of them received EGFR tyrosine kinase inhibitors (EGFR-TKIs) as a third-line treatment. Seven patients (17.1 %) received EGFR-TKIs as a second-line treatment and 2 of them received cytotoxic chemotherapies as a third-line treatment. Among the 10 patients (24.4 %) who were treated with an EGFR-TKI in the second- or third-line, five had an EGFR gene mutation. One patient with EGFR gene mutation did not receive an EGFR-TKI.
Response to treatment
All 41 patients were assessable for tumor responses. Fifteen patients exhibited PR, 20 patients exhibited SD, and disease progressed in six cases, resulting in a RR of 36.6 % [95 % confidence interval (CI) 22.1–53.1 %] and a DCR of 85.4 % (95 % CI, 70.8–94.4 %) (Table 2). The lower limit of the 95 % CI of the RR exceeded the threshold RR of 20 %; thus, the primary endpoint was achieved.
The final survival assessment was conducted in July 2014, 4 years and 8 months after the last patient’s enrollment. With a median follow-up time of 16.2 months (range of 2.4–54.1 months), median PFS and median survival time (MST) were 4.7 months (95 % CI 3.9–5.6 months) and 16.2 months (95 % CI 6.1–26.2 months), respectively (Fig. 1). The one-year survival rate was 53.6 % (95 % CI 37.4–69.3 %).
Retrospective subanalysis of PFS and OS of patients with or without an EGFR gene mutation showed that EGFR gene mutation status did not influence the PFS (a median of 5.9 months for the mutation-positive group, as compared to 4.6 months for the mutation-negative group; p = 0.738), but patients with an EGFR gene mutation were associated with a trend of longer OS than those without an EGFR gene mutation (a median of 16.2 months for the mutation-positive group, as compared to 11.7 months for the mutation-negative group; p = 0.06).
Adverse events
All 41 eligible patients were also evaluable for toxicity analysis. The rates of grade 3 and 4 hematologic and non-hematologic adverse events during the treatment were as follows: eight patients (19.5 %) had grade ≥3 leukopenia; 12 patients (29.3 %) had grade ≥3 neutropenia; 14 patients (34.1 %) had grade ≥3 anemia; seven patients (17.1 %) had grade ≥3 thrombocytopenia. Only one patient (2.4 %) experienced grade 3 febrile neutropenia. Granulocyte colony stimulating factors (G-CSF) were given to three patients (7.3 %). The most common non-hematologic adverse event was anorexia (29/41 patients, 70.7 %); other non-hematologic adverse events were rare (Table 3). No interstitial lung disease was reported. No treatment-related death was observed.
Genetic polymorphisms and clinical indices
Blood samples were collected from 37 patients (90.2 %). Gene polymorphisms of C677T and A1298C in the MTHFR were in Hardy–Weinberg equilibrium according to Pearson’s Chi-square test (C677T: χ 2 = 0.182 < χ 2 [0.05] = 3.84; A1298C: χ 2 = 0.946 < χ 2 [0.05]) (Table 4). Patients with 3R/3R and 3R/4R of the tandem repeat in 5′-UTR of TYMS had experienced significantly more grade 3/4 anemia (p = 0.047) or grade 3/4 thrombocytopenia (p = 0.038) than those with 2R/3R. Other variants explored in this study did not significantly correlate with hematologic toxicities (Table 5). Non-hematologic toxicities were mild, and the relationship between those events and gene variants was not evaluated. Gene variants did not significantly correlate with RR, DCR (Table 5), PFS, or OS (data not shown).
Discussion
Although CDDP and CBDCA have substantially different toxicity profiles, a meta-analysis comparing CDDP- and CBDCA-based chemotherapy failed to establish which regimen is associated with superior survival [5, 22–24]. CBDCA was used in this study, as non-hematologic toxicity and strong subjective symptoms (e.g., nausea, vomiting, and general fatigue) predominate in patients treated with CDDP, whereas hematologic toxicity with relatively less symptoms is observed more commonly in patients treated with CBDCA [20, 23, 24]. In a phase III study conducted in Norway, an AUC of 5.0 mg/mL × min of CBDCA was selected for patients in the CBDCA plus PEM arm [9] and resulted in good clinical efficacy with a tolerable toxicity profile. We also adopted the same dose of CBDCA in this study, and the treatment efficacy of our CBDCA plus PEM regimen was favorable. Further, the toxicity profile was mild and tolerable. The PFS in this study seemed to be shorter than that in the other studies for Japanese patients [25, 26]. A continuation maintenance therapy with PEM was not adopted in our protocol due to the fact that the maintenance strategy [27] had not been established when our trial was launched. Compared to other Japanese phase II trials (from 5–6 cycles) [25, 26], there were fewer chemotherapy cycles in the current study, while Norwegian study reported even fewer (a median of 3.3 cycles) [9]. In our study, PD was the major cause (13 patients, 52 %) of undergoing 4 or less cycles of the chemotherapy. The dose of CBDCA may be associated with the fewer cycle of chemotherapy, providing another reason for the shorter PFS.
PEM targets folate-dependent reactions and acts on TS, a key enzyme for DNA synthesis [28, 29]. Alteration of TS activity due to polymorphisms in the cognate coding gene influences outcomes in patients with NSCLC [13, 30, 31]. MTHFR is an essential enzyme for one-carbon metabolism needed for DNA synthesis, repair, and methylation [32]. The alteration of MTHFR activity plays a role in carcinogenesis [33, 34], which supports the notion that MTHFR polymorphisms may affect patient outcomes [13, 15, 32]. Only an increasing repeat number of VNTR in 5′-UTR of TYMS correlated with anemia and thrombocytopenia, suggesting that this genetic marker might be useful for the prediction of hematologic toxicity in response to PEM. In order to avoid the severe hematologic toxicities in patients with the 3R/3R variant, we should consider switching the regimen, reducing the initial dose of PEM, or upwardly adjusting the minimum number of red blood cells or platelets required for starting the treatment.
A significant correlation between the MTHFR-C677T allele and improved clinical outcome has been found in another study [16]. However, we were not able to show the same result. One of the reasons appears to be race/ethnicity in those variants. In breast cancer, race/ethnicity has been reported to modify the association between the two SNPs of MTHFR and breast cancer survival [35]. It is known that PEM targets another enzyme associated with folate metabolism other than TS, such as dihydrofolate reductase (DHFR), which inhibits a cytotoxic effect of antifolates, thereby reducing treatment efficacy [36]. Indeed, a previous study showed the association between PFS and either of TS or of DHFR [37]. Collectively, a comprehensive analysis of polymorphisms of all enzymes associated with folate metabolism is required.
In terms of toxicity profile, the two Japanese studies of CBDCA (AUC = 6.0 mg/mL × min) plus PEM (500 mg/m2) followed by maintenance PEM (500 mg/m2) conducted by the Kyoto Thoracic Oncology Research Group (KTOGT0902) [25] and by Okamoto et al. [26], demonstrated that grade 3/4 neutropenia, anemia, and thrombocytopenia were seen in 33, 31, and 18 % and 56, 29.4, and 41.3 %, respectively. In the former study, red blood cell and platelet transfusions were required for 6.1 and 4.1 %, respectively. In our study, grade 3/4 neutropenia, anemia, and thrombocytopenia were seen, respectively, in 29.3, 34.1, and 17.1 %, whereas packed red blood cell transfusions were given to three patients (7.3 %) and platelet concentrate given to one patient (2.4 %). Non-hematologic toxicities were also mild, and our study achieved a good treatment completion rate over three courses. AUC of 5 or 6 mg/mL × min of CBDCA should be adjusted for individual patients in terms of the balance between efficacy and toxicity. In clinical practice, we assume that CBDCA plus PEM regimen can be selected for patients with non-squamous and EGFR wild-type NSCLC as a first-line therapy, particularly for those unfit for CDDP or with an ECOG performance status (PS) of two as demonstrated in a previous study [38] or elderly patients with good PS who have also a benefit of CBDCA-based platinum doublet [39, 40].
Recent phase III trials have shown favorable efficacies of EGFR-TKI in NSCLC patients with active EGFR gene mutation [41, 42]. There was no significant difference of either PFS or OS according to EGFR gene mutation status in our study, but OS of the patients with EGFR gene mutation tended to be longer than those without the mutation, presumably due to the post-treatment therapy using EGFR-TKIs. The smaller size of patients with EGFR mutation (6/40, 15 %) in our study was due to the fact that several institutes were also participating in another ongoing trial, which was recruiting NSCLC patients with EGFR gene mutation. However, our data implicate the importance of the use of EGFR-TKIs for treating patients with an active EGFR gene mutation. CBDCA plus PEM regimen seems also appropriate for those with EGFR mutant-positive NSCLC, who failed the initial EGFR-TKI therapy.
Although the development of effective treatment strategies against advanced NSCLC has progressed swiftly over the last decade, minimization of toxicities remains important for QOL purposes. The toxicity profile of chemotherapy can vary according to race, ethnicity, and genetic makeup. Gene analyses in this study are the first to demonstrate a possibility of correlation between genetic polymorphisms and hematologic toxicity in non-squamous NSCLC patients treated with PEM. Further studies to confirm this evidence are warranted.
References
Jemal A, Bray F, Center MM, Farley J, Ward E, Frorman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
NSCLC Meta-Analyses Collaborative Group (2008) Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol 26:4617–4625
Hotta K, Fujiwara Y, Matsuo K, Suzuki T, Kiura K, Tabata M, Takigawa N, Ueoka H, Tanimoto M (2007) Recent improvement in the survival of patients with advanced non-small-cell lung cancer enrolled in phase III trials of first-line systemic chemotherapy. Cancer 109:939–948
Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S, Rolski J, Goksel T, de Marinis F, Simms L, Sugarman KP, Gandara D (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naïve patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26:3543–3551
Manegold C, Gatzemeier U, von Pawel J, Pirker R, Malayeri R, Blatter J, Krejcy K (2000) Front-line treatment of advanced non-small-cell lung cancer with MTA (LY231514, pemetrexed disodium, ALIMTA) and cisplatin: A multicenter phase II trial. Ann Oncol 11:435–440
Treat J, Scagliotti GV, Peng G, Monberg MJ, Obasaju CK, Socinski MA (2012) Comparison of pemetrexed plus cisplatin with other first-line doublets in advanced non-small cell lung cancer (NSCLC): a combined analysis of three phase 3 trials. Lung Cancer 76:222–227
Al-Saleh K, Quinton C, Ellis PM (2012) Role of pemetrexed in advanced non-small-cell lung cancer: meta-analysis of randomized controlled trials, with histology subgroup analysis. Curr Oncol 19:e9–e15
Shepherd FA, Dancey J, Arnold A, Neville A, Rusthoven J, Johnson RD, Fisher B, Eisenhauer E (2001) Phase II study of pemetrexed disodium, a multitargeted antifolate, and cisplatin as first-line therapy in patients with advanced nonsmall cell lung carcinoma: A study of the National Cancer Institute of Canada Clinical Trials Group. Cancer 92:595–600
Grønberg BH, Bremnes RM, Fløtten O, Amundsen T, Brunsvig PF, Hjelde HH, Kaasa S, von Plessen C, Stornes F, Tollåli T, Wammer F, Aasebø U, Sundstrøm S (2009) Phase III study by the Norwegian Lung Cancer Study Group: Pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non–small-cell lung cancer. J Clin Oncol 27:3217–3224
Zinner RG, Fossella FV, Gladish GW, Glisson BS, Blumenschein GR Jr, Papadimitrakopoulou VA, Pisters KM, Kim ES, Oh YW, Peeples BO, Ye Z, Curiel RE, Obasaju CK, Hong WK, Herbst RS (2005) Phase II study of pemetrexed in combination with carboplatin in the first-line treatment of advanced non-small cell lung cancer. Cancer 104:2449–2456
Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M (2004) Meta-analysis of randomized clinical trials comparing cisplatin to carboplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol 22:3852–3859
Muhsin M, Gricks C, Kirkpatrick P (2004) Pemetrexed disodium. Nat Rev Drug Discov 3:825–826
Takehara A, Kawakami K, Ohta N, Oyama K, Ota Y, Oda M, Watanabe G (2005) Prognostic significance of the polymorphisms in thymidylate synthase and methylenetetrahydrofolate reductase gene in lung cancer. Anticancer Res 25:4455–4462
Maring JG, Groen HJ, Wachters FM, Uges DR, de Vries EG (2005) Genetic factors influencing pyrimidine-antagonist chemotherapy. Pharmacogenomics J 5:226–243
Ishihama H, Chida M, Araki O, Karube Y, Seki N, Tamura M, Umezu H, Honma K, Masawa N, Miyoshi S (2008) Comparison of 5-fluorouracil-related gene expression levels between adenocarcinomas and squamous cell carcinomas of the lung. Jpn J Clin Oncol 39:33–36
Smit EF, Burgers SA, Biesma B, Smit HJ, Eppinga P, Dingemans AM, Joerger M, Schellens JH, Vincent A, van Zandwijk N, Groen HJ (2009) Randomized phase II and pharmacogenetic study of pemetrexed compared with pemetrexed plus carboplatin in pretreated patients with advanced non-small-cell lung cancer. J Clin Oncol 27:2038–2045
Rose MG, Farrell MP, Schmitz JC (2002) Thymidylate synthase: a critical target for cancer chemotherapy. Clin Colorectal Cancer 1:220–229
Okamoto I, Hirabayashi N, Kitano M, Nakagawa K (2011) Thymidylate synthase and dihydropyrimidine dehydrogenase expression levels are associated with response to S-1 plus carboplatin in advanced non-small cell lung cancer. Lung Cancer 73:103–109
Ozasa H, Oguri T, Uemura T, Miyazaki M, Maeno K, Sato S, Ueda R (2010) Significance of thymidylate synthase for resistance to pemetrexed in lung cancer. Cancer Sci 101:161–166
Tanaka F, Wada H, Fukui Y, Fukushima M (2011) Thymidylate synthase (TS) gene expression in primary lung cancer patients: a large-scale study in Japanese population. Ann Oncol 22:1791–1797
Kawakami K, Watanabe G (2003) Identification and functional analysis of single nucleotide polymorphism in the tandem repeat sequence of thymidylate synthase gene. Cancer Res 63:6004–6007
Go RS, Adjei AA (1999) Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol 17:409–422
Jiang J, Liang X, Zhou X, Huang R, Chu Z (2007) A meta-analysis of randomized controlled trials comparing carboplatin-based to cisplatin-based chemotherapy in advanced non-small cell lung cancer. Lung Cancer 57:348–358
Ardizzoni A, Boni L, Tiseo M, Fossella FV, Schiller JH, Paesmans M, Radosavljevic D, Paccagnella A, Zatloukal P, Mazzanti P, Bisset D, Rosell R, CISCA (CISplatin versus CArboplatin) Meta-analysis Group (2007) Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst 99:847–857
Kim YH, Hirabayashi M, Togashi Y, Hirano K, Tomii K, Masago K, Kaneda T, Yoshimatsu H, Otsuka K, Mio T, Tomioka H, Suzuki Y, Mishima M (2012) Phase II study of carboplatin and pemetrexed in advanced non-squamous, non-small-cell lung cancer: Kyoto Thoracic Oncology Research Group Trial 0902. Cancer Chemother Pharmacol 70:271–276
Okamoto I, Aoe K, Kato T, Hosomi Y, Yokoyama A, Imamura F, Kiura K, Hirashima T, Nishio M, Nogami N, Okamoto H, Saka H, Yamamoto N, Yoshizuka N, Sekiguchi R, Kiyosawa K, Nakagawa K, Tamura T (2013) Pemetrexed and carboplatin followed by pemetrexed maintenance therapy in chemo-naïve patients with advanced nonsquamous non-small-cell lung cancer. Invest New Drugs 31:1275–1282
Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, Corral J, Melemed S, John W, Chouaki N, Zimmermann AH, Visseren-Grul C, Gridelli C (2012) Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomized controlled trial. Lancet Oncol 13:247–255
Takimoto CH (1996) New antifolates: pharmacology and clinical applications. Oncologist 1:68–81
Hanauske AR, Chen V, Paoletti P, Niyikiza C (2001) Pemetrexed disodium: a novel antifolate clinically active against multiple solid tumors. Oncologist 6:363–373
Hashimoto H, Ozeki Y, Sato M, Obara K, Matsutani N, Nakagishi Y, Ogata T, Maehara T (2006) Significance of thymidylate synthase gene expression level in patients with adenocarcinoma of the lung. Cancer 106:1595–1601
Kaira K, Ohde Y, Nakagawa K, Okumura T, Murakami H, Takahashi T, Kondo H, Nakajima T, Endo M, Yamamoto N (2012) Thymidylate synthase expression is closely associated with outcome in patients with pulmonary adenocarcinoma. Med Oncol 29:1663–1672
Lee MS, Asomaning K, Su L, Wain JC, Mark EJ, Christiani DC (2012) MTHFR polymorphisms, folate intake and carcinogen DNA adducts in the lung. Int J Cancer 131:1203–1209
Suzuki T, Matsuo K, Hiraki A, Saito T, Sato S, Yatabe Y, Mitsudomi T, Hida T, Ueda R, Tajima K (2007) Impact of one-carbon metabolism-related gene polymorphisms on risk of lung cancer in Japan: a case control study. Carcinogenesis 28:1718–1725
Liu H, Jin G, Wang H, Wu W, Liu Y, Qian J, Fan W, Ma H, Miao R, Hu Z, Sun W, Wang Y, Jin L, Wei Q, Shen H, Huang W, Lu D (2008) Association of polymorphisms in one-carbon metabolizing genes and lung cancer risk: a case-control study in Chinese population. Lung Cancer 61:21–29
Martin DN, Boersma BJ, Howe TM, Goodman JE, Mechanic LE, Chanock SJ, Ambs S (2006) Association of MTHFR gene polymorphism with breast cancer survival. BMC Cancer 6:257
Askari BS, Krajinovic M (2010) Dihydrofolate reductase gene variations in susceptibility to disease and treatment outcomes. Curr Genomics 11:578–583
Chen CY, Chang YL, Shih JY, Lin JW, Chen KY, Yang CH, Yu CJ, Yang PC (2011) Thymidylate synthase and dehydrofolate reductase expression in non-small cell lung cancer: the association with treatment efficacy of pemetrexed. Lung Cancer 74:132–138
Zukin M, Barrios CH, Pereira JR, Ribeiro RDA, Beato CA, do Nascimento YN, Murad A, Franke FA, Precivale M, Araujo LH, Baldotto CS, Vieira FM, Small IA, Ferreira CG, Lilenbaum RC (2013) Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol 31:2849–2853
Quoix E, Zalcman G, Oster JP, Westeel V, Pichon E, Lavolé A, Dauba J, Debieuvre D, Souquet PJ, Bigay-Game L, Dansin E, Poudenx M, Molinier O, Vaylet F, Moro-Sibilot D, Herman D, Bennouna J, Tredaniel J, Ducoloné A, Lebitasy MP, Baudrin L, Laporte S, Milleron B, Intergroupe Francophone de Cancérologie Thoracique (2011) Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet Oncol 378:1079–1088
Gervais R, Robinet G, Clément-Duchêne C, Denis F, El Kouri C, Martin P, Chouaki N, Bourayou N, Morère JF (2013) Pemetrexed and carboplatin, an active option in first-line treatment of elderly patients with advanced non-small cell lung cancer (NSCLC): a phase II trial. Lung Cancer 80:185–190
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T, North-East Japan Study Group (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. Engl J Med 362:2380–2388
Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M, West Japan Oncology Group (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 11:121–128
Acknowledgments
We thank all the patients who participated in this study and also thank all institutions for recruiting patients.
Conflict of interest
Satoshi Oizumi received an honorarium from AstraZeneca and a grant from Eli Lilly. Others had no conflict of interest to declare.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Kanazawa, K., Yokouchi, H., Wang, X. et al. Phase II trial of carboplatin and pemetrexed as first-line chemotherapy for non-squamous non-small cell lung cancer, and correlation between the efficacy/toxicity and genetic polymorphisms associated with pemetrexed metabolism: Hokkaido Lung Cancer Clinical Study Group Trial (HOT) 0902. Cancer Chemother Pharmacol 74, 1149–1157 (2014). https://doi.org/10.1007/s00280-014-2589-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2589-3