Abstract
The number of patients who are administered immunosuppressive agents has been increasing. Accordingly, more patients face higher risks for developing immunodeficiency-associated lymphoproliferative disorders (LPD). Although immunodeficiency-associated LPD are distinct from other lymphoid neoplasms in terms of their immunocompromised backgrounds, little is known about the impact of lymphopenia at diagnosis on survival in patients with these LPD. Seventy-one immunodeficiency-associated LPD in Kyoto University Hospital (post-transplant LPD (PTLD), n = 26; other iatrogenic immunodeficiency-associated LPD, n = 45) were reviewed and analyzed. The median age at diagnosis was 63 years (range, 3–83). Diffuse large B cell lymphoma was the most common subtype (n = 33), followed by Hodgkin lymphoma (n = 12), B cell monomorphic LPD not specified (n = 11), and polymorphic LPD or early-phase diseases (n = 15). The median follow-up period for survivors was 2.5 years and overall survival (OS) and progression-free survival (PFS) at 2.5 years were 75% and 67%, respectively. Multivariate analysis showed that lymphopenia (≤ 800/μL) at diagnosis predicted inferior OS (HR, 3.72; P = 0.043) and PFS (HR, 3.82; P = 0.012). Serum albumin values also strongly affected OS (> 3.18 g/dL vs. ≤ 3.18 g/dL; HR, 0.21; P = 0.010) and PFS (HR, 0.26; P = 0.013). Lymphopenia at diagnosis is suggested to predict inferior OS and PFS in patients with immunodeficiency-associated LPDs. Immunocompromised status might affect disease progression in these distinct lymphoid neoplasms growing under immunocompromised backgrounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a result of recent advances in medical care, the number of patients who receive various immunosuppressive agents has been increasing. These patients are known to be at risk for the development of immunodeficiency-associated lymphoproliferative disorders (LPD) under an iatrogenic immunocompromised status [1,2,3]. Although aberrant infection of Epstein-Barr virus (EBV) in immunosuppressed lymphocytes has been suggested to play a key role in the pathogenesis of these LPD [4,5,6,7], the overall context of this unique disease entity is not yet completely understood.
This insufficient understanding of iatrogenic immunodeficiency-associated LPD is partly attributed to their clinical, histopathological, and genetic heterogeneity [1, 8,9,10]. They include post-transplant lymphoproliferative disorders (PTLD) that arise in patients after solid organ transplantations or hematopoietic stem cell transplantation (HSCT) and other iatrogenic immunodeficiency-associated LPD that arise in patients treated with various immunosuppressive agents for any reason. They include various pathological subtypes including non-destructive hyperplasia of lymphocytes, polymorphic LPDs, and several aggressive types of malignant lymphomas. Their genetic landscapes have not yet been completely revealed. In recent studies, PTLD has been considered to consist of genetically distinct populations: EBV-related or others [6, 11,12,13] and germinal center B cell-like (GCB) or non-GCB subtype [14]. There was also a hypothesis that some subtypes of PTLD might actually be a coincidental occurrence of lymphoid neoplasms among post-HSCT patients [11, 15], although this view has not yet reached a consensus. The genetic backgrounds of other iatrogenic immunodeficiency-associated LPD such as MTX-associated LPD have been scarcely examined.
Regardless of this heterogeneity, LPD growing with an immunocompromised background are known to present worse clinical outcomes than those without such a background [1, 16, 17]. In the analysis of PTLD, the International Prognostic Index (IPI) score, hypoalbuminemia, and the treatment response for rituximab have been suggested as prognostic factors for survival [1, 16, 18, 19]. However, these prognostic factors have been less discussed in other iatrogenic immunodeficiency-associated LPDs [20, 21]. Moreover, although all these iatrogenic immunodeficiency-associated LPDs share immunocompromised backgrounds [22] with some pathological features derived from aberrant viral infection [7, 23, 24], the impact of the immunosuppressive status in each patient on clinical outcomes has rarely been assessed [25]. This should be more carefully examined since it reflects not only the patient’s morbidity but also the anti-viral or anti-tumor effects of lymphocytes.
In this study, we analyzed the impact of lymphopenia on survival in patients with iatrogenic immunodeficiency-associated LPDs. We chose total lymphocyte count as a clinical factor to evaluate patients’ immunosuppressive status since it is easy to obtain and is always examined as an index for immune reconstitution in routine practice.
Methods
Data collection
Clinical data of patients who were pathologically and clinically diagnosed with PTLD or other iatrogenic immunodeficiency-associated LPD over the past 20 years were collected from electronic medical records in Kyoto University Hospital.
Diagnosis was based on the WHO classification at the time and also reviewed according to the WHO classification of 2017 (revised 4th edition) when analyzed. Details of lymphomas and the results of blood examinations such as total lymphocyte count and serum albumin value at diagnosis were also collected from the records. Those associated with primary immune disorders or human immunodeficiency virus (HIV) infections were excluded. All patients gave their informed consent prior to their inclusion in the study. The Institutional Review Board of Kyoto University Hospital, where this study was organized, approved this study.
Statistics
The primary endpoint of this study was overall survival (OS) and the secondary endpoint was progression-free survival (PFS). OS was examined by calculating deaths from any cause; survivors at the last follow-up were censored. PFS was examined by calculating progression/relapse of LPD/lymphomas or death from any cause. Descriptive statistics were used to summarize variables related to patient characteristics. OS and PFS were evaluated by Kaplan-Meier methods and the Cox regression hazards model was used in univariate and multivariate analyses to assess the prognostic significance of the total lymphocyte count at diagnosis. Multivariate analysis was performed using covariates that were selected by preceding stepwise selection in the Cox model with a P value threshold of under 0.2. Covariates assessed were recipients’ sex, clinical background (PTLD, other iatrogenic immunodeficiency-associated LPDs), histological characteristics (monomorphic, polymorphic, or early-phase diseases), International Prognostic Index (IPI) value, primary treatment (rituximab-containing chemotherapies, other chemotherapies or focal radiation, no treatments or reduction in immunosuppressive agents), EBER positivity, serum albumin value at diagnosis, and year at diagnosis (1998–2013, 2013–2017).
Results
Patient characteristics (Table 1)
A total of 71 patients (PTLD, n = 26; other iatrogenic immunodeficiency-associated LPD, n = 45) were included, 65 of whom had data of total lymphocyte counts at the diagnosis of LPD (52–87,412/μL). The median age at transplantation was 63 years (range, 3–83) and the median follow-up period for survivors was 2.5 years. Diffuse large B cell lymphoma was diagnosed in 33 patients (PTLD, n = 12; others, n = 21), Hodgkin lymphoma in 12 (PTLD, n = 3; others, n = 9), monomorphic B cell LPD not specified in 11 (PTLD, n = 3; others, n = 7), and polymorphic LPD or early-phase diseases in 15 (PTLD, n = 7; others, n = 8). As for the initial treatment, immunosuppressive agents were reduced in 34 patients (PTLD, n = 4; others, n = 30), rituximab-containing chemotherapies were given in 23 patients (PTLD, n = 14, others, n = 9), other chemotherapies were given in 7 (PTLD, n = 4; others, n = 3), and radiation or nothing was given in 5 (PTLD, n = 4: others, n = 1). The median value of serum albumin at diagnosis was 3.18 g/dL. We set a threshold value of 800/μL (10 [9]/L) absolute lymphocyte counts as the lymphopenia definition by calculating the optimal threshold value using a receiver-operating characteristic (ROC) curve. A total of 26 patients (PTLD, n = 14; others, n = 12) were diagnosed with lymphopenia (≤ 800/μL) and 39 patients were not (PTLD, n = 11; others, n = 28).
Impact of total lymphocyte count at diagnosis on OS
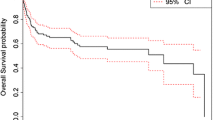
The impact of total lymphocyte count on OS was illustrated with reference to a lymphopenia group (total lymphocyte count ≤ 800 /μL at diagnosis) and a no-lymphopenia group (total lymphocyte count > 800/μL at diagnosis) (Fig. 1a). Overall, the 2.5-year OS was 74.8% (lymphopenia group, 38.8%; no-lymphopenia group, 93.1%).
Overall survival. Probability of overall survival for total patients (a), post-transplant lymphoproliferative disorders (PTLD) (b), and other iatrogenic lymphoproliferative disorders (c) with reference to a lymphopenia group (total lymphocyte count < = 800/μL at diagnosis) and a no-lymphopenia group (total lymphocyte count > 800/μL at diagnosis)
In the multivariate analysis, lymphopenia at diagnosis was associated with inferior OS (HR, 3.72; P = 0.043; Table 2). Serum albumin values (> 3.18 g/dL vs. ≤ 3.18 g/dL; HR, 0.21; P = 0.010) and high IPI (high vs. low to high-intermediate; HR, 4.37; P = 0.003) also affected the OS. A subgroup multivariate analysis to assess the impact of lymphopenia according to the clinical background showed a similar trend (Fig. 1b, c), although statistical significance was observed only in patients with other iatrogenic immunodeficiency-associated LPD (HR, 26.67; P = 0.012). Progression of lymphoma or LPD was the most common cause of death in the lymphopenia group (PTLD, n = 3; others, n = 6), followed by transplant-related mortality (PTLD, n = 5) (Table 3).
Impact of lymphopenia at diagnosis on PFS
The impact of the total lymphocyte count on PFS was illustrated with reference to a lymphopenia group and a no-lymphopenia group (Fig. 2a). Overall, the 2.5-year PFS was 67.1% (lymphopenia group, 36.7%; no-lymphopenia group, 86.6%).
Progression-free survival. Probability of progression-free survival for total patients (a), post-transplant lymphoproliferative disorders (PTLD) (b), and other iatrogenic lymphoproliferative disorders (c) with reference to a lymphopenia group (total lymphocyte count < = 800/μL at diagnosis) and a no-lymphopenia group (total lymphocyte count > 800/μL at diagnosis)
In a multivariate analysis, lymphopenia was independently associated with inferior PFS (HR, 3.82; P = 0.012; Table 4). Serum albumin values also showed a strong impact (> 3.18 g/dL vs. ≤ 3.18 g/dL; HR, 0.26; P = 0.013). Trends of inferior PFS in patients with high IPI (high vs. low to high-intermediate; HR, 3.04; P = 0.067) and superior PFS in those who received rituximab-containing chemotherapy as primary treatment (rituximab-containing chemotherapies vs. other chemotherapies; HR, 0.16; P = 0.081) were suggested. Although the non-lymphopenia group showed a trend of superior PFS (Fig. 2b, c), its statistical impact was apparent only in patients with other iatrogenic immunodeficiency-associated LPDs (HR, 9.66; P = 0.010), but not in patients with PTLD (HR, 0.66; P = 0.703) (Table 5).
Discussion
The results of our study demonstrated that lymphopenia at diagnosis may predict inferior survival in patients with immunodeficiency-associated LPD, despite its histological heterogeneity. Contrary to the expectation that this high mortality among patients with lymphopenia reflects their fragility with respect to various infections or intensive chemotherapy [21], the major cause of death was disease progression. Whereas the possibility of rituximab as a primary treatment might have had some impacts on disease suppression, the impact of lymphopenia was independently associated with a higher risk of mortality.
These results suggest that lymphopenia itself could influence disease progression among immunocompromised patients. Several biological expectations could support this hypothesis. First, tumor pathogenesis of these LPD depends partially on the underlying infection of oncoviruses such as EBV. Immunocompromised status in lymphopenia patients could progress aberrant expansion of these oncoviruses. Second, anti-tumor effects of lymphocytes are thought to be less efficient in patients with fewer lymphocytes. Studies on graft-versus-lymphoma (GVL) effects [26] or programmed cell death 1 (PD1)-programmed cell death 1-ligand 1 (PDL1) inhibition [27] have revealed that the anti-tumor effects of lymphocytes play important roles in suppressing tumor cells. As suggested in several malignant diseases [28], the total number of lymphocytes might reflect their tumor-suppressive efficacy against lymphoma cells as well, with a clear impact especially among immunocompromised patients. These considerations that follow our results could explain why some immunodeficiency-associated LPD shrink after the cessation or reduction of immunosuppressive agents [24, 29, 30]. They also support a previous suggestion that earlier recovery of lymphocytes after the cessation or reduction of immunosuppressive agents can predict a lower frequency of disease progression [31].
Possibility of rituximab application, higher IPI value, and hypoalbuminemia were reconfirmed as strong prognostic factors for overall survival in our analysis. However, similar to the results of the phase 2 PTLD-1 trial, [32] rituximab-containing chemotherapies such as R-CHOP did not dramatically improve overall survival. The investigation of risk-dependent strategies and the results of other regimens examined in ongoing clinical trials are awaited [16, 33]. Based on our hypothesis, the promotion of the anti-tumor effects of lymphocytes might be another potent strategy to improve clinical outcomes of iatrogenic immunodeficiency-associated LPD. Since frequent somatic alterations in genes encoding PD-L1/PD-L2 were suggested to contribute to the tumor pathogenesis of lymphomas associated with prior EBV infection, [34]PD1-PDL1 inhibitors could be considered as a therapeutic option in EBV-related immunodeficiency-associated LPD [35, 36]. Although more detailed investigation is warranted, modulation of tumor microenvironment should be a potent target in the treatment strategy of immunodeficiency-associated LPD including PTLD [37]. Nevertheless, it is often a big issue to improve and balance immunoreactivities of lymphocytes among patients in post-transplant status or with autoimmune diseases [38, 39]. EBV targeted cell therapies using virus-specific T-cells derived from patients’ own lymphocytes or from third party T-cells have been suggested to be an emerging option with favorable outcomes in patients with EBV-related PTLD [40,41,42,43,44]. Since similar efficacy could be expected for EBV-related immunodeficiency-associated LPD other than PTLD, there is a call for clinical trials for refractory/relapsed cases. Off-the-shelf products are awaited to broaden the application of these novel agents.
Our study has several limitations. First, this was a retrospective analysis in a small, heterogeneous population and some factors could not be collected from clinical records. As shown via the indefinite impact of lymphopenia in the subgroup analysis of patients with PTLD, the heterogeneity and small number of cases might have obscured the results of multivariate analysis. A prospective study using a larger cohort is mandatory to confirm the reproducibility of our findings.
Second, although clinically suggestive, our proposed explanation of our results has not yet been proved biologically and a more detailed biological approach is necessary. Third, lymphocyte subsets were not evaluated in this study. To discuss the anti-tumor effects of lymphocytes more in detail, evaluation of T lymphocyte subsets might be of importance.
Conclusion
Lymphopenia at diagnosis may potentially predict inferior OS and PFS in patients with immunodeficiency-associated LPDs. It might reflect the characteristics of the mechanism of disease progression for these distinct lymphoid neoplasms growing under immunocompromised backgrounds. A more detailed analysis in a larger cohort is needed to clarify the tumor pathology of these LPD and to investigate better risk-stratified treatment strategies against them.
References
Dierickx D, Tousseyn T, Gheysens O (2015) How i treat posttransplant lymphoproliferative disorders. Blood 126(20):2274–2283
Mariette X, Cazals-Hatem D, Warszawki J, Liote F, Balandraud N, Sibilia J (2002) Lymphomas in rheumatoid arthritis patients treated with methotrexate: a 3-year prospective study in France. Blood 99(11):3909–3915
Hasserjian RP, Chen S, Perkins SL, de Leval L, Kinney MC, Barry TS, Said J, Lim MS, Finn WG, Medeiros LJ, Harris NL, O'Malley DP (2009) Immunomodulator agent-related lymphoproliferative disorders. Mod Pathol 22(12):1532–1540
Chetty R, Biddolph SC, Kaklamanis L et al (1996) EBV latent membrane protein (LMP-1) and bcl-2 protein expression in Reed-Sternberg-like cells in post-transplant lymphoproliferative disorders. Histopathology 28(3):257–260
Stevens SJC, Verschuuren EAM, Pronk I, van der Bij W, Harmsen MC, The TH, Meijer CJLM, van den Brule AJC, Middeldorp JM (2001) Frequent monitoring of Epstein-Barr virus DNA load in unfractionated whole blood is essential for early detection of posttransplant lymphoproliferative disease in high-risk patients. Blood 97(5):1165–1171
Timms JM, Bell A, Flavell JR, Murray PG, Rickinson AB, Traverse-Glehen A, Berger F, Delecluse HJ (2003) Target cells of Epstein-Barr-virus (EBV)-positive post-transplant lymphoproliferative disease: similarities to EBV-positive Hodgkin’s lymphoma. Lancet 361(9353):217–223
Miyazaki T, Fujimaki K, Shirasugi Y, Yoshiba F, Ohsaka M, Miyazaki K, Yamazaki E, Sakai R, Tamaru JI, Kishi K, Kanamori H, Higashihara M, Hotta T, Ishigatsubo Y (2007) Remission of lymphoma after withdrawal of methotrexate in rheumatoid arthritis: relationship with type of latent Epstein-Barr virus infection. Am J Hematol 82(12):1106–1109
Al-Mansour Z, Nelson BP, Evens AM (2013) Post-transplant lymphoproliferative disease (PTLD): risk factors, diagnosis, and current treatment strategies. Curr Hematol Malig Rep 8(3):173–183
Evens AM, Choquet S, Kroll-Desrosiers AR, Jagadeesh D, Smith SM, Morschhauser F, Leblond V, Roy R, Barton B, Gordon LI, Gandhi MK, Dierickx D, Schiff D, Habermann TM, Trappe R (2013) Primary CNS posttransplant lymphoproliferative disease (PTLD): an international report of 84 cases in the modern era. Am J Transplant 13(6):1512–1522
Rosenberg AS, Klein AK, Ruthazer R, Evens AM (2016) Hodgkin lymphoma post-transplant lymphoproliferative disorder: a comparative analysis of clinical characteristics, prognosis, and survival. Am J Hematol 91(6):560–565
Morscio J, Dierickx D, Ferreiro JF, Herreman A, van Loo P, Bittoun E, Verhoef G, Matthys P, Cools J, Wlodarska I, de Wolf-Peeters C, Sagaert X, Tousseyn T (2013) Gene expression profiling reveals clear differences between EBV-positive and EBV-negative posttransplant lymphoproliferative disorders. Am J Transplant 13(5):1305–1316
Menter T, Dickenmann M, Juskevicius D, Steiger J, Dirnhofer S, Tzankov A (2017) Comprehensive phenotypic characterization of PTLD reveals potential reliance on EBV or NF-κB signalling instead of B-cell receptor signalling. Hematol Oncol 35(2):187–197
Menter T, Juskevicius D, Alikian M, Steiger J, Dirnhofer S, Tzankov A, Naresh KN (2017) Mutational landscape of B-cell post-transplant lymphoproliferative disorders. Br J Haematol 178(1):48–56
Vakiani E, Basso K, Klein U, Mansukhani MM, Narayan G, Smith PM, Murty VV, Dalla-Favera R, Pasqualucci L, Bhagat G (2008) Genetic and phenotypic analysis of B-cell post-transplant lymphoproliferative disorders provides insights into disease biology. Hematol Oncol 26(4):199–211
Margolskee E, Jobanputra V, Jain P, Chen J, Ganapathi K, Nahum O, Levy B, Morscio J, Murty V, Tousseyn T, Alobeid B, Mansukhani M, Bhagat G (2016) Genetic landscape of T- and NK-cell post-transplant lymphoproliferative disorders. Oncotarget 7(25):37636–37648
DeStefano CB, Desai SH, Shenoy AG, Catlett JP (2018) Management of post-transplant lymphoproliferative disorders. Br J Haematol 182(3):330–343
Hoshida Y, Xu JX, Fujita S, Nakamichi I, Ikeda J, Tomita Y, Nakatsuka S, Tamaru J, Iizuka A, Takeuchi T, Aozasa K (2007) Lymphoproliferative disorders in rheumatoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medication. J Rheumatol 34(2):322–331
Xu L-P, Zhang C-L, Mo X-D, Zhang XH, Chen H, Han W, Chen YH, Wang Y, Yan CH, Wang JZ, Wang FR, Zhao T, Liu YR, Liu KY, Huang XJ (2015) Epstein-Barr virus–related post-transplantation lymphoproliferative disorder after unmanipulated human leukocyte antigen haploidentical hematopoietic stem cell transplantation: incidence, risk factors, treatment, and clinical outcomes. Biol Blood Marrow Transplant 21(12):2185–2191
Montanari F, Radeski D, Seshan V, Alobeid B, Bhagat G, O’Connor OA (2015) Recursive partitioning analysis of prognostic factors in post-transplant lymphoproliferative disorders (PTLD): a 120 case single institution series. Br J Haematol 171(4):491–500
Lam GY (2015) Lymphoproliferative disorders in inflammatory bowel disease patients on immunosuppression: lessons from other inflammatory disorders. World J Gastrointest Pathophysiol 6(4):181
Trusson R, Serre JE, Szwarc I, Brunot V, Garrigue V, Delmas S, Kanouni T, Cartron G, Mourad G (2016) Treatment response and outcomes in post-transplantation lymphoproliferative disease vs lymphoma in Immunocompetent patients. Transplant Proc 48(6):1927–1933
Balandraud N, Meynard JB, Auger I, Sovran H, Mugnier B, Reviron D, Roudier J, Roudier C (2003) Epstein-Barr virus load in the peripheral blood of patients with rheumatoid arthritis: accurate quantification using real-time polymerase chain reaction. Arthritis Rheum 48(5):1223–1228
Baecklund E, Backlin C, Iliadou A, Granath F, Ekbom A, Amini RM, Feltelius N, Enblad G, Sundström C, Klareskog L, Askling J, Rosenquist R (2006) Characteristics of diffuse large B cell lymphomas in rheumatoid arthritis. Arthritis Rheum 54(12):3774–3781
Niitsu N, Okamoto M, Nakamine H, Hirano M (2010) Clinicopathologic correlations of diffuse large B-cell lymphoma in rheumatoid arthritis patients treated with methotrexate. Cancer Sci 101(5):1309–1313
Zimmermann H, Babel N, Dierickx D, Morschhauser F, Mollee P, Zaucha JM, Dreyling MH, Dührsen U, Reinke P, Verhoef G, Subklewe M, Hüttmann A, Tousseyn T, Bachy E, Hauser IA, Tarella C, van den Neste E, Gheysens O, Anagnostopoulos I, Leblond V, Riess H, Choquet S, Trappe RU (2018) Immunosuppression is associated with clinical features and relapse risk of B cell posttransplant lymphoproliferative disorder. Transplantation 102(11):1914–1923
Grigg A, Ritchie D (2004) Graft-versus-lymphoma effects: clinical review, policy proposals, and immunobiology. Biol Blood Marrow Transplant 10(9):579–590
Okazaki T, Honjo T (2007) PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol 19(7):813–824
Ménétrier-Caux C, Ray-Coquard I, Blay J-Y, Caux C (2019) Lymphopenia in cancer patients and its effects on response to immunotherapy: an opportunity for combination with cytokines? J Immunother Cancer 7(1):85
Baird RD, Van Zyl-Smit RN, Dilke T, Scott SE, Rassam SMB (2002) Spontaneous remission of low-grade B-cell non-Hodgkin’s lymphoma following withdrawal of methotrexate in a patient with rheumatoid arthritis: case report and review of the literature. Br J Haematol 118(2):567–568
Salloum E, Cooper DL, Howe G, Lacy J, Tallini G, Crouch J, Schultz M, Murren J (1996) Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rheumatoid arthritis and other rheumatic diseases. J Clin Oncol 14(6):1943–1949
Inui Y, Matsuoka H, Yakushijin K, Okamura A, Shimada T, Yano S, Takeuchi M, Ito M, Murayama T, Yamamoto K, Itoh T, Aiba K, Minami H (2015) Methotrexate-associated lymphoproliferative disorders: management by watchful waiting and observation of early lymphocyte recovery after methotrexate withdrawal. Leuk Lymphoma 56(11):3045–3051
Trappe R, Oertel S, Leblond V, Mollee P, Sender M, Reinke P, Neuhaus R, Lehmkuhl H, Horst HA, Salles G, Morschhauser F, Jaccard A, Lamy T, Leithäuser M, Zimmermann H, Anagnostopoulos I, Raphael M, Riess H, Choquet S, German PTLD Study Group, European PTLD Network (2012) Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol 13(2):196–206
DeStefano CB, Malkovska V, Rafei H et al (2017) DA-EPOCH-R for post-transplant lymphoproliferative disorders. Eur J Haematol 99(3):283–285
Kataoka K, Miyoshi H, Sakata S, Dobashi A, Couronné L, Kogure Y, Sato Y, Nishida K, Gion Y, Shiraishi Y, Tanaka H, Chiba K, Watatani Y, Kakiuchi N, Shiozawa Y, Yoshizato T, Yoshida K, Makishima H, Sanada M, Onozawa M, Teshima T, Yoshiki Y, Ishida T, Suzuki K, Shimada K, Tomita A, Kato M, Ota Y, Izutsu K, Demachi-Okamura A, Akatsuka Y, Miyano S, Yoshino T, Gaulard P, Hermine O, Takeuchi K, Ohshima K, Ogawa S (2019) Frequent structural variations involving programmed death ligands in Epstein-Barr virus-associated lymphomas. Leukemia 33(7):1687–1699
Kinch A, Sundström C, Baecklund E, Backlin C, Molin D, Enblad G (2019) Expression of PD-1, PD-L1, and PD-L2 in posttransplant lymphoproliferative disorder after solid organ transplantation. Leuk Lymphoma 60(2):376–384
Miyoshi H, Kiyasu J, Kato T, Yoshida N, Shimono J, Yokoyama S, Taniguchi H, Sasaki Y, Kurita D, Kawamoto K, Kato K, Imaizumi Y, Seto M, Ohshima K (2016) PD-L1 expression on neoplastic or stromal cells is respectively a poor or good prognostic factor for adult T-cell leukemia/lymphoma. Blood 128(10):1374–1381
Marcelis L, Tousseyn T (2019) The tumor microenvironment in post-transplant lymphoproliferative disorders. Cancer Microenviron 12(1):3–16
Ashrafi F, Shahidi S, Ebrahimi Z, Mortazavi M (2015) Outcome of rapamycin therapy for post-transplant-lymphoproliferative disorder after kidney transplantation: case series. Int J Hematol Stem Cell Res 9(1):26–32
Crane GM, Powell H, Kostadinov R, Rocafort PT, Rifkin DE, Burger PC, Ambinder RF, Swinnen LJ, Borowitz MJ, Duffield AS (2015) Primary CNS lymphoproliferative disease, mycophenolate and calcineurin inhibitor usage. Oncotarget 6(32):33849–33866
Burns DM, Crawford DH (2004) Epstein-Barr virus-specific cytotoxic T-lymphocytes for adoptive immunotherapy of post-transplant lymphoproliferative disease. Blood Rev 18(3):193–209
Ricciardelli I, Blundell MP, Brewin J, Thrasher A, Pule M, Amrolia PJ (2014) Towards gene therapy for EBV-associated posttransplant lymphoma with genetically modi fied EBV-specific cytotoxic T cells. Blood 124(16):2514–2523
Barker JN, Doubrovina E, Sauter C, Jaroscak JJ, Perales MA, Doubrovin M, Prockop SE, Koehne G, O'Reilly RJ (2010) Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood 116(23):5045–5049
Bollard CM, Savoldo B, Rooney CM, Heslop HE (2003) Adoptive T-cell therapy for EBV-associated post-transplant lymphoproliferative disease. Acta Haematol 110(2–3):139–148
Bollard CM, Rooney CM, Heslop HE (2012) T-cell therapy in the treatment of post-transplant lymphoproliferative disease. Nat Rev Clin Oncol 9(9):510–519
Acknowledgments
We are grateful to Emi Furusaka, Tomoko Okuda, and Maki Shindo for their expert data management and secretarial assistance. We also thank all the members of our clinical teams at Kyoto University Hospital for their dedicated care of the patients and their collaborative support.
Funding
This work was funded in part by the Takeda Science Foundation (JK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Article Summary
Little is known about the impacts of immunocompromised status on clinical outcomes in patients with immunodeficiency-associated LPD. We found that lymphopenia at the diagnosis of LPDs could be a novel predictive factor for inferior OS and PFS in these patients.
Rights and permissions
About this article
Cite this article
Watanabe, M., Kanda, J., Hishizawa, M. et al. Lymphopenia at diagnosis predicts survival of patients with immunodeficiency-associated lymphoproliferative disorders. Ann Hematol 99, 1565–1573 (2020). https://doi.org/10.1007/s00277-020-04084-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-020-04084-5