Abstract
From April 2016, carfilzomib, in combination with lenalidomide and dexamethasone (KRD), became available for use in the daily practice in Italy for patients with relapsed or refractory multiple myeloma (RRMM). We performed a retrospective survey at 14 different institutions from Southern Italy in order to evaluate patient characteristics and treatment results from an unselected series of patients treated accordingly so far. One hundred and twenty-three consecutive patients were included, with a median of 2 previous lines of therapy (range 1–9) and a median age of 63 years (range 39–82). At the time of analysis, median number of courses administered is 11 (range 1–34), and all patients are evaluable for response. Overall response rate including complete remission, very good partial remission, and partial remission is 85%. After a median follow-up of 27 months, median overall and progression-free survival are 33 and 23 months, respectively. Sixty-three patients are alive and between them, 45 (37%) are in continuous remission. Sixty patients have died (49%), mainly from progressive disease. There were 6 treatment-related deaths (5% of the whole patient population). Overall, hematological and non-hematological toxicity were manageable, mostly on outpatient basis. Arterial hypertension has been observed in 43 cases (35%) but did not lead to treatment interruption. Our data demonstrate that in real life, KRD is highly effective and well tolerated in the majority of patients with RRMM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the recent years, several new drugs have become available for use in routine practice in the setting of relapsed or refractory multiple myeloma (RRMM) [1,2,3]. Data leading to approval derive from large prospective randomized trials, in which conspicuous numbers of patients are enrolled and evaluated. Nonetheless, it is well known that patient population in clinical trials may differ from the real-world setting both in patient and disease characteristics, due to restrictive inclusion and exclusion criteria. That is why knowledge deriving from post-approval surveys in unselected patients treated outside registration trials can corroborate and implement previous data [4]. In turn, potential biases like center and patient selection and accuracy of collected data must be taken into account when evaluating retrospective data.
Following the publication of data from the Aspire trial [5], carfilzomib, an irreversible second-generation proteasome inhibitor [6, 7], was approved for patients with RRMM in combination with lenalidomide and dexamethasone (KRD) in USA and Europe [8], and from April 2016, it was available in Italy for such indication.
In this retrospective survey, we report clinical characteristics and therapeutic results in a cohort of 123 consecutive patients treated with KRD in 14 different hematologic institutions from Southern Italy.
Materials and methods
For the purpose of this retrospective survey and with approval of local independent ethical committee (n.10/2019), a database was created with main clinical characteristics of the patients at diagnosis and at the time of KRD start, including previous therapy history, response to KRD, toxicity, and survival. The necessity of dose reductions and number of accesses to clinic during KRD was also recorded. The study was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent for data processing was obtained from all patients and, when applicable, for the purpose of this analysis.
All the 12 Hematologic Divisions of Campania region in Southern Italy participated in the survey, plus 2 Hematologic Divisions of Calabria region. All patients with RRMM treated with KRD in the period of observation were included in the survey, while patients treated with KD only were excluded.
Treatment schedule was as follows: programmed eighteen 28-day cycles; intravenous carfilzomib on days 1, 2, 8, 9, 15, and 16 of each cycle (starting dose: 20 mg/sqm on days 1 and 2 of cycle 1; target dose 27 mg/sqm thereafter) from cycle 1 to 12, and on days 1, 2, 15, and 16 from cycle 13 to 18; oral lenalidomide (25 mg) from day 1 to 21 of each cycle; dexamethasone at two possible different schedules, according to clinician choice: 40 mg on days 1, 8, 15, and 22; or 20 mg on days 1, 2, 8, 9, 15, 16, 22, and 23. After completion of 18 courses, carfilzomib could be stopped and lenalidomide and dexamethasone continued, according to original Aspire Trial [5], or continued along with the other two drugs in compliant patients until progression or unacceptable toxicity, depending on clinician choice and in accordance with AIFA (Agenzia Italiana del Farmaco) indications.
Blood pressure was routinely evaluated at baseline and while on treatment at every infusion on carfilzomib. Echocardiogram with evaluation on left ventricular ejection fraction (L-VEF) was performed in every patient before start of KRD, including those with baseline normal blood pressure. In all patients on KRD, antibacterial, antiviral, and antithrombotic prophylaxis according to local standard was prescribed.
Results
The survey includes patients that started therapy with KRD from April 2016 to April 2018. Data cut-off for last follow up was May 2020. Main clinical characteristics are summarized in Table 1. A total of 123 consecutive patients (74 males and 49 females) have been evaluated. More in detail, 3 of the participating centers contributed with more than 10 patients each; 9 centers with 3 to 10 patients each; and the remaining 2 centers with 2 and 1 patient, respectively. Median age at diagnosis was 60 years (range 34–81). At the time of KRD beginning, median age was 63 years (range 39–82), with 33 patients (27%) aged over 65 years. Median number of previous lines of therapy was 2 (range 1–9), and autologous stem cell transplantation (ASCT) had been formerly performed in 78 patients (63%). Many different regimens including chemotherapy and/or new agents were administered during previous treatments. In particular, lenalidomide and bortezomib had already been used in 54 (44%) and 119 (97%) cases, respectively. No patient had been treated with carfilzomib before. Status of MM at the beginning of KRD was biochemical relapse, symptomatic relapse, and refractory to last therapy in 18 (15%), 57 (46%), and 48 (39%) cases, respectively. FISH analysis data were available in few patients and are not shown. Baseline echocardiogram showed mild and not clinically significant anomalies in 10 (8%) of patients; median L-VEF evaluated at baseline was 60% (range 50–67); no cases of pre-existing cardiac amyloidosis were found. Dexamethasone starting schedule used was 20 mg twice weekly in 73 patients, and 40 mg once weekly in the remaining 50 patients.
Median number of cycles administered so far is 11 (range 1–34). All patients are evaluable for response. We obtained an overall response rate (ORR) of 85%. More in detail, complete remission (CR) was achieved in 30 patients (24%) after a median of 5 cycles (range 2–12). Very good partial response (VGPR) and partial response (PR) were obtained in 50 (41%) and 25 (20%) cases, respectively. Finally, 4 patients (3%) remained in stable disease (SD) and 14 patients (11%) were refractory to KRD.
At last follow up recorded, 98 patients (80%) have discontinued the treatment for the following reasons: normal termination of the programmed 18 KRD cycles: 18 patients (in 16 cases treatment was continued with RD only, which was programmed until progression or unacceptable toxicity; in 2 cases, lenalidomide had previously been stopped for toxicity); consolidation with ASCT after a median of 4 cycles (range 3–11): 6 patients; relapse or progression during KRD: 36 patients; no response: 14 patients; toxicity: 19 patients; other neoplasia: 3 patients; own decision: 2 patients. Of note, 18 patients have continued KRD also after completion of the 18 cycles scheduled according to original ASPIRE design, while among the 6 patients consolidated with ASCT after KRD, 2 of them started over KRD 2 months after recovery from transplantation as prolonged continuous treatment.
Neutropenia WHO grade ≥ 2 was observed in 29 cases (24%) and use of G-CSF was necessary at least once in 24 patients. Anemia grade ≥ 2 was already present in 34 patients at the beginning of KRD, while in 22 cases (18%), it occurred during treatment, leading to necessity of RBC tranfusions for at least one time in 5 of them. Thrombocytopenia grade ≥ 3 occurred in 16 patients (13%), with PLT transfusion requirement in 3 cases. Main non-hematologic toxicity included arterial hypertension grade ≥ 2 recorded for at least one episode in 43 patients (35%), and managed according to cardiologist indications with addition or change of antihypertensive drugs, in no case leading to discontinuation of therapy; other cardiologic toxicity in 7 cases (N = 1 myocardial infarction in a patient with previous cardiomyopathy and uncontrolled diabetes; N = 1 atrial fibrillation; N = 3 heart failures; N = 1 cardiac arrest, resolved with electric defibrillation, in a patient with recent episode of pulmonary thromboembolism; N = 1 reduction of L-VEF, which finally resulted as consequence of secondary cardiac amyloidosis); FUO or infection, present for at least one episode, in 36 patients; thrombosis, recorded in 15 cases (12%; more in detail; we observed 2 cases of pulmonary thromboembolism, and 13 cases of deep vein thrombosis); hemoptysis resulted in death in one severely thrombopenic patient; tumor lysis syndrome in one patient. Occurrence of other primary malignancies was observed in 4 patients, in 3 cases while on KRD, and in 1 case during lenalidomide continuous treatment (one fatal cholangiocarcinoma; one fatal pancreatic adenocarcinoma; one esophageal carcinoma; one recurrence of previously treated prostatic carcinoma). Finally, no relevant toxicity at all was recorded in 43 patients (35%). Toxicity findings are summarized in Table 2. Delay to subsequent course administration was recorded in 50 patients (41%), while reduction of doses was needed in 57 patients: in detail, carfilzomib was reduced to 20 mg/sqm in a total of 22 cases (8 from the beginning for poor performance status and/or advanced age, 16 during treatment; lenalidomide in 27 cases (8 from the beginning, mainly for renal insufficiency, and 19 during treatment); and dexamethasone in 26 cases for hyperglycemia and/or patient intolerance. Regarding access to hospital during first 18 KRD courses, an overall medium 0.63 additional visits not related to drug infusion per cycle per patient have been recorded. After splitting our cases in subgroups according to chronological period of treatment (patient 1 to 50, 51 to 100, and 101 to 123), we observed that medium additional visits were 0.76, 0.51, and 0.3, respectively. Hospitalization for KRD administration was needed in one patient only, due to poor MM-related performance status.
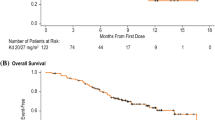
At the time of writing, after a median follow up of 27 months from the beginning of KRD (range 2–50), 37 out of 105 responding patients have relapsed (35%); between all patients requiring subsequent line of treatment; in 15 cases, it was not possible to start any therapy for MM because of poor performance status and/or very aggressive course of disease; above all, 63/123 (51%) patients are alive, and between them, 45/123 (37%) are in continuous remission; 60/123 patients (49%) have died. More frequent cause of death was progressive disease (49 cases). In the remaining 11 cases, there were N = 5 sepsis, N = 2 s neoplasia, N = 1 hemoptysis, N = 1 progression of renal failure, and N = 2 sudden death of unknown reason (both in patients with controlled disease). Median overall survival (OS) is 33 months and progression-free survival (PFS) is 23 months, as shown in Fig. 1. We also evaluated PFS according to various different factors, and in particular in Fig. 2, PFS according to number of previous lines of treatment, previous use of lenalidomide, age, and status of disease at the beginning of KRD are shown. Statistically significant difference (p 0.018) was found when comparing age (≤ 65 vs > 65 years).
Progression-free survival according to different factors. A PFS by number of previous lines of therapy (blue: 1 line; green: 2 lines; yellow: ≥3 lines). B PFS by previous exposure to lenalidomide (blue: yes; green: no). C PFS by age (blue: ≤ 65 years; green: > 65 years). D PFS by disease status at KRD (blue: relapse; green: refractory to previous treatment)
Discussion
In recent years, a large number of new drugs with new and different mechanism of action have been tested in the setting of MM [1, 2]. In particular, carfilzomib has demonstrated a significant clinical benefit in patients with RRMM, in combination with either dexamethasone or lenalidomide and dexamethasone [5, 9]. Results deriving from experimental trials demonstrated significant advantage in terms of response rate and PFS, and approval of KRD in the routine practice represented a turning point in the treatment of RRMM. In the last months, in order to confirm clinical results in the real-life setting, some single or multi-center experiences with KRD have been presented, mainly as abstracts [10,11,12,13,14,15,16]. To our knowledge, our survey is one of the largest KRD series in terms of number of patients evaluated.
First of all, considering the number of consecutive patients included in our survey (123 patients during a 24-months period), we can assert that at our institutions’ KRD has rapidly become a standard of care in RRMM at least in patients under 70 years of age (median age 63 years in our series).
Therapeutic results observed in this analysis are encouraging, taking into account that ours is a highly unselected real-life population of RRMM. ORR is 85%, which is a similar rate as compared to the ASPIRE study [5] (87%) and other real-life data. Median PFS in our population is 21 months, and this suggests a possible correlation with programmed interruption of carfilzomib after 18 cycles, as we will discuss later in the section. OS is 33 months, which is less than that observed in the ASPIRE study (48 months). Our speculation is that the aforementioned unselected nature of our survey and a not irrelevant proportion of patients that could not be given any further treatment may have accounted for a significant role in this result.
We performed subgroup analysis in order to search any parameter possibly related to efficacy of treatment. PFS was statistically different between patients aged ≤ 65 or > 65 years (28 vs 16 months, p 0.018). We were not able to find any other significant differences according to selected patient or disease characteristics, including number and type of previous therapy lines, in particular lenalidomide-based ones. Nevertheless, we have to stress that these results may be partly affected by the relative small number of patients and the extreme heterogeneity of our population and have to be interpreted very cautiously. Overall, toxicity was well manageable. In particular, 19 patients (15%) stopped KRD due to side effects, leading to death in 5 cases, but this figure can be expected following any treatment in such category of patients. Hematologic toxicity grade ≥ 2 was recorded in approximately one-third of patients and easily managed with adequate support. Arterial hypertension is a well-known and peculiar toxicity that can occur during carfilzomib treatment and needs to be routinely and carefully monitored [17]. In our survey, we recorded at least one episode of hypertension in 43 patients (35%); 31/43 (72%) had a previous known history of hypertension, while in the other 12/43 cases (28%), hypertension occurred for the first time after carfilzomib infusion; permanent addition or change of antihypertensive drugs was necessary in 16/43 (37%); in no case, hypertension led to discontinuation of therapy; nearly double rate of hypertension in our population as compared to ASPIRE trial (14.3%) could be possibly explained by the unselected nature of our patients. Thrombosis was recorded in 15 patients (12%), and this is comparable to most published data of patients treated with lenalidomide, meaning that addition of carfilzomib do not seem to increase the risk. Finally, no toxicity at all was documented in 35% of patients, suggesting that in a relevant proportion of cases, KRD treatment can be easily administered without any further complications also in the real-life setting. Once again, due to the number and heterogeneity of our group, we were not able to demonstrate any predisposing factor for different types of toxicity.
In our population, reduction of at least one drug was necessary in 57 patients (46%). More precisely, the rates of reduction of carfilzomib in our survey and in the original ASPIRE trial were 17% vs 11%, while those of lenalidomide were 21% vs 43.4%, respectively. The higher reduction rate of carfilzomib could be possibly related to a less selected population while the lower reduction rate of lenalidomide to a significantly shorter follow up.
According to clinician choice, patients were treated with two different schedules of dexamethasone (20 mg twice weekly in 73 patients, 40 mg once weekly in the remaining 50 patients). We found no difference either in response rate or in necessity of dexamethasone reduction between the two groups.
In our survey, 6 patients did stop KRD after a median of 4 cycles and underwent mobilization of stem cells followed by ASCT [18, 19]. In no case, there was excessive or unexpected toxicity from ASCT, confirming that in RRMM, KRD can be safely used as part of a therapeutic program that includes a subsequent consolidation with ASCT. Such program can be preferentially proposed to selected patients with prolonged duration of remission after a previous transplantation procedure. At the moment of the last evaluation, two patients started over KRD and are in continuous remission, two are in observation only and still maintaining remission, while two have relapsed.
In the original trial, KRD treatment plan contemplated interruption of carfilzomib after 18 cycles. In our population, 18 patients actually stopped carfilzomib after the initially programmed 18 courses. After the publication of updated data from ASPIRE trial [20] which suggested a possible benefit of continuation of carfilzomib therapy after the 18th cycle, in some centers, it was decided to keep on treatment responding and complying patients with all three drugs continuously until progression or unacceptable intolerance. At the moment of data cut-off, a total of 18 responding patients are continuing KRD, with no further toxicity, but patients are too few to evaluate any improvement of results.
We evaluated number of accesses to clinic needed for every patient during KRD treatment. Worthy of note, the number of additional visits not related to carfilzomib infusions decreased during time from 0.76 to 0.3 per cycle per patient, and this is mainly related to the experience that each institution has gained during time. In addition, confirmation of preliminary data on efficacy and tolerability of a weekly infusion of carfilzomib 70 mg/sqm [21, 22] could further reduce hospital accesses, substantially improving patient quality of life and work schedule for nurses and doctors.
In conclusion, our data confirm that KRD combination is effective and safe in patients with RRMM, including those previously given lenalidomide. Data from real life on ORR, survival, and toxicity are in line with those described in the registration trial and strongly encourage the use of this combination. What remains uncertain, given the recent availability of other highly effective combinations for RRMM, namely, daratumumab, lenalidomide, and dexamethasone [23], is how to select among different options on individual basis.
References
Jagannath S, Abonour R, Durie BGM, Gasparetto C, Hardin JW, Narang M, Terebelo HR, Toomey K, Wagner L, Srinivasan S, Kitali A, Yue L, Flick ED, Agarwal A, Rifkin RM (2018) Heterogeneity of second-line treatment for patients with multiple myeloma in the connect MM registry (2010-2016). Clin Lymphoma Myeloma Leuk 18(7):480–485
Anderson KC (2016) Progress and paradigms in multiple myeloma. Clin Cancer Res 22(22):5419–5427
Agarwal A, Chow E, Bhutani M, Voorhees PM, Friend R, Usmani SZ (2017) Practical considerations in managing relapsed multiple myeloma. Clin Lymphoma Myeloma Leuk 17(2):69–77
Mohty M, Terpos E, Mateos MV, Cavo M, Lejniece S, Beksac M, Bekadja MA, Legiec W, Dimopoulos M, Stankovic S, Durán MS, de Stefano V, Corso A, Kochkareva Y, Laane E, Berthou C, Salwender H, Masliak Z, Pečeliūnas V, Willenbacher W, Silva J, Louw V, Nemet D, Borbényi Z, Abadi U, Pedersen RS, Černelč P, Potamianou A, Couturier C, Feys C, Thoret-Bauchet F, Boccadoro M, Bekadja M, Hamladji RM, Ali HA, Hamdi S, Touhami H, Mansour NS, Willenbacher W, Linkesch W, Nemet D, Pedersen RS, Abildgaard N, Laane E, Hein M, Mohty M, Eveillard JR, Yamani A, Moreau P, Sanhes L, Lepeu G, Laribi K, Jourdan E, Fitoussi O, Allangba O, Fleury J, Escoffre M, Benramdane R, Cartron G, Dine G, Legouffe E, Harich HD, Illmer T, Dörfel S, Hannig CV, Koenigsmann M, Prange-Krex G, Salwender H, Tamm I, Zeller W, Maasberg M, Schlag R, Klausmann M, Uhlig J, Alkemper B, Schütz S, Tessen HW, Mohr B, Schmidt P, Heinrich B, Hebart H, Seipelt G, Zoeller T, Heits F, Müller-Naendrup C, Hansen R, Repp R, von Weikersthal LF, Schmits R, Heßling J, Krammer-Steiner B, Janzen V, Schauer M, Grüner MW, Kisro J, Denzlinger C, Freier W, Junghanss C, Görner M, Laichinger K, Ostermann H, Dürk H, Hess G, Reich G, Terpos E, Dimopoulos M, Matsouka P, Pouli A, Anagnostopoulos A, Masszi T, Borbényi Z, Ivanyi J, Szomor A, Abadi U, Nagler A, Magen H, Avivi I, Quitt M, Palumbo A, Boccadoro M, de Stefano V, Za T, Vallisa D, Foa R, Corso A, Bosi A, Vacca A, Lanza F, Palazzo G, Avvisati G, Cavo M, Ferrara F, Consoli U, Cantonetti M, Angelucci E, Califano C, di Raimondo F, Guarini A, Musso M, Pizzuti M, Giuliani N, Ardizzoia A, di Renzo N, Gaidano G, Gozzetti A, Pitini V, Farina G, Centurioni R, de Fabritiis P, Iuliano F, la Nasa G, la Verde G, Pane F, Recine U, la Targia M, Mineo G, Cangialosi C, Fagnani D, Federici A, Romano A, Specchia G, Storti S, Bongarzoni V, Bacigalupo A, Gobbi M, Latte G, Mannina D, Capalbo S, Lejniece S, Pečeliūnas V, Jurgutis M, Stankovic S, Legiec W, Woszczyk D, Hołojda J, Gornik S, Pluta A, Morawiec-Szymonik E, Kyrcz-Krzemien S, Homenda W, Grosicki S, Sulek K, Lange A, Kloczko J, Starzak-Gwozdz J, Hellmann A, Komarnicki M, Kuliczkowski K, Viveiros C, Gonçalves C, Esefyeva N, Kochkareva J, Kaplanov K, Volodicheva E, Laricheva E, Dergacheva V, Chukavina M, Volchenko N, Nazarova I, Anchukova L, Ovanesova E, Gritsenko T, Salogub G, Magomedova L, Kuznetsova I, Osyunikhina S, Serdyuk O, Karyagina E, Ivanova V, Černelč SP, Louw V, Coetzee C, Gunther K, Moodley D, Duran S, Gutiérrez AE, de Oteyza JP, Capote FJ, Casanova M, Sanchez JM, Rios-Herranz E, Ibañez-Garcia J, Herranz MJ, Hernandez B, Sanchez SS, Escalante F, Carnicero F, Lleonart JB, Gironella M, Martínez R, de la Guia AL, Palomera L, Iglesias R, Ramos FS, de la Serna J, Sanchez PG, Vidal JB, Mateos MV, Morfa MD, Beksac T–M, Vural F, Aydin Y, Unal A, Goker H, Bilgir O, Guvenc B, Turgut M, Ozet GG, Ali R, Masliak Z, Kyselyova M, Glushko N, Vybyrana R, Skrypnyk I, Tretyak N, Kharchevska T, Dyagil I, Popovs'ka T, Shimanskiy V, Lysa T, Oliynyk H, Vilchevskaya K, Kryachok I, Popovych Y, Romanyuk N, Yushchenko N, Kaplan P, Rekhtman G, Pylypenko H, Kozlov V, Mohty M, Terpos E, Mateos MV, Palumbo A, Drach J, Boccadoro M, Harousseau JL, Einsele H, Goldschmidt H, Facon T, Michalet M, Savchenko VG, de la Rubia J, Cook G, Mellqvist UH, Ludwig H (2018) Multiple myeloma treatment in real-world clinical practice: results of a prospective, multinational, noninterventional study. Clin Lymphoma Myeloma Leuk 18(10):e401–e419. https://doi.org/10.1016/j.clml.2018.06.018
Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Špička I, Oriol A, Hájek R, Rosiñol L, Siegel DS, Mihaylov GG, Goranova-Marinova V, Rajnics P, Suvorov A, Niesvizky R, Jakubowiak AJ, San-Miguel JF, Ludwig H, Wang M, Maisnar V, Minarik J, Bensinger WI, Mateos MV, Ben-Yehuda D, Kukreti V, Zojwalla N, Tonda ME, Yang X, Xing B, Moreau P, Palumbo A, ASPIRE Investigators (2015) Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med 372(2):142–152
Niesvizky R, Martin TG III, Bensinger WI et al (2013) Phase Ib dose-escalation study (PX-171-006) of carfilzomib, lenalido- mide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Clin Cancer Res 19:2248–2256
Wang M, Martin T, Bensinger W, Alsina M, Siegel DS, Kavalerchik E, Huang M, Orlowski RZ, Niesvizky R (2013) Phase 2 dose-expansion study (PX-171- 006) of carfilzomib, lenalidomide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Blood 122:3122–3128
Tzogani K, Camarero Jiménez J, Garcia I, Sancho-López A, Martin M, Moreau A, Demolis P, Salmonson T, Bergh J, Laane E, Ludwig H, Gisselbrecht C, Pignatti F (2017) The European medicines agency review of carfilzomib for the treatment of adult patients with multiple myeloma who have received at least one prior therapy. Oncologist 22(11):1339–1346
Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hájek R, Facon T, Ludwig H, Oriol A, Goldschmidt H, Rosiñol L, Straub J, Suvorov A, Araujo C, Rimashevskaya E, Pika T, Gaidano G, Weisel K, Goranova-Marinova V, Schwarer A, Minuk L, Masszi T, Karamanesht I, Offidani M, Hungria V, Spencer A, Orlowski RZ, Gillenwater HH, Mohamed N, Feng S, Chng WJ, ENDEAVOR Investigators (2016) Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol 17(1):27–38
Muchtar E, Gatt ME, Rouvio O, Ganzel C, Chubar E, Suriu C, Tadmor T, Shevetz O, Lavi N, Shochat T, Cohen YC, Avivi I, Raanani P, Magen H (2016) Efficacy and safety of salvage therapy using carfilzomib for relapsed or refractory multiple myeloma patients: a multicentre retrospective observational study. Br J Haematol 172(1):89–96
Rifkin RM, Medhekar E, Amirian SE et al (2019) A real-world comparative analysis of carfilzomib and other systemic multiple myeloma chemotherapies in a US community oncology setting. Ther Adv Hematol 10:1–10
Antonioli E, Staderini M, Nozzoli C et al (2017) “Real-life” experience of carfilzomib combined with lenalidomide and dexamethasone in relapsed/refractory multiple myeloma patients. Haematologica 102(s3):131–132
Barilà G, Meneghini V, Bonalumi A et al (2018) KRD treatment of relapsed/refractory multiple myeloma patients: a real life experience. Hemasphere 2(s1):978–979
Calafiore V, Martino E, Parisi M et al (2018) Efficacy, safety and tolerability of carfilzomib- lenalidomide-dexamethasone (KRD) regimen in RR/MM. Haematologica 103(s1):35–36
Guidotti F, Ferla V, Gregorini AI, Rossi FG, Pompa A (2018) Carfilzomib-lenalidomide-dexamethasone in relapsed/refractory multiple myeloma: a single centre real-life experience. Haematologica 103(s1):38
Terpos E, Caers J, Sohne M et al (2018) Real-world evidence of the use of carfilzomib among patients with relapsed multiple myeloma in Europe: an interim analysis for a prospective observational study. Hemasphere 2(s1):962
Dimopoulos MA, Roussou M, Gavriatopoulou M, Psimenou E, Ziogas D, Eleutherakis-Papaiakovou E, Fotiou D, Migkou M, Kanellias N, Panagiotidis I, Ntalianis A, Papadopoulou E, Stamatelopoulos K, Manios E, Pamboukas C, Kontogiannis S, Terpos E, Kastritis E (2017) Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood Adv 1(7):449–454
Garderet L, Iacobelli S, Koster L, Goldschmidt H, Johansson JE, Bourhis JH, Krejci M, Leleu X, Potter M, Blaise D, Koenecke C, Peschel C, Radocha J, Metzner B, Lenain P, Schäfer-Eckart K, Pohlreich D, Grasso M, Caillot D, Einsele H, Ladetto M, Schönland S, Kröger N (2018) Outcome of salvage third autologous stem cell transplantation in multiple myeloma. Biol Blood Marrow Transplant 24(7):1372–1378
Hagen PA, Stiff P (2018) The role of salvage second autologous hematopoietic cell transplantation in relapsed multiple myeloma. Biol Blood Marrow Transplant 25:e98–e107. https://doi.org/10.1016/j.bbmt.2018.12.002
Dimopoulos M, Wang M, Maisnar V, Minarik J, Bensinger W, Mateos MV, Obreja M, Blaedel J, Moreau P (2018) Response and progression-free survival according to planned treatment duration in patients with relapsed multiple myeloma treated with carfilzomib, lenalidomide, and dexamethasone (KRd) versus lenalidomide and dexamethasone (Rd) in the phase III ASPIRE study. J Hematol Oncol 11(1):49. https://doi.org/10.1186/s13045-018-0583-7
Moreau P, Mateos MV, Berenson JR, Weisel K, Lazzaro A, Song K, Dimopoulos MA, Huang M, Zahlten-Kumeli A, Stewart AK (2018) Once weekly versus twice weekly carfilzomib dosing in patients with relapsed and refractory multiple myeloma (A.R.R.O.W.): interim analysis results of a randomised, phase 3 study. Lancet Oncol 19(7):953–964
Bringhen S, Mina R, Cafro AM, Liberati AM, Spada S, Belotti A, Gaidano G, Patriarca F, Troia R, Fanin R, de Paoli L, Rossi G, Lombardo A, Bertazzoni P, Palumbo A, Sonneveld P, Boccadoro M (2018) Once-weekly carfilzomib, pomalidomide, and low-dose dexamethasone for relapsed/refractory myeloma: a phase I/II study. Leukemia 32(8):1803–1807
Dimopoulos MA, Oriol A, Nahi H, POLLUX Investigators et al (2016) Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 375(14):1319–1331
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Palmieri, S., Rocco, S., Vitagliano, O. et al. KRD (carfilzomib and lenalidomide plus dexamethasone) for the treatment of relapsed or refractory multiple myeloma in the real-life: a retrospective survey in 123 patients. Ann Hematol 99, 2903–2909 (2020). https://doi.org/10.1007/s00277-020-04158-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-020-04158-4