Abstract
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is an effective and curative treatment for acute myeloid leukemia (AML). We explored the outcome of haploidentical donor (HID) transplantation for intermediate-risk AML and compared to that of matched sibling donor (MSD) transplants. One hundred twenty-seven consecutive patients with intermediate-risk AML in the first complete remission (CR1) who underwent allo-HSCT between January 1, 2015, and August 1, 2016, were enrolled. Thirty-seven patients received MSD grafts, and 90 received HID grafts. The 2-year leukemia-free survival (LFS) of the HID group was comparable to that of the MSD group: 82.0% ± 4.1% versus 82.7% ± 6.4%, P = 0.457. The 2-year cumulative incidences of relapse and transplantation-related mortality (TRM) were comparable between the HID and MSD groups (relapse, 4.5% ± 0.1%, versus 11.5% ± 0.3%, P = 0.550; TRM, 13.4% ± 0.1% vs. 5.8% ± 0.2%, P = 0.154). The HID recipients had a trend of a lower 2-year cumulative incidence of positive posttransplant flow cytometry (FCM+) and relapse than the MSD recipients (5.6% ± 0.1% vs. 19.9% ± 0.5%, P = 0.092). These results suggest that the outcomes of allo-HSCT with HIDs are comparable to those with MSDs in terms of LFS, TRM, and relapse for intermediate-risk AML in CR1. HIDs could be an alternative to MSDs for intermediate-risk AML.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukemia (AML) remains the most frequent indication for allogeneic hematopoietic stem cell transplantation (allo-HSCT) [1,2,3]. AML patients with unfavorable cytogenetics are recommended to undergo HSCT in the first complete remission (CR1) due to their high risk of relapse. However, the recommendations for HSCT in intermediate-risk AML were less clear. The risk-benefit ratio in regard to patient fitness, donor source, minimal residual disease (MRD) status, and transplant center experience must be evaluated when making a decision on HSCT. The 2017 European LeukemiaNet (ELN) recommendations and the 2018 National Comprehensive Cancer Network (NCCN) Guidelines recommended that allo-HSCT could be used as postremission therapy for intermediate-risk AML patients [4, 5]. An increasing number of studies have suggested that adults with intermediate-risk AML in CR1 could benefit from allo-HSCT [6,7,8,9,10]. However, the lack of a matched sibling donor (MSD) and the difficulty in finding a matched unrelated donor (MUD) limited the application of allo-HSCT.
For patients who lack an MSD, a haploidentical donor (HID) could be an option. A multicenter, prospective study in China demonstrated similar survival after HID-HSCT and MSD-HSCT for patients with intermediate- or high-risk AML in CR1 [11]. The haploidentical group had a 3-year overall survival (OS) of 79%, which was comparable to the 82% 3-year OS in the HLA-identical group (P = 0.36). A publication on intermediate- and high-risk AML by Yoon et al. reported a 5-year leukemia-free survival (LFS) of 65.9% for MSD and 68.5% for HID in intermediate-risk AML; however, in the posttransplant cyclophosphamide (PT-CY) setting, a worse LFS was observed by Salvatore et al. in HID-HSCT than in MSD-HSCT for intermediate-risk AML (56% versus 70%, respectively, P < 0.01) [12]. In recent years, more safety and efficacy data were obtained on HID transplants than on MSD/MUD transplants [5, 13,14,15,16,17]. While remarkable improvements have been made in HID-HSCT, the role of HID-HSCT for intermediate-risk AML is somewhat controversial. The NCCN guidelines suggested that patients with intermediate-risk AML could receive alternative donor transplantation in the absence of matched donors [5], while haploidentical allo-HSCT is not listed as a consolidation option in the ELN recommendations [4]. Our previous study reported that HID-HSCT was superior to chemotherapy alone as a postremission treatment for intermediate-risk AML [7, 8]. As published data on HID-HSCT for intermediate-risk AML are limited, whether transplantation from an HID is equivalent to that from an MSD for intermediate-risk AML is still a matter of debate.
Here, we report the results of the comparison of HID transplants and MSD transplants for homogeneous patients with intermediate-risk AML in CR1.
Patients and methods
Patients
Between January 1, 2015, and August 1, 2016, 127 consecutive patients aged ≥ 15 years with intermediate-risk AML in CR1 received HID (N = 90) or MSD (N = 37) allo-HSCT according to donor availability at the Peking University Institute of Hematology. Fourteen patients who received a haplo-HCT from maternal donors or collateral relatives with low-dose posttransplant cyclophosphamide (PT-CY) [18] were excluded. The study protocol was approved by the Institutional Review Board of Peking University. All patients provided their written informed consent for this procedure.
Risk status
Patients were stratified as intermediate-risk AML based on the NCCN guidelines [5]. Included criteria are (1) wild-type NPM1 without FLT3-ITD mutation; (2) t(9;11); and (3) cytogenetic abnormalities not classified as favorable or adverse. Excluded criteria are (1) the favorable-risk cytogenetics t(8;21), t(15;17), inv(16), or t(16;16); (2) mutated NPM1 without FLT3-ITD mutation; (3) the poor-risk cytogenetics including complex karyotypes (≥ 3 clonal chromosomal abnormalities), monosomal karyotypes, − 5, 5q-, − 7, 7q-, 11q23-non t(9;11), inv(3), t(3;3), t(6;9), and t(9;22); and (4) wild-type NPM1 and mutated FLT3-ITD.
Transplant protocols
The transplantation procedure was described in previous studies [11, 19, 20]. For HID transplants, the busulfan (BU)-based conditioning regimen consisted of cytarabine (Ara-C; 4 g/m2/day, intravenous, days − 10 and − 9), BU (3.2 mg/kg/day, intravenous, days − 8 to − 6), cyclophosphamide (CY; 1.8 g/m2/day, days − 5 and − 4), rabbit antithymoglobulin (ATG; 2.5 mg/kg/day, days − 5 to − 2), and semustine (Me-CCNU; 250 mg/m2, oral, day − 3). For MSD transplants, patients received hydroxyurea (Hu; 40 mg/kg, two doses, oral, day − 10), a lower dose of Ara-C (2 g/m2/day, intravenous, day − 9), and no ATG; otherwise, the regimen was identical to that of haploidentical patients. Bone marrow (BM) cells and/or peripheral blood (PB) cells were collected after G-CSF mobilization. Day 1 was the first day of donor cell infusion. All transplantation recipients received cyclosporine A (CsA), mycophenolate mofetil (MMF), and short-term methotrexate (MTX) as graft-versus-host disease (GVHD) prophylaxis. The haploidentical graft recipients received G-CSF (5 μg/kg, subcutaneously, daily) from day + 6 until myeloid recovery [21, 22].
Monitoring of MRD
BM assessments were performed to assay for MRD before transplantation. After transplantation, BM samples were examined at + 1, + 2, + 3, + 4.5, + 6, + 9, + 12, + 18, and + 24 months, as well as once a year thereafter. More frequent analyses were performed if the MRD status became positive. Eight-color multiparameter FCM was used to detect leukemia-associated antigen phenotypes (LAIPs). More than 0.01% of previously identified LAIPs were defined as FCM-positive (FCM+) [23,24,25].
Definitions
Neutrophil recovery was defined as the first day of 3 consecutive days when an absolute neutrophil count (ANC) ≥ 0.5 × 109/L was achieved, and platelet recovery was defined as the first day of 7 consecutive days when a platelet count ≥ 20 × 109/L was achieved without transfusion. Relapse was defined as hematological relapse or extramedullary relapse [5]. TRM was defined as death due to any cause other than relapse. OS was calculated from the date of HSCT to the date of death from any cause. LFS was calculated from the date of HSCT to the date of relapse or death. GVHD-free/relapse-free survival (GRFS) was calculated from the date of HSCT to the date of events that included grades III–IV aGVHD, cGVHD requiring systemic therapy, relapse, or death [26].
Statistical analysis
The last follow-up date was March 1, 2019. The primary endpoint for the study was OS, and secondary endpoints included LFS, relapse, and TRM. The Mann-Whitney U rank sum test was used for continuous variables, and a chi-square test or Fisher’s exact test was used for categorical variables. All tests were two-sided. Kaplan-Meier outcome curves were constructed for the OS and LFS of patients. The log-rank test was used to identify prognostic factors, and a Cox proportional hazards regression model was used to assess the relative impact of previously defined risk factors with multivariate analysis. The cumulative incidences of relapse and GVHD were calculated with a completing-risk model, with TRM as the competing event. The forced factor (haploidentical vs. HLA-identical) and all factors with P < 0.20 in the univariate analysis were included in a multivariate regression. P < 0.05 was considered significant. Data analyses were primarily conducted with SPSS software (SPSS, Chicago, IL) and R software (version 2.6.1) (http://www.r-project.org).
Results
Patient characteristics
Baseline characteristics for the subjects are summarized in Table 1. In comparison to MSD recipients, the HID recipients were younger (P = 0.011). The median time from the diagnosis of AML to transplantation in the HID group was longer than that in the MSD group (P = 0.003).
Engraftment
All patients achieved neutrophil recovery. The median time to neutrophil recovery was 16 days (range, 12–27 days) in the MSD group and was 13 days (range, 9–25 days) in the HID group (P = 0.000). Platelet engraftment was observed in 126 cases, at a median of 13 days (range, 8–43 days) in the MSD group and 15 days (range, 9–784 days) in the HID group (P = 0.032). One HID recipient had persistent thrombocytopenia and died of severe pneumonia at 38 days posttransplant. All patients exhibited complete donor chimerism at 1 month after transplantation.
Relapse
Of the 127 patients, 11 patients (8.7%) relapsed, with a median time of 355 days after transplantation (range, 50–969 days). The median time to relapse was 254.5 days (range, 126–727 days) for the MSD group and 473 days (range, 50–969 days) for the HID group. In the competing-risk model, the 2-year rate of relapse in the HID group was not significantly different from that in the MSD group (4.5% ± 0.1%, versus 11.5% ± 0.3%, P = 0.550) (Fig. 1). If either a positive posttransplant FCM status or a morphological relapse was considered relapse at the MRD level, the HID group had a tendency to have a lower relapse rate than the MSD group (5.6% ± 0.1% vs. 19.9% ± 0.5%, P = 0.092). Fifteen patients were posttransplant MRD+ or experienced disease recurrence. Of them, one received palliative care, and 14 received interventions. Of them, 4 patients in the MSD group and 5 patients in the HID group received a preemptive/therapeutic donor lymphocyte infusion (DLI) at a median time of 295 days (range, 70–1070 days) posttransplant. At last follow-up, 9 (60%) patients died of relapse.
As shown in Table 1, 5 MSD patients and 12 HID patients had positive pre-MRD status. Two MSD patients and three HID patients experienced MRD reactivation after transplantation.
In the multivariable analysis, a WBC count at diagnosis ≥ 100 × 109/L remained the only independent risk factor for higher risk of relapse (Table 2). Other variables including the donor type, recipient age, and cycles of induction to achieve CR1 (1 cycle vs. ≥ 2 cycles) were not significantly associated with the risk of relapse.
TRM
At the last follow-up, 6 MSD recipients and 18 HID recipients died. The causes of death included relapse (n = 9) and TRM (n = 15). The leading cause of death after transplantation was severe pneumonia (n = 10), accounting for the cause of death in 8 HID recipients and in two MSD recipients. The cumulative incidence of TRM at 2 years was not significantly different between HID and MSD transplants (13.4% ± 0.1% vs. 5.8% ± 0.2%, P = 0.154). The 2-year probability of TRM was significantly higher for recipients > 50 years than those ≤ 50 years (29.3% ± 1.7% vs. 9.0% ± 0.1%, P = 0.005). In the multivariate analysis, recipient age > 50 years was confirmed as the only independent prognostic factor for TRM (Table 2).
GVHD
The cumulative incidence of grades II-IV aGVHD at 100 days posttransplant was 30.0% after HID-HSCT and 5.4% after MSD-HSCT (P = 0.002). Severe aGVHD tended to occur more frequently in HID-HSCT than in MSD-HSCT (8.9% vs. 0%, P = 0.062). Other factors including female donor to male recipient (F-M) were not associated with the development of aGVHD.
The 2-year rate of cGVHD was lower in the HID-HSCT group than in the MSD-HSCT group (25.1% ± 0.2% vs. 45.6% ± 0.7%, P = 0.007), and the 2-year incidence of extensive cGVHD was not significantly different between the HID- and MSD-HSCT groups (10.3% ± 0.1% vs. 22.6% ± 0.5%, P = 0.068). In the multivariable analysis, the following factors were associated with the development of cGVHD: cycles of induction to achieve CR1 ≥ 2 (≥ 2 cycles vs. 1 cycle, HR = 2.175, 95% CI 1.040–4.547, P = 0.039), and HLA matching (matched vs. mismatched, HR = 2.408, 95% CI 1.316–4.408, P = 0.004). Female donor/male recipient was not a risk factor for cGVHD (F-M vs. others, HR = 1.260, 95% CI 0.620–2.562, P = 0.523). No risk factors for the occurrence of extensive cGVHD were found.
Survival after transplantation
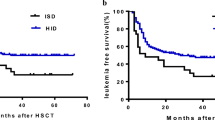
The 2-year OS after transplantation was 83.1% ± 4.0% in the HID group and 88.5% ± 5.4% in the MSD group (P = 0.623) (Fig. 2a). The 2-year LFS of the HID group was comparable to that of the MSD group (82.0% ± 4.1% versus 82.7% ± 6.4%, respectively, P = 0.457) (Fig. 2b). Furthermore, the 2-year GRFS was not significantly different between the HID and MSD groups (71.8% ± 4.8% vs. 65.9% ± 8.0%, P = 0.769) (Fig. 2c).
Survival. a Overall survival. The 2-year OS after transplantation was 83.1% ± 4.0% in the HID group and 88.5% ± 5.4% in the MSD group. b Leukemia-free survival. The 2-year LFS was 82.0% ± 4.1% in the HID group and 82.7% ± 6.4% in the MSD group. c GVHD, relapse-free survival. The 2-year GRFS was 71.8% ± 4.8% in the HID group and 65.9% ± 8.0% in the MSD group. GVHD, graft-versus-host disease; HID, haploidentical donor; MSD, matched sibling donor
The survival was significantly different between patients who were > 50 years old and younger patients (OS, P = 0.011; and LFS, P = 0.018). In the subgroup analysis, worse outcomes for patients > 50 years old were seen in MSD transplants (OS, P = 0.032; and LFS, P = 0.009) but not in HID transplants (OS, P = 0.195; and LFS, P = 0.297). Patients who had positive pretransplant MRD (pre-MRD+) had significantly worse OS and LFS than the negative pre-MRD (pre-MRD−) group (OS, P = 0.002; and LFS, P = 0.012), but the GRFS was not significantly different between these two groups (P = 0.153).
In the Cox regression, pre-MRD+ status and recipient age > 50 years were independent risk factors of OS and LFS. For GRFS, recipient age > 50 years remained the only adverse factor for GRFS in the multivariable analysis (Table 2).
Discussion
HID-HSCT has been established as an alternative for patients who lack an HLA-identical donor. Several studies have described HID transplants for AML patients and have compared HID transplants with MUD or MSD transplants [11, 13, 27, 28]. However, most of these studies examined AML patients as a whole population without stratifying by cytogenetics, and other studies focused on HID transplants for high-risk AML. In this study, we compared the outcomes of HID transplants with that of MSD transplants for homogeneous intermediate-risk AML patients in CR1. Our data demonstrated similar LFS and OS with HID and MSD, which is consistent with a report from Korean researchers and with our previous study on intermediate-risk and high-risk AML [11, 29].
Notably, HID transplants had a trend of decreased relapse probability at the MRD level, suggesting a potential graft-versus-leukemia (GVL) effect of HID. There are conflicting data on whether HID-HSCT might have a superior GVL effect than MSD or MUD-HSCT [13, 27, 29,30,31,32]. While no superior GVL effect of HID-HSCT has been confirmed in a large population [30], there has been some evidence that HID grafts might yield better GVL effects in some specific populations [30, 31]. Ringden et al. reported a lower relapse rate in the HID group for acute leukemia (AL) in CR2/3 than that in the MSD group [30]. Wang et al. showed that the cumulative incidence of relapse was 26% after HID transplants for relapsed/refractory AL, which was lower than that after MSD transplants (49%, P = 0.008) [31]. Yoon et al. also observed a lower relapse rate (18.5%) in HID than in MSD transplants (23.5%) for intermediate-to-poor risk AML in CR1 [29]. Our findings showed that HID grafts seemed to have a stronger GVL effect in this intermediate-risk population, although we found no significant difference in relapse rates between HID- and MSD-HSCT, probably due to the small sample size. In addition, it is worth noting that MSD recipients exhibited an increased risk of a positive FCM status posttransplant. Preemptive interventions for patients who are MRD+ might reduce relapse after transplantation and reduce the difference in relapse rates between MSD- and HID-HSCT [23, 33]. It could be argued that there were some unbalanced baseline factors, including patient age, and WBC count at diagnosis, which might also have influenced the risk of relapse. However, among patients with a WBC count at diagnosis ≥ 100 × 109/L, three of the eight MSD patients relapsed, and none of the five HID recipients experienced recurrence; however, the sample size was not large enough to draw a valid conclusion. These observations confirmed that HID-HSCT might have better protection against relapse than MSD-HSCT in this population.
With regard to GVHD, our data suggested that there was a higher incidence of aGVHD in the HID cohort than in the MSD cohort, as reported in our previous studies comparing MSD- and HID-HSCT [11, 34]. However, the MSD group showed a relatively higher cumulative incidence of cGVHD than the HID group. A higher proportion of cGVHD was also observed after MSD transplants than after HID transplants in our previous study on myelodysplastic syndrome (MDS) [35], which is in agreement with results from PT-CY-based transplantation [27]. The reason for the higher rate of cGVHD in MSD transplants is unclear, but there are some possible explanations. In the present study, we noted that male recipients with female grafts were significantly more common in the MSD group than in the HID group. The combination of female donors and male recipients was associated with an increased risk of GVHD, which was supported by studies from Randolph et al. and was observed by our group in haploidentical transplant settings [36,37,38].
While a higher frequency of aGVHD may theoretically lead to a high risk of TRM following HSCT, the analyses from Yoon et al. and those from our center failed to show a higher TRM among HID transplants than among MSD recipients [29], although Salvatore and colleagues previously conducted a pair-matched analysis of recipients from the European Society for Blood and Marrow Transplantation (EBMT) and found that HID-HSCT resulted in more TRM than MSD [12]. Notably, the aforementioned EBMT results were mainly based on the PT-CY protocol for GVHD prophylaxis, and this distinction might have partially contributed to the differences among these results. Although there were more F-M transplants in the MSD group, F-M was not associated with aGVHD, cGVHD, or TRM in this population.
In accordance with our previous reports, no correlations between donor type and survival were found in the multivariable analysis for OS, LFS, and GRFS, and older age (> 50 years old) was an adverse factor for OS, LFS, and GRFS. Previous reports showed that patients > 50 years old had a worse survival than other patients because of their increased TRM [11, 39]. In the current study, patients > 50 years had a higher TRM rate than younger recipients. Nevertheless, the disadvantage of old age was obvious for only the MSD recipients, which is in line with our previous reports that age > 50 years old had no influence on survival and TRM among HID recipients [40]. Increasing experience has demonstrated that these patients might benefit from HID-HSCT [8], and our results suggest that when an MSD is not available for adults with intermediate-risk AML, HID may be used in both in young and older patients.
For ethical and practical reasons, the patients were not randomized to receive HID or MSD grafts. Although homogeneous patients received HID- or MSD-HSCT transplants according to donor availability, there were still some unbalanced factors. The patients in the HID cohort were younger and had a longer time to transplant than those in the MSD cohort. However, the median interval from diagnosis to transplant was 7 versus 6 months between the two cohorts. Despite these limitations, our data supported HID-HSCT as a postremission strategy for intermediate-risk AML in CR1.
In summary, haploidentical and HLA-identical donor transplantation have similar survival for patients with intermediate-risk AML in CR1. These results showed that haploidentical donors could be an alternative for AML patients who lack an HLA-identical donor.
References
D’Souza A, Lee S, Zhu X, Pasquini M (2017) Current use and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant 23(9):1417–1421
Passweg JR, Baldomero H, Bader P et al (2017) Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant 52(6):811–817
Xu L, Chen H, Chen J, Han M, Huang H, Lai Y, Liu D, Liu Q, Liu T, Jiang M, Ren H, Song Y, Sun Z, Wang J, Wu D, Zhou D, Zou P, Liu K, Huang X (2018) The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China-recommendations from the Chinese Society of Hematology. J Hematol Oncol 11(1):33
Dohner H, Estey E, Grimwade D et al (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 129(4):424–447
https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf. Accessed Feb 1, 2019
Burnett AK, Wheatley K, Goldstone AH, Stevens RF, Hann IM, Rees JHK, Harrison G, the Medical Research Council Adult and Paediatric Working Parties (2002) The value of allogeneic bone marrow transplant in patients with acute myeloid leukaemia at differing risk of relapse: results of the UK MRC AML 10 trial. Br J Haematol 118(2):385–400
Huang XJ, Zhu HH, Chang YJ, Xu LP, Liu DH, Zhang XH, Jiang B, Jiang Q, Jiang H, Chen YH, Chen H, Han W, Liu KY, Wang Y (2012) The superiority of haploidentical related stem cell transplantation over chemotherapy alone as postremission treatment for patients with intermediate- or high-risk acute myeloid leukemia in first complete remission. Blood. 119(23):5584–5590
Lv M, Wang Y, Chang Y-J, Zhang XH, Xu LP, Jiang Q, Jiang H, Lu J, Chen H, Han W, Wang FR, Wang JZ, Chen Y, Yan CH, Zhang YY, Sun YQ, Mo XD, Zhu HH, Jia JS, Zhao T, Wang J, Liu KY, Huang XJ (2019) Myeloablative haploidentical transplantation is superior to chemotherapy for patients with intermediate-risk acute myelogenous leukemia in first complete remission. Clin Cancer Res 25(6):1737–1748
Cornelissen JJ, van Putten WL, Verdonck LF et al (2007) Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood. 109(9):3658–3666
Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, Wadleigh M, DeAngelo DJ, Stone RM, Sakamaki H, Appelbaum FR, Döhner H, Antin JH, Soiffer RJ, Cutler C (2009) Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. Jama. 301(22):2349–2361
Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, Fan ZP, Wu DP, Huang XJ (2015) Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 125(25):3956–3962
Salvatore D, Labopin M, Ruggeri A et al (2018) Outcomes of hematopoietic stem cell transplantation from unmanipulated haploidentical versus matched sibling donor in patients with acute myeloid leukemia in first complete remission with intermediate or high-risk cytogenetics: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 103(8):1317–1328
Ciurea SO, Zhang M-J, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, Armand P, Antin JH, Chen J, Devine SM, Fowler DH, Luznik L, Nakamura R, O’Donnell PV, Perales MA, Pingali SR, Porter DL, Riches MR, Ringdén OTH, Rocha V, Vij R, Weisdorf DJ, Champlin RE, Horowitz MM, Fuchs EJ, Eapen M (2015) Haploidentical transplant with post-transplant cyclophosphamide versus matched unrelated donor transplant for acute myeloid leukemia. Blood. 126(8):1033–1040
McCurdy SR, Kasamon YL, Kanakry CG et al (2017) Comparable composite endpoints after HLA-matched and HLA-haploidentical transplantation with post-transplantation cyclophosphamide. Haematologica. 102(2):391–400
Bashey A, Zhang X, Sizemore CA et al (2011) T-cell replete haploidentical transplantation using post-transplant cyclophosphamide results in equivalent non-relapse mortality and disease-free survival compared to transplantation from HLA-identical sibling and matched unrelated donors: a stratified cox model analysis of two hundred and sixty contemporaneous allogeneic transplants from a single center. Blood. 118(21):833–833
Raiola AM, Dominietto A, di Grazia C, Lamparelli T, Gualandi F, Ibatici A, Bregante S, van Lint MT, Varaldo R, Ghiso A, Gobbi M, Carella AM, Signori A, Galaverna F, Bacigalupo A (2014) Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant 20(10):1573–1579
Versluis J, Labopin M, Ruggeri A, Socie G, Wu D, Volin L, Blaise D, Milpied N, Craddock C, Yakoub-Agha I, Maertens J, Ljungman P, Huynh A, Michallet M, Deconinck E, Chevallier P, Passweg J, Ciceri F, Mohty M, Cornelissen JJ, Nagler A (2017) Alternative donors for allogeneic hematopoietic stem cell transplantation in poor-risk AML in CR1. Blood Adv 1(7):477–485
Wang Y, Chang YJ, Chen L, Xu LP, Bian ZL, Zhang XH, Yan CH, Liu KY, Huang XJ (2017) Low-dose post-transplant cyclophosphamide can mitigate GVHD and enhance the G-CSF/ATG induced GVHD protective activity and improve haploidentical transplant outcomes. Oncoimmunology. 6(11):e1356152
Wang Y, Liu DH, Liu KY, Xu LP, Zhang XH, Han W, Chen H, Chen YH, Wang FR, Wang JZ, Sun YQ, Huang XJ (2013) Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: nine years of experience at a single center. Cancer. 119(5):978–985
Huang X-J, Liu D-H, Liu K-Y, Xu LP, Chen H, Han W, Chen YH, Wang JZ, Gao ZY, Zhang YC, Jiang Q, Shi HX, Lu DP (2006) Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant 38(4):291–297
Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W, Chen YH, Zhang XH, Lu DP (2009) Treatment of acute leukemia with unmanipulated HLA-mismatched/haploidentical blood and bone marrow transplantation. Biol Blood Marrow Transplant 15(2):257–265
Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, Chen YH, Han W, Wang FR, Wang JZ, Liu KY, Huang XJ (2017) Haploidentical hematopoietic stem cell transplantation for myelodysplastic syndrome. Biol Blood Marrow Transplant 23(12):2143–2150
Yan CH, Liu DH, Liu KY, Xu LP, Liu YR, Chen H, Han W, Wang Y, Qin YZ, Huang XJ (2012) Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood. 119(14):3256–3262
Yan CH, Wang Y, Wang JZ et al (2016) Minimal residual disease- and graft-vs.-host disease-guided multiple consolidation chemotherapy and donor lymphocyte infusion prevent second acute leukemia relapse after allotransplant. J Hematol Oncol 9(1):87
Wang Y, Chen H, Chen J, Han M, Hu JD, Jiong Hu, Huang H, Lai Y, Liu D, Liu Q, Liu T, Jiang M, Ren H, Song Y, Sun Z, Wang C, Wang J, Wu D, Xu K, Zhang X, Xu L, Liu K, Huang X (2018) The consensus on the monitoring, treatment, and prevention of leukemia relapse after allogeneic hematopoietic stem cell transplantation in China. Cancer Lett 438:63–75
Holtan SG, DeFor TE, Lazaryan A et al (2015) Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 125(8):1333–1338
Di Stasi A, Milton DR, Poon LM et al (2014) Similar transplantation outcomes for acute myeloid leukemia and myelodysplastic syndrome patients with haploidentical versus 10/10 human leukocyte antigen-matched unrelated and related donors. Biol Blood Marrow Transplant 20(12):1975–1981
Ciceri F, Labopin M, Aversa F, Rowe JM, Bunjes D, Lewalle P, Nagler A, di Bartolomeo P, Lacerda JF, Lupo Stanghellini MT, Polge E, Frassoni F, Martelli MF, Rocha V (2008) A survey of fully haploidentical hematopoietic stem cell transplantation in adults with high-risk acute leukemia: a risk factor analysis of outcomes for patients in remission at transplantation. Blood. 112(9):3574–3581
Yoon JH, Kim HJ, Park SS, Jeon YW, Lee SE, Cho BS, Eom KS, Kim YJ, Lee S, Min CK, Cho SG, Kim DW, Lee JW, Min WS (2017) Long-term clinical outcomes of hematopoietic cell transplantation for intermediate-to-poor-risk acute myeloid leukemia during first remission according to available donor types. Oncotarget. 8(25):41590–41604
Ringden O, Labopin M, Ciceri F et al (2016) Is there a stronger graft-versus-leukemia effect using HLA-haploidentical donors compared with HLA-identical siblings? Leukemia. 30(2):447–455
Wang Y, Liu DH, Xu LP, Liu KY, Chen H, Chen YH, Han W, Shi HX, Huang XJ (2011) Superior graft-versus-leukemia effect associated with transplantation of haploidentical compared with HLA-identical sibling donor grafts for high-risk acute leukemia: an historic comparison. Biol Blood Marrow Transplant 17(6):821–830
Yu S, Fan Q, Sun J, Fan Z, Zhang Y, Jiang Q, Huang F, Xuan L, Dai M, Zhou H, Liu H, Liu QF (2016) Haploidentical transplantation without in vitro T-cell depletion results in outcomes equivalent to those of contemporaneous matched sibling and unrelated donor transplantation for acute leukemia. Medicine. 95(11):e2973
Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, Chen YH, Han W, Wang FR, Wang JZ, Liu KY, Huang XJ (2017) IFN-alpha is effective for treatment of minimal residual disease in patients with acute leukemia after allogeneic hematopoietic stem cell transplantation: results of a registry study. Biol Blood Marrow Transplant 23(8):1303–1310
Mo XD, Xu LP, Liu DH, Chen YH, Han W, Zhang XH, Chen H, Wang Y, Wang JZ, Liu KY, Huang XJ (2012) Patients receiving HLA-haploidentical/partially matched related Allo-HSCT can achieve desirable health-related QoL that is comparable to that of patients receiving HLA-identical sibling Allo-HSCT. Bone Marrow Transplant 47(9):1201–1205
Wang Y, Wang HX, Lai YR, Sun ZM, Wu DP, Jiang M, Liu DH, Xu KL, Liu QF, Liu L, Wang JB, Gao F, Ou-Yang J, Gao SJ, Xu LP, Huang XJ (2016) Haploidentical transplant for myelodysplastic syndrome: registry-based comparison with identical sibling transplant. Leukemia. 30(10):2055–2063
Randolph SS, Gooley TA, Warren EH, Appelbaum FR, Riddell SR (2004) Female donors contribute to a selective graft-versus-leukemia effect in male recipients of HLA-matched, related hematopoietic stem cell transplants. Blood. 103(1):347–352
Chang YJ, Luznik L, Fuchs EJ, Huang XJ (2016) How do we choose the best donor for T-cell-replete, HLA-haploidentical transplantation? J Hematol Oncol 9:35
Wang Y, Wu DP, Liu QF, Xu LP, Liu KY, Zhang XH, Xu Y, Huang F, Huang XJ (2018) Donor and recipient age, gender and ABO incompatibility regardless of donor source: validated criteria for donor selection for haematopoietic transplants. Leukemia. 32(2):492–498
Jagasia M, Arora M, Flowers ME et al (2012) Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 119(1):296–307
Chen Y, Wang Y, Xu LP, Liu KY, Chen H, Chen YH, Zhang XH, Wang FR, Han W, Wang JZ, Yan CH, Zhang YY, Sun YQ, Huang XJ (2015) Haploidentical stem cell transplantation in patients aged 50 yr and older with leukemia: similar outcomes compared to younger adults. Clin Transpl 29(6):523–530
Acknowledgments
We thank the principal investigators and the skilled teams at each of the participating sites. We thank all colleagues for participating in the research.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 81770189, 82070189), the National Key Research and Development Program of China (2019YFC0840606) from the Ministry of Science and Technology, the Science and Technology Project of Guangdong Province of China 2016B030230003, the project of health collaborative innovation of Guangzhou city (no. 201704020214).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, YR., Xu, LP., Zhang, XH. et al. Allogeneic hematopoietic stem cell transplantation for intermediate-risk acute myeloid leukemia in the first remission: outcomes using haploidentical donors are similar to those using matched siblings. Ann Hematol 100, 555–562 (2021). https://doi.org/10.1007/s00277-020-04359-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-020-04359-x