Abstract
Although the current standard of care for diffuse large B-cell lymphoma (DLBCL) is six cycles of rituximab/cyclophosphamide/doxorubicin/vincristine/prednisolone combination chemotherapy (R-CHOP), a larger than expected number of patients cannot complete planned six cycles for various reasons in the real world. We aimed to evaluate the prognosis of patients with DLBCL after incomplete treatment by analyzing the chemotherapy response and survival according to the cause of discontinuation and the number of cycles. We analyzed a retrospective cohort of patients diagnosed with DLBCL who underwent incomplete cycles of R-CHOP at Seoul National University Hospital and Boramae Medical Center from January 2010 to April 2019. A total of 1183 patients were diagnosed with DLBCL, of which 260 (22%) did not complete six cycles of R-CHOP. The most common cause of discontinuation of chemotherapy was life-threatening infection, and the most common pathogen was Pneumocystis jirovecii. Overall survival (OS) and progression-free survival (PFS) were significantly better in patients who achieved complete response (CR) or partial response (PR) at the first response evaluation. Patients underwent three or more cycles of chemotherapy had a longer OS than those who did not. In patients with limited-stage disease, consolidative radiotherapy showed a significant improvement in OS and PFS. Advanced stage, high comorbidity score, and poor primary response to chemotherapy were poor prognostic factors in patients with unplanned treatment shortening. This study provides real-world outcomes for patients who could not complete the planned six cycles of R-CHOP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma (NHL), accounting for approximately 30% of NHL cases [1-3]. Although DLBCL has an aggressive disease course and poor prognosis, its clinical outcome and prognosis have improved since the era of rituximab therapy [1, 4]. Over the past 10 years, six cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone combination chemotherapy (R-CHOP), administered every 3 weeks, have been the current standard-of-care for previously untreated patients with DLBCL [5, 6].
As life expectancy increases, the number of elderly patients with DLBCL and the median age at diagnosis of DLBCL also increases. One-third of patients with DLBCL are older than 75 years of age [3]. Even in elderly patients with DLBCL, it is recommended to fully complete six cycles of R-CHOP to obtain favorable outcomes despite high treatment-related mortality and intolerance to R-CHOP [7-9]. However, as the patients’ comorbidity increased, a group of patients could not maintain R-CHOP with high intensity. In order to improve clinical outcomes and complete six cycles of chemotherapy in this group of patients, several attempts have been made, including the prophylactic administration of granulocyte colony-stimulating factor (G-CSF), change to modified regimen such as “R-mini-CHOP” in which the dose of cytotoxic agents is reduced, and application of the other novel agents [8-11]. Unfortunately, such standardized or reliable methods were not satisfactory for the patients who could not complete all six cycles of R-CHOP. Although considerable numbers of patients belong to this group in the real world, research on their clinical outcomes is scarce.
To address this knowledge gap, we analyzed the clinical characteristics and outcomes of patients who could not complete the planned six cycles of R-CHOP. In particular, we focused on the group of patients who had a durable survival, despite a shorter treatment cycle than planned. In addition, prognostic factors and leading causes of chemotherapy discontinuation were analyzed.
Materials and methods
Study population
Patients with histopathological confirmed DLBCL from January 2010 to April 2019 at Seoul National University Hospital (SNUH) and Seoul National University Boramae Medical Center (SNU-BMC) were identified, and their medical records were retrospectively reviewed. Patients who did not complete all six cycles of R-CHOP or R-mini-CHOP were included in this study. Patients who completed six cycles of R-CHOP or R-mini-CHOP were excluded from the main analysis and used as a control group for analyzing the treatment efficacy and relative risk of clinical outcomes. Patients who died within 21 days after the start of the first cycle were excluded to focus on those with a durable clinical outcome without the completion of six cycles of R-CHOP. Patients confirmed with disease progression during R-CHOP, classified as having primary resistance to R-CHOP, were also excluded from this analysis. All data, including patients’ demographics, laboratory, radiological, and histopathological findings, were obtained from electronic medical records. The Institutional Review Board of SNU-BMC approved this study, considering this study to be a retrospective, non-invasive study in patients (IRB No. 10–2020-290).
Treatment and assessment
All patients received at least one cycle of R-CHOP or R-mini-CHOP, which was well known as R-CHOP-21. R-CHOP regimen consisted of an intravenous dose of rituximab 375 mg/m2 on day 1, cyclophosphamide 750 mg/m2 on day 1, doxorubicin 50 mg/m2 on day 1, vincristine 1.4 mg/m2 on day 1, and oral prednisolone 100 mg daily on days 1–5. R-mini-CHOP was a modified version of R-CHOP, with an intravenous dose of rituximab 375 mg/m2 on day 1, cyclophosphamide 400 mg/m2 on day 1, doxorubicin 25 mg/m2 on day 1, vincristine 1 mg fixed dose on day 1, and prednisolone 40 mg/m2 on days 1–5. The first tumor response was assessed after two or three cycles using the Lugano response criteria evaluated by 18F-fluorodeoxyglucose-positron emission tomography/computed tomography scan (PET/CT). Only those with follow-up CT among patients who did not undergo follow-up PET/CT were evaluated using the CT-based Lugano criteria.
Statistical analysis
Overall survival (OS) was defined as the time from the start of R-CHOP to any cause of death. Progression-free survival (PFS) was defined as the time from the initiation of R-CHOP chemotherapy to disease progression or death. Statistical analysis was performed using the SPSS software for Windows, ver. 25.0 (SPSS Inc., Chicago, IL, USA) and R version 4.1.0. Data were described as mean ± standard deviation, median (range), or number (proportion) as appropriate. Survival analysis was estimated using the Kaplan–Meier method. Differences in survival between subgroups were compared using the log-rank test. P value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
In total, 1183 patients were diagnosed with DLBCL at SNUH and SNU-BMC during the study period, of which 923 patients (78.0%) completed six cycles of R-CHOP or R-mini-CHOP chemotherapy. Two hundred sixty patients (22.0%) could not complete the planned six cycles of chemotherapy. Based on the exclusion criteria, 165 patients were finally included in this analysis (Fig. 1). The baseline characteristics are summarized in Table 1. The median age was 70.8 years. 56.9% of the patients were male, and 52.1% of the patients were in Ann Arbor stage I or II. Most patients had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1 at diagnosis, and more than half of patients were low or low-intermediate risk group as per the international prognostic index (IPI). About 78.2% of patients had a Charlson comorbidity index (CCI) ≥ 2. Compared with patients who completed six cycles of R-CHOP, patients with an inevitable discontinuation of chemotherapy tended to be older, have higher IPI scores, and have higher CCI (Supplementary table 1).
Most patients received R-CHOP, and 23.0% of patients received R-mini-CHOP; the chemotherapy regimen was decided by the treating physician according to the patients’ age and performance status. Among 136 patients who were evaluated for disease status after chemotherapy, 83.8% were evaluated using PET/CT. Only 22 patients were evaluated using CT, and most of which were patients using CT for the unplanned assessment.
Efficacy of incomplete R-CHOP or R-mini-CHOP chemotherapy
The median follow-up duration was 24.5 months. The 5-year OS in this population was 48.6% (95% confidence interval (CI), 41.4–57.1), which was lower than 5-year OS of 81.4% (95% CI, 78.9–83.9) in patients completed six cycles of chemotherapy (Supplementary figure 1 (A)(B)). Even when propensity score matching was performed by controlling for demographic variables, including age, sex, ECOG PS, stage, and comorbidities (Supplementary table 2), the clinical outcome of patients who could not complete 6 cycles of R-CHOP was lower than that of patients who have completed chemotherapy (Supplementary figure 1 (C)(D)). Interestingly, this tendency was maintained even when only patients with limited-stage DLBCL were analyzed separately (Supplementary figure 2 (A)(B)). However, patients who met FLYER inclusion criteria for 16.4% of the total (194 patients) showed good clinical outcomes regardless of the number of the chemotherapy cycle (Supplementary figure 2 (C)(D)).
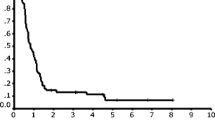
The response distribution according to the number of chemotherapy cycles is shown in Table 2. The median number of chemotherapy cycles was three, and the overall response rate was 80.6%. Comparing survival according to the best response, patients who achieved complete response (CR) or partial response (PR) after R-CHOP had significantly better OS (5-year OS rate, 56.9% vs. 14.1%, P < 0.001) and PFS (P < 0.001) than those who did not (Fig. 2). Even when the groups were analyzed according to the response evaluation tools, patients who achieved CR had significantly better OS in both PET/CT and CT groups (Supplementary figures 3 and 4). Patients who had undergone three or more cycles of chemotherapy showed superior survival compared to those who had not (5-year OS rate, 58.5% vs. 24.2%, P < 0.001) (Fig. 3). In addition, patients who received R-CHOP showed better OS and PFS than those who received R-mini-CHOP (Supplementary figure 5).
Cause of interruption of chemotherapy
The factors that caused the patients to discontinue chemotherapy are summarized in Table 3. In the excluded patients who died within 21 days after the beginning of the first chemotherapy cycle, the cause of death was similar to the cause of interrupting chemotherapy in the study patients, such as life-threatening infections. However, half of those excluded patients died of multi-organ failure due to high tumor burden (Supplementary table 3). In 78.2% of the analyzed cases, the treating physician decided to stop the planned chemotherapy. The cause of discontinuation by the treating physician was a life-threatening infection in 51 patients and failure to maintain the ECOG PS after chemotherapy in 48 patients. Twenty-one patients underwent the local treatment after early termination of chemotherapy without other reasons for discontinuation of chemotherapy, such as life-threatening infections. The most common life-threatening infection was Pneumocystis jirovecii pneumonia (PCP), followed by imipenem-resistant Acinetobacter baumannii, methicillin-sensitive Staphylococcus aureus, Klebsiella, Pneumococcus, and Actinomycosis. In addition, R-CHOP was terminated prematurely due to systemic bacteremia (three cases of Escherichia coli bacteremia, two cases of vancomycin-resistant Enterococcus bacteremia, and methicillin-resistant Staphylococcus aureus (MRSA) bacteremia) and intra-abdominal infection. The median age of patients who experienced life-threatening infections was 76.5 years, which was higher than that of the study population. In the first cycle of R-CHOP or R-mini-CHOP, more than half of the patients (59.3%) received prophylactic G-CSF. However, except for nine patients who received prophylactic sulfamethoxazole/trimethoprim (TMP-SMX) from the first cycle, other prophylaxis, such as acyclovir or ciprofloxacin, was not used.
In addition to infection, 15 patients experienced adverse events. Prolonged neutropenia occurred in seven cases, rituximab hypersensitivity in three cases, pulmonary toxicity confirmed by pulmonary function test in three cases, and renal toxicity in two cases. As for cases of worsening PS due to reasons unrelated to chemotherapy, cognitive impairment due to aging, stroke, intestinal perforation, hip fracture, toxic hepatitis due to herbal medicines, sudden gastrointestinal bleeding, cardiac arrest, and liver failure due to underlying liver cirrhosis were identified.
Thirty-six patients refused further cycles of chemotherapy by themselves, and half of them complained of decreased physical strength after chemotherapy. Four patients requested treatment termination as post-treatment care was difficult due to a lack of caregivers after chemotherapy. One patient complained of extreme fear and anxiety after one cycle of chemotherapy and was referred to the department of neuropsychiatry. The other 13 patients were lost to follow-up without any recorded adverse events.
Among the patients whose treating physician decided to terminate chemotherapy, 65.1% were evaluated as CR at the first response evaluation and 20.9% as PR at the first response evaluation. Thus, the physicians tend to decide whether to discontinue chemotherapy after the first response evaluation. Contrarily, among patients who initiated early termination of chemotherapy, 13 patients (36.1%) did not evaluate the response after chemotherapy, and in most cases, follow-up was voluntarily lost without discussion with their doctors, and those with a very short median OS (4.87 months, 95% CI, 2.03-NA). There were no significant differences in OS and PFS when survival was analyzed according to whether the decision to discontinue chemotherapy was made by the physician or patient (Supplementary figure 6). The median age of the 129 patients for whom the treating physician decided to stop chemotherapy was 72.8 years. Among them, 108 patients, excluding 21 patients who planned local treatment, had a median age of 74.23 years, higher than the median age of patients who refused further cycles of chemotherapy by themselves (66.15 years, P = 0.005).
Relative risk factors for OS and PFS in unplanned shortening treatment
The result of univariate and multivariate analyses for OS are described in Table 4. As expected, factors such as old age, advanced stage, poor ECOG performance status, and increased serum lactate dehydrogenase (LDH) levels that made up the IPI risk score were identified as poor prognostic factors for OS. The use of prophylactic G-CSF did not improve OS. Rather, in univariate analysis, prophylactic G-CSF use was identified as a poor prognostic factor for OS. However, in multivariate analysis, there was no significant relationship with the OS because there was a tendency to use prophylactic G-GCF in the case of old age. In terms of treatment strategy, lower dose intensity, such as an R-mini-CHOP regimen or fewer cycles of chemotherapy, were confirmed as poor relative risk factors; however, in multivariate analysis, their effect was not significant. On the other hand, the achievement of CR status for chemotherapy was an independent good prognostic factor that significantly improved OS in multivariate analysis. The same trend was maintained in the univariate and multivariate analysis of PFS in patients with DLBCL who had experienced unplanned shortening of chemotherapy (Supplementary table 4).
Applying other modalities
Twenty-eight patients underwent radiotherapy after the early termination of chemotherapy, and all had stage I or II DLBCL (Supplementary table 5). Seven additional patients also received radiotherapy for consolidation treatment after the decision to discontinue chemotherapy; chemotherapy was stopped for other reasons such as infection or poor performance status. When subgroup analysis of stage I or II patients was performed, most patients who underwent radiotherapy received more than three cycles of chemotherapy, and most patients had already achieved CR before radiotherapy. When the survival of each group was compared with and without radiotherapy, the patients who received radiotherapy showed significantly better clinical outcomes in OS and PFS than those who did not (Fig. 4).
Twenty-six patients who underwent surgery apart from chemotherapy were identified; in all patients, surgery was performed for diagnostic purposes or before the histological diagnosis. Among them, 14 patients underwent R0 resection, and there were eight patients with gastrointestinal tract DLBCL, four patients with head and neck involvements, one patient with neck node involvement, and one patient with lung nodule (Supplementary table 6).
Discussion
Although R-CHOP is the standard of care for patients with treatment-naïve DLBCL, in real-world practice, it is not possible to administer six cycles of R-CHOP to patients with high comorbidity or old age. Various efforts such as prophylactic administration of G-CSF or dose modification have been made to improve survival outcomes and complete six cycles of R-CHOP in this group of patients [8-10]. However, no standardized method has been established yet. Although the phase III FLYER trial, which compared six cycles of R-CHOP with four cycles of R-CHOP followed by two additional rituximab monotherapies, was published [12], this study targeted young-age, low relapse-risk patients. Even in elderly DLBCL patients, it is still recommended to complete six cycles of R-CHOP to obtain favorable outcomes [13]. To bridge the gap between real-world practice and the recommended guideline, our study was conducted for prognostic analysis that can provide advice to physicians in the clinical setting.
From January 2010 to April 2019, 1183 patients were diagnosed with DLBCL, of whom, 260 patients (22.0%) were treated with incomplete cycles of R-CHOP. Disease progression was confirmed in 57 patients (4.8%) in the first response evaluation, and 33 patients (2.8%) died within 21 days after the beginning of the first cycle of R-CHOP (Fig. 1). This proportion was observed at a rate similar to that of the large population-based study of the Swedish group [14]. In these cases, treatment could not be continued regardless of doctor-patient discussions about treatment discontinuation during chemotherapy. Therefore, these patients were not suitable for evaluating the natural course of patients who had a durable survival outcome after early termination of R-CHOP, which was the main purpose of this study, and were excluded from the final analysis.
The failure to complete high-intensity chemotherapy was due to host factor. In a large population-based cohort study of a Swedish group, advanced age and poor performance status strongly predicted failure to complete planned treatment for reasons other than non-response [14]. Our study also confirmed the same trend when the entire cohort was analyzed. The median age of patients who discontinued treatment was 70.78 years, and showed a high comorbidity score with a CCI of 2 or more and a high IPI score compared to the patients who completed treatment. However, in the multivariate analysis of OS and PFS, patients who had already achieved CR as an interim response during R-CHOP had a relatively good prognosis compared with those who did not, and the chemotherapy intensity, including the number of cycles and dose reduction, was not an independent factor affecting prognosis. Therefore, it would be important to establish a treatment strategy tailored to individual patients through an understanding of the specific causes of early discontinuation of chemotherapy and insight into a patient group with a relatively good prognosis.
As a single factor, the cause of early termination in most patients was a life-threatening infection. The most common pathogen was Pneumocystis jirovecii, and neutropenic fever was documented in 18 of 51 cases with life-threatening infections. According to the reimbursement system in Korea, prophylactic long-acting G-CSF has been used for patients with DLBCL since 2014. Thus, in our study population, 98 patients (59.4%) used prophylactic G-CSF, and grade three or four neutropenia was prevented relatively well using prophylactic G-CSF. However, in our study population, the use of prophylactic antibiotics was not frequently implemented, and only nine patients received prophylactic TMP-SMX from the first cycle of R-CHOP. Since 2016, our center’s protocol recommended the use of prophylactic TMP-SMX in patients aged over 60 years who started the fourth cycle of R-CHOP. Based on this, of the 23 patients who discontinued treatment due to PCP, 18 received R-CHOP chemotherapy before 2015. And four of the five patients who received R-CHOP after 2016 developed PCP after the second or third cycle of R-CHOP. Moreover, prophylaxis using TMP-SMX is highly effective against PCP infection [15]. Thus, prophylactic TMP-SMX should be considered from the first cycle in patients with poor performance status and high comorbidity scores.
The decision to discontinue chemotherapy was mostly made by the treating physicians. Irrespective of whether the doctor or the patient decided on early termination of chemotherapy, the main reason for not completing six cycles of R-CHOP was worsening performance status after chemotherapy (Table 3). Although more than half of the patients had a limited stage in our study population, it was difficult to tolerate high-dose chemotherapy because they had a CCI of 2 or higher (Table 1). According to the FLYER trial [12], in patients with limited-stage DLBCL, four cycles of R-CHOP followed by two additional rituximab monotherapies were not inferior to six cycles of R-CHOP. In the entire retrospective cohort, 194 patients (16.4% of all patients, 36.1% of the limited-stage patients) fulfilled the FLYER inclusion criteria. Among them, 23 patients did not complete 6 cycles of R-CHOP, and 171 patients completed 6 cycles of R-CHOP. In 23 patients who did not complete 6 cycles of chemotherapy, the 5-year OS in this subgroup was surprisingly 100% despite insufficient chemotherapy intensity, and there was no difference in survival compared with patients who completed 6 cycles (Supplementary figure 2 (C)(D)). However, the 5-year OS rate in patients with a limited stage who did not fulfill the FLYER inclusion criteria was 61.1% (95% CI, 49.9–74.7) in the chemotherapy discontinuation group and 84.9% (95% CI, 80.8–89.2) in the chemotherapy complete group. From this point of view, another retrospective study suggested that dose modification or alternative regimens could provide comparable efficacy in elderly patients with limited-stage DLBCL [8]. Unfortunately, during the study period, the reimbursement program in Korea did not permit the use of rituximab monotherapy maintenance or alternative agents; therefore, in our cohorts, there were no cases who received alternative treatment replacing R-CHOP. However, similar to previous studies, in our study, despite the early termination of planned R-CHOP, patients who had a good response to R-CHOP or received more than three cycles of R-CHOP had comparable and acceptable survival outcomes. Taken together, a new modified regimen for elderly patients with a limited-stage disease may be considered.
The role of additional radiotherapy after R-CHOP is controversial [16-18]. The Southwest Oncology Group study S0014 showed promising results regarding additional radiotherapy [16], and in patients with old age and limited-stage DLBCL, additional radiation treatment showed a survival gain [18]; however, there was no comparison with chemotherapy cycles. Afterward, six cycles of R-CHOP alone were non-inferior to the addition of radiotherapy [19]. In our study, 21 patients (14.1%) underwent local treatment and a discontinued R-CHOP after discussion between the treating physicians and the patients (Table 3). In addition, seven more patients discontinued further cycle of R-CHOP for other reasons such as life-threatening infections, and received additional consolidative radiotherapy. Thus, a total 28 patients in this study received radiotherapy. All of these patients had stage I or II limited diseases, and their treatment response was at least PR. Interestingly, when comparing survival outcomes with or without radiotherapy among a total of 86 patients with stage I or II DLBCL in our study population, patients who underwent radiation showed a significantly better outcome than those who did not, even though more patients had undergone up to four or five cycles of R-CHOP among those who did not receive radiotherapy. From this point of view, in patients with stage I or II DLBCL who cannot complete six cycles of R-CHOP, additional radiotherapy should be considered for a survival benefit [20].
Another local treatment modality is surgery. However, considering the pathogenesis of DLBCL, systemic chemotherapy alone can be curative, and curative intent surgery is not recommended unlike solid tumors. In real-world practice, some patients undergo curative intent surgery before confirmation of the pathologic diagnosis, and it is not easy to encourage these patients to continue high-intensity chemotherapy after surgery. In our study, 26 patients underwent surgery, and 14 patients underwent R0 resection (Supplementary table 4). Except for one case, most patients received more than three cycles of R-CHOP, and all patients underwent surgery before the start of chemotherapy to confirm the pathologic diagnosis. Therefore, it is difficult to estimate the additive role of surgery in this small population.
This study had several limitations. First, since it was impossible to predict which patients will not receive six cycles of R-CHOP when they start chemotherapy, this study had to be constructed as a retrospective design. Second, one of the most representative factors based on the electronic medical record per patient was described as the cause of discontinuation of chemotherapy. However, in general, various factors, such as patients’ performance status, tolerability, or compliance, are considered in the decision-making process regarding the discontinuation of chemotherapy. Third, there was a lead time bias to exclude some patients who died within 21 days after the first cycle of R-CHOP. However, this study aimed to determine the long-term prognosis of unexpected early termination of chemotherapy in actual practice. Therefore, this population was excluded from the final analysis because of the increasing likelihood of underestimation of clinical outcomes. Thus, with some limitations, our study provided essential clinical information about prognosis after incomplete standard treatment in real-world practice.
When R-CHOP is interrupted, patients who achieve primary response have better survival outcomes than those who do not. Response evaluation using PET/CT or other modalities should be performed before deciding to terminate chemotherapy. In addition, in patients with stage I or II DLBCL, a change to local treatment such as radiotherapy could be considered. These findings could provide the clinical outcome after early termination of R-CHOP to clinicians and provide evidence to explain to patients the prognosis after the inevitable discontinuation of R-CHOP.
Data Availability
The data that support the findings of this study are available on request from the corresponding author, KHK. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
References
Liu Y, Barta SK (2019) Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol 94(5):604–616. https://doi.org/10.1002/ajh.25460
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. https://doi.org/10.3322/caac.21660
Sehn LH, Salles G (2021) Diffuse large B-cell lymphoma. N Engl J Med 384(9):842–858. https://doi.org/10.1056/NEJMra2027612
Tanimura A, Hirai R, Nakamura M, Takeshita M, Hagiwara S, Miwa A (2018) The prognostic impact of dose-attenuated R-CHOP therapy for elderly patients with diffuse large B-cell lymphoma. Intern Med 57(24):3521–3528. https://doi.org/10.2169/internalmedicine.0990-18
Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, Lefort S, Marit G, Macro M, Sebban C, Belhadj K, Bordessoule D, Ferme C, Tilly H (2010) Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood 116(12):2040–2045. https://doi.org/10.1182/blood-2010-03-276246
Poeschel V, Schmitz N, Ruebe C, Feller AC, Loeffler M, German High-Grade Non-Hodgkin Lymphoma Study G (2008) Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol 9(2):105–116. https://doi.org/10.1016/S1470-2045(08)70002-0
Lin RJ, Behera M, Diefenbach CS, Flowers CR (2017) Role of anthracycline and comprehensive geriatric assessment for elderly patients with diffuse large B-cell lymphoma. Blood 130(20):2180–2185. https://doi.org/10.1182/blood-2017-05-736975
Cheng CL, Liu JH, Chou SC, Yao M, Tang JL, Tien HF (2018) Retrospective analysis of frontline treatment efficacy in elderly patients with diffuse large B-cell lymphoma. Eur J Haematol 101(1):28–37. https://doi.org/10.1111/ejh.13069
Juul MB, Jensen PH, Engberg H, Wehberg S, Dessau-Arp A, Haziri D, Kristensen HB, Baech J, Schurmann L, Clausen MR, Valentin R, Knudsen LM, Munksgaard L, El-Galaly TC, Frederiksen H, Larsen TS (2018) Treatment strategies and outcomes in diffuse large B-cell lymphoma among 1011 patients aged 75 years or older: a Danish population-based cohort study. Eur J Cancer 99:86–96. https://doi.org/10.1016/j.ejca.2018.05.006
Kumar A, Fraz MA, Usman M, Malik SU, Ijaz A, Durer C, Durer S, Tariq MJ, Khan AY, Qureshi A, Faridi W, Nasar A, Anwer F (2018) Treating diffuse large B cell lymphoma in the very old or frail patients. Curr Treat Options Oncol 19(10):50. https://doi.org/10.1007/s11864-018-0565-6
Peyrade F, Jardin F, Thieblemont C, Thyss A, Emile JF, Castaigne S, Coiffier B, Haioun C, Bologna S, Fitoussi O, Lepeu G, Fruchart C, Bordessoule D, Blanc M, Delarue R, Janvier M, Salles B, Andre M, Fournier M, Gaulard P, Tilly H, Grouped’Etude des Lymphomes de l’Adultei (2011) Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol 12(5):460–468. https://doi.org/10.1016/S1470-2045(11)70069-9
Poeschel V, Held G, Ziepert M, Witzens-Harig M, Holte H, Thurner L, Borchmann P, Viardot A, Soekler M, Keller U, Schmidt C, Truemper L, Mahlberg R, Marks R, Hoeffkes HG, Metzner B, Dierlamm J, Frickhofen N, Haenel M, Neubauer A, Kneba M, Merli F, Tucci A, de Nully BP, Federico M, Lengfelder E, di Rocco A, Trappe R, Rosenwald A, Berdel C, Maisenhoelder M, Shpilberg O, Amam J, Christofyllakis K, Hartmann F, Murawski N, Stilgenbauer S, Nickelsen M, Wulf G, Glass B, Schmitz N, Altmann B, Loeffler M, Pfreundschuh M, Investigators FT, German Lymphoma A (2019) Four versus six cycles of CHOP chemotherapy in combination with six applications of rituximab in patients with aggressive B-cell lymphoma with favourable prognosis (FLYER): a randomised, phase 3, non-inferiority trial. Lancet 394(10216):2271–2281. https://doi.org/10.1016/S0140-6736(19)33008-9
Khan Y, Brem EA (2019) Considerations for the treatment of diffuse large B cell lymphoma in the elderly. Curr Hematol Malig Rep 14(4):228–238. https://doi.org/10.1007/s11899-019-00519-7
Wasterlid T, Harrysson S, Andersson TM, Ekberg S, Enblad G, Andersson PO, Jerkeman M, Eloranta S, Smedby KE (2020) Outcome and determinants of failure to complete primary R-CHOP treatment for reasons other than non-response among patients with diffuse large B-cell lymphoma. Am J Hematol 95(7):740–748. https://doi.org/10.1002/ajh.25789
Lee JY, Kang M, Suh KJ, Kim JW, Kim SH, Kim JW, Kim YJ, Song KH, Kim ES, Kim HB, Lee KW, Kim JH, Bang SM, Lee JS, Lee JO (2021) Pneumocystis jirovecii pneumonia in diffuse large B-cell Lymphoma treated with R-CHOP. Mycoses 64(1):60–65. https://doi.org/10.1111/myc.13184
Persky DO, Unger JM, Spier CM, Stea B, LeBlanc M, McCarty MJ, Rimsza LM, Fisher RI, Miller TP, Southwest Oncology G (2008) Phase II study of rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited-stage aggressive B-cell lymphoma: Southwest Oncology Group study 0014. J Clin Oncol 26(14):2258–2263. https://doi.org/10.1200/JCO.2007.13.6929
Kwon J, Kim IH, Kim BH, Kim TM, Heo DS (2015) Additional survival benefit of involved-lesion radiation therapy after R-CHOP chemotherapy in limited stage diffuse large B-cell lymphoma. Int J Radiat Oncol Biol Phys 92(1):91–98. https://doi.org/10.1016/j.ijrobp.2014.12.042
Parikh RR, Yahalom J (2017) Older patients with early-stage diffuse large B-cell lymphoma: the role of consolidation radiotherapy after chemoimmunotherapy. Leuk Lymphoma 58(3):614–622. https://doi.org/10.1080/10428194.2016.1205739
Lamy T, Damaj G, Soubeyran P, Gyan E, Cartron G, Bouabdallah K, Gressin R, Cornillon J, Banos A, Le Du K, Benchalal M, Moles MP, Le Gouill S, Fleury J, Godmer P, Maisonneuve H, Deconinck E, Houot R, Laribi K, Marolleau JP, Tournilhac O, Branger B, Devillers A, Vuillez JP, Fest T, Colombat P, Costes V, Szablewski V, Bene MC, Delwail V, Group L (2018) R-CHOP 14 with or without radiotherapy in nonbulky limited-stage diffuse large B-cell lymphoma. Blood 131(2):174–181. https://doi.org/10.1182/blood-2017-07-793984
Gine E, Sehn LH (2016) Diffuse large B-cell lymphoma: should limited-stage patients be treated differently? Hematol Oncol Clin North Am 30(6):1179–1194. https://doi.org/10.1016/j.hoc.2016.07.010
Author information
Authors and Affiliations
Contributions
JY and KHK designed the analysis, collected data, and performed the analysis. KHK, JSK, JMB, JH, DYS, YK, TMK, IK, SSY, DSH, HP, and JHP contributed data collection and interpreted the results of the analysis. JY and KHK wrote the initial version of the manuscript and revised it. All authors approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Institutional Review Board of SNU-BMC (IRB No. 10–2020-290).
Conflict of interest
Author SSY has received research grants from Roche-Genentech, Kyowahako-Kirin, and Yuhan Pharma, and author SSY is an advisory role of Tikaros, ProtanBio, AbbVie, BMS-Celgene, Janssen, Novartis, and Takeda. JY, KHK, JSK, JMB, JH, DYS, YK, TMK, IK, DSH, HP, and JHP declares that they have no financial relationship to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yoon, J., Kim, K.H., Kim, J.S. et al. Clinical outcomes after incomplete cycles of R-CHOP for diffuse large B-cell lymphoma: 10 years’ real-world experience in a single institute. Ann Hematol 102, 1467–1476 (2023). https://doi.org/10.1007/s00277-023-05179-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05179-5