Abstract
Sickle cell anemia (SCA) is the most severe form of sickle cell disease caused by homozygosity of the βS-gene (S/S or βSβS) and has worldwide distribution. Six polymorphic sites in the β-globin gene cluster were analyzed from a sample of 56 chromosomes of patients with SCA from the state of Maranhão, northeastern Brazil. PCR-RFLP showed that the CAR haplotype was predominant with a frequency of 64.28%, followed by the BEN haplotype (28.57%). Atypical haplotypes were identified at a frequency of 7.15%. Genotypes CAR/CAR, BEN/BEN, and CAR/BEN were present in 46.43%, 10.71%, and 35.71% of patients, respectively. β-Globin haplotype determination is important not only for the monitoring and prognosis of patients with SCA, but it also serves to inform anthropological studies that contribute to elucidating any peculiarities associated with African influences that contributed to the ethnological, economic, cultural, and social formation of Brazil. The high frequency of the CAR/CAR and CAR/BEN haplotypes in this study, which are associated with low levels of fetal hemoglobin, may ultimately reflect a severe clinical course and poor prognosis in patients with SCA in Maranhão.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sickle cell anemia (SCA) is the most severe form of sickle cell disease caused by homozygous hemoglobin S (α2βS2) that derives from a point mutation in the seventh codon (GAG→GTG) of the β-globin gene on chromosome 11, resulting in the substitution of the amino acid valine for glutamic acid (p.Glu6Val) [1, 2].
The polymorphic regions of a chromosome are referred to as a haplotype. The sickle mutation exists in Africa, as it is most prevalent in populations with diverse genetic haplotypes. Haplotype diversity in the β-globin gene probably represents separate occurrences of the HbS mutation; individuals affected by this haplotype variant show minor differences in disease characteristics, depending on the DNA structures in which such mutations occurred [3].
The clinical variability of SCA is influenced by genetic and non-genetic factors that modify disease expression. Polymorphic studies in groups of individuals affected by this disease are possible given the ability to map the African origins of the population and to determine the haplotype classifications associated with specific geographic regions [4].
Haplotypes have been useful markers in anthropological studies; they can be used to define the flow of the βS allele in human populations. Haplotypes have different ethnic and geographical origins; specifically, the βS allele can be found in the Central African Republic (CAR), or Bantu, and in South-Central and Eastern Africa. Other examples of different haplotypes include the Benin type (BEN), which originated in the African Midwest; the Senegal type (SEN), characteristic of Atlantic Africa; the Cameroon type (CAM), found within the geographical boundaries of Cameroon and in a small region in the west coast of Africa; and the Arabian-Indian or Asian type, present in the Arabian Peninsula and India. Haplotype characterization may serve as a parameter when determining the prognosis of patients living with SCA, as this method can be used to determine case severity and patient group characteristics, particularly since the latter are associated with clinical heterogeneity of the disease [2].
Hematological differences that were originally noted among African populations with varied haplotypes have suggested that these markers may be responsible for the phenotypic heterogeneity of SCA. Haplotypes associated with high levels of fetal hemoglobin would be associated with a less severe disease course. Thus, the SEN, CAM, and ARB haplotypes are commonly associated with high levels of fetal hemoglobin (> 15%) and a moderate to mild clinical course. In the BEN haplotype, fetal hemoglobin levels are intermediate (ranging from 5 to 15%) and the clinical course is less pronounced [5]. Conversely, the CAR haplotype presents lower levels of fetal hemoglobin (< 5%) and is associated with a severe clinical course [6]. SCA patients with higher levels of fetal hemoglobin are associated with a milder clinical course. These patients have a lower risk of developing painful seizures, acute chest syndromes, leg ulcer, cholelithiasis, and likely to experience higher survival rates. However, the genetic mechanisms underlying this different haplotype have not yet been clearly defined [4].
In addition to genetic factors, environmental factors are also important in determining the outcomes associated with SCA. They are well established: air quality, altitude, climate, skin cooling, precipitating bone pain, contact with Streptococcus pneumonia or parvovirus B19, nutrition, access to public health measures such as immunization, and socioeconomic status, which may determine families’ access to communication, transport, and medical care [4].
The clinical course of SCA involves vaso-occlusive hospitalizations, stroke episodes, infections, renal failure, cholelithiasis, etc. that can vary widely among patients with SCA [3].
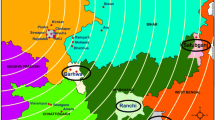
HbSS haplotypes were studied in different Brazilian populations to clarify their origins and to demonstrate how disease prevalence in different populations is directly related to miscegenation [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23]. For instance, according to Ministério da Saúde [24] and Lopes et al. [25], the frequency of individuals with the sickle cell trait and sickle cell disease in Maranhão is really elevated, corresponding, respectively, to 1/23 and 1/1.400 live births, behind only the states of Bahia and Rio de Janeiro (Fig. 1). Considering that no research has been conducted on patients’ haplotype analyses in association with SCA in this state, this study aimed to determine the haplotypes of patients with SCA who were followed at the public ambulatory clinic from Centro de Hematologia e Hemoterapia do Maranhão (HEMOMAR) in São Luís, Maranhão, northeastern Brazil.
Incidence of live births diagnosed with sickle cell disease in some Brazilian states undergoing neonatal screening (Ministry of Health, 2012). The states of Pará (PA), Ceará (CE), and Rio Grande do Norte (RN), despite not having updated data on neonatal screening to date, present haplotype characterization studies (Table 1)
Materials and methods
The sample population in this study consisted of 28 patients with SCA who were selected based on convenience sampling. Patients comprised both sexes and they ranged in age from 2 to 39 years old; patients were non-consanguineous, and they did not receive a blood transfusion 3 months prior to data collection. All patients were evaluated from April to June 2008, and they had been receiving treatment and follow-up at the HEMOMAR in São Luís, Maranhão, northeastern Brazil, at the time the study was conducted. This study was approved by the Research Ethics Committee of the University Hospital of the Federal University of Maranhão (HUUFMA), under number 111/2008. All patients and/or their caregivers signed a free and informed consent form; data collection was performed only after this form was signed.
Genomic DNA was isolated from peripheral blood samples [26]. To identify the haplotypes associated with a βS mutation, we used polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) and analyzed the following six polymorphic restriction sites, according to the methods described by Sutton et al. [27]: (1) 5′γG XmnI; (2) HindIII γG; (3) Hind III γA; (4) Hinc IIψβ; (5) Hinc II 3′δ; and (6) Hinf I 5′β.
For to compare the HbF levels of the different haplotypes groups (CAR/CAR; CAR / Benin; Benin/Benin), was used the statistical test one-way ANOVA (variance test) by the PAST program with a significance level at p < 0.05.
Results
The identified haplotypes in the 28 patients studied are presented in Table 1. The CAR haplotype was the most frequently found (64.28%), followed by the BEN haplotype (28.57%). Atypical haplotypes were found in 7.15% of patients (Table 1). The SEN, CAM, and ARB haplotypes were not observed in this population. Analyzed patients were considered homozygous for CAR and BEN with at 46.43% and 10.71%, respectively; 35.71% were heterozygous for these haplotypes.
Discussion
Considering the high frequency of individuals with the sickle cell trait and sickle cell disease in Maranhão, Brazil [25], and given the fact that no studies have explored the haplotypes of SCA in our state, it is clear that conducting the present study was highly necessary.
The βs haplotypes were found in 28 patients in São Luís, which is the capital of Maranhão state, located in northeastern Brazil; this haplotype is typical of African origin.
In the present study, historical records that showed how the first slaves to arrive in Brazil did so via the ports of São Luís, mainly Recife-PE [13] reinforced CAR haplotype predominance, which was followed by BEN predominance. The frequencies found for the CAR and BEN haplotypes (64.28% and 28.57%, respectively) in our study are in agreement with the findings from other studies based out of other states in Brazil, such as Rio de Janeiro [6, 16], Ceará [11, 12], Pará [15], and Rio Grande do Sul [5]. The mean rates obtained in these states were 63.05% and 28.95% for the CAR and BEN haplotypes, respectively. In Bahia, there is a high prevalence of the BEN haplotype when compared with that of CAR, as this state received a number of slaves from Nigeria and Ghana, regions where the BEN haplotype prevails [7, 8].
When exploring historical reports on the slave trade to Brazil, it is estimated that in the period between 1701 and 1816, 68% of imported slaves came from Angola and the rest came from the Benin region. From 1843 to 1871, 90% of slaves came from Congo, Angola, and Mozambique [28]. The historical trade of slaves from populations in Atlantic West Africa to northern Brazil (10%) may have served as justification for the findings of Cardoso and Guerreiro (2006) in Belém, the Pará state capital, where the authors found a 10.9% frequency of the SEN haplotype, which is common in Senegal. The SEN haplotype is reported to be the third most present haplotype in Brazil [15]. Studies such as those by Lindeau et al. (2016) [5], who evaluated 220 patients with SCA, identified only one individual with the SEN haplotype (0.5%). This haplotype is irregularly distributed in Brazil as a result of a small slave trade that occurred from Senegal, Gambia, Sierra Leone, and Guinea. Interestingly, Senegambia and West–Central Africa accounted for 97% of slaves who landed in the state of Maranhão-Grão Pará (at this time [1621–1772], Pará, Maranhão, Amapá, Amazonas, and Roraima were considered a single state). However, there is a greater frequency of the CAR haplotype in the population of Maranhão, which was under intense influence of the slave trade, primarily from Guinea-Bissau, Togo, Benin, Nigeria, and Angola from 1655 to 1822 [28, 29].
Although the present study haven't record of the SEN, CAM, and ARB haplotypes, some studies have already reported the presence at least one of these haplotypes in the Brazilian population; however, their rates have been found at low frequencies [5, 6, 8, 9, 11, 13,14,15,16,17, 19,20,21,22,23] (Table 1).
In the North, Central, and South American countries, the most frequently found haplotype is BEN, unlike in Brazil, where CAR is more prevalent. The SEN haplotype shows an irregular distribution in Latin America, with a higher prevalence in Cuba, Mexico, Venezuela, and north/northeastern Brazil. The CAM haplotype can be found in 50% of American countries; however, in Africa, its distribution is restricted to the mid-west region, more specifically to the Ethom ethnic group of Cameroons [20].
The 7.15% prevalence rate of atypical haplotypes recorded in this study likely reflects the various genetic mechanisms that may be associated with the sickle cell gene. These haplotypes are those that differ from most of the five common haplotypes observed worldwide. These data corroborate the hypothesis that all these different structures are generated by the recombination, point substitutions, or non-reciprocal transfer (conversion) into preexisting common haplotypes; they do not appear to be the result of de novo mutations in the β-globin gene [30].β-Globin haplotype determination is significantly important not only for the monitoring and prognosis of patients with SCA, but it also serves to further inform anthropological studies that contribute to clarifying the nuances associated with the African influence that contributed to the ethnological, economic, cultural, and social formation of Brazil.
The high frequency of the CAR/CAR and CAR/BEN haplotypes in this study, which were associated with low levels of fetal hemoglobin, may reflect a severe clinical course and poor prognosis in patients with SCA in Maranhão, Brazil. The mean HbF levels in the CAR/CAR, CAR/Benin, and Benin/Benin haplotypes in our study were 4.5%, 8.9%, and 14.3%, respectively, demonstrating a significant difference between the three groups: CAR/CAR × CAR/Benin × Benin/Benin (p = 0.0019); CAR/CAR × CAR/Benin (p = 0.019); CAR/CAR × Benin/Benin (p = 0.003); and CAR/Benin × Benin/Benin (p = 0.041). These data reinforce the claim that low HbF levels are associated with a worse clinical outcome, since most patients associated with the CAR haplotype in our state had an unfavorable clinical outcome. Specifically, among the CAR patients with lower HbF, two of them developed end-stage renal failure (TRI) and started dialysis, which is similar to those presented in the study by [30], where individuals who developed TRI had HbF < 5.0%, and none of them had HbF levels > 20%. In our case series, in general, patients with haplotype CAR had more vaso-occlusive crisis, stroke episodes, infections, and cholelithiasis and consequently showed more hospital admissions. Most patients in this study evolved with frequent painful seizures requiring hospitalization, transcranial Doppler abnormalities, ischemic stroke (CVA), and renal failure. Due to the unfavorable evolution and according to the complications that these individuals developed, they started to use hydroxyurea, monthly transfusion, and renal replacement therapy. Our results corroborate literature data associated with CAR haplotypes.
References
Kato GJ, Piel FB, Reid CD, Gaston MH, Ohene FK, Krishnamurti L et al (2018) Sickle cell disease. Nat Rev Dis Primers 4
Leal AS, Martins PRJ, Balarin MAS, Pereira GA, Resende GAD (2016) Haplotypes βs-globin and its clinical-haematological correlation in patients with sickle-cell anemia in Triângulo Mineiro, Minas Gerais, Brazil. J Bras Patol Med Lab 52(1):6–10
Serjeant GR (2013) The natural history of sickle cell disease. Cold Spring Harb Perspect Med 3:1–11
Piel FB, Steinberg MH, Rees DC (2017) Sickle cell disease. New Engl J Med 376(16):1561–1573
Lindenau JD, Wagner SC, Castro SMD, Hutz MH (2016) The effects of old and recent migration waves in the distribution of HBB* S globin gene haplotypes. Genet Mol Biol 39(4):515–523
Fleury MK (2007) Haplótipos do cluster da globina beta em pacientes com anemia falciforme no Rio de Janeiro: aspectos clínicos laboratoriais. Rev Bras Anal Clin 32:89–93
Lyra IM, Gonçalves MS, Braga JA, Gesteira Mde F, Carvalho MH, Saad ST et al (2005) Clinical, hematological, and molecular characterization of sickle cell anemia pediatric patients from two different cities in Brazil. Cad Saúde Pública 21(4):1287–1290
Adorno EV, Zanette A, Lyra I, Seixas MO, Reis MG, Gonçalves MS (2008) Clinical and molecular characteristics of sickle cell anemia in northeast of Brazil. Genet Mol Biol 31:621–625
Silva WDS, Klautau-Guimarães MDN, Grisolia CK (2010) β-Globin haplotypes in normal and hemoglobinopathic individuals from Reconcavo Baiano, state of Bahia, Brazil. Genet Mol Biol 33(3):411–417
Aleluia MM, Fonseca TCC, Souza RQ, Neves FI, da Guarda CC, Santiago RP, Cunha BLA, Figueiredo CVB, Santana SS, da Paz SS, Ferreira JRD, Cerqueira BAV, Gonçalves MS (2017) Comparative study of sickle cell anemia and hemoglobin SC disease: clinical characterization, laboratory biomarkers and genetic profiles. BMC Hematol 15(17):15
Galiza Neto GC, Pitombeira MS, Vieira HF, Vieira MLC, Farias DAB (2005) Analysis of Sglobin gene haplotypes in Ceará, Brazil. J Bras Patol Med Lab 41:315–321
Silva LB, Gonçalves RP, Rabenhorst SHB (2009) Analysis of sickle cell anemia haplotypes in Fortaleza reveals the ethnic origins of Ceará state population. J Bras Patol Med Lab 45:115–118
Bezerra MAC, Santos MNN, Araújo AS, Gomes YM, Abath FGC, Bandeira MGC (2007) Molecular variations linked to the grouping of β and α-globin genes in neonatal patients with sickle cell disease in the state of Pernambuco, Brazil. Hemoglobin 31:1–6
Cabral CHK, Serafim ÉSS, de Medeiros WRDB, Fernandes TAAM, Kimura EM, Costa FF (2011) Determination of S haplotypes in patients with sickle-cell anemia in the state of Rio Grande do Norte, Brazil. Genet Mol Biol 34:421–424
Cardoso GL, Guerreiro JF (2006) African gene flow to North Brazil as revealed by HbSS gene haplotype analysis. Am J Hum Biol 18:93–98
Okumura JV, Lobo CL de C, Bonini-Domingos CR (2013) Beta-S globin haplotypes in patients with sickle cell anemia: one approach to understand the diversity in Brazil. Rev Bras Hematol Hemoter 35(1):71–72
Zago MA, Figueiredo MS, Ogo SH (1992) Bantu beta s cluster haplotype predominates among Brazilian blacks. Am J Phys Anthropol 88(3):295–298
Figueiredo MS, Kerbauy J, Gonçalves MS, Arruda VR, Saad ST, Sonati MF et al (1996) Effect of alpha-thalassemia and beta-globin gene cluster haplotypes on the hematological and clinical features of sickle-cell anemia in Brazil. Am J Hematol 53(2):72–76
Auricchio MT, Vicente JP, Meyer D, Mingroni-Netto RC (2007) Frequency and origins of hemoglobin S mutation in African-derived Brazilian populations. Hum Biol 79(6):667–677
Belisário AR, Martins ML, Brito AM, Rodrigues CV, Silva CM, Viana MB (2010) B-Globin gene cluster haplotypes in a cohort of 221 children with sickle cell anemia or S beta0-thalassemia and their association with clinical and hematological features. Acta Haematol 124(3):162–170 Erratum in: Acta Haematol. 2011; 125(3):120
Leal AS, Martins PRJ, Balarin MAS (2015) Haplotype of the βS-globin cluster in patients with sickle cell anemia at a University Hospital in the Triangulo Mineiro, Minas Gerais. Rev Bras Hematol Hemoter 37(2):140–141
Shimauti ELT, Silva DGH, Souza EMA, Eduardo A, Leal FP, Bonini-Domingos CR (2015) Prevalence of βS-globin gene haplotypes, α-thalassemia (3.7 kb deletion) and redox status in patients with sickle cell anemia in the state of Paraná, Brazil. Genet Mol Biol 38(3):316–323
Watanabe AM, Pianovski MD, Lenzi L, Cat R (2017) The frequency of βS-globin haplotypes in the state of Paraná, Brazil, and clinical manifestations of sickle cell anemia. J Bras Patol Med Lab 53(1):24–30
Brasil (2012) Ministério da Saúde. Secretaria de Atenção à Saúde. Departamento de Atenção Especializada. Doença falciforme: condutas básicas para tratamento / Ministério da Saúde, Secretaria de Atenção à Saúde, Departamento de Atenção Especializada. Ministério da Saúde, Brasília
Lopes TC, Sarmento LDM, Fróz RC, Marinho HT, Noronha EP, Oliveira RAG (2011) A avaliação do Programa Nacional de Triagem Neonatal para Hemoglobinopatias. Rev Inst Adolfo Lutz 70(3):417–421
Salazar LA, Hirata MH, Cavalli SA, Machado MO, Hirata RDC (1998) Optimized procedure for DNA isolation from fresh and cryopreserved clotted human blood useful in clinical molecular testing. Clin Chem 44:1748–1750
Sutton M, Bouhassra EE, Nagel RL (1989) Polymerase chain reaction applied to the determination of β-like globin gene cluster haplotypes. Am J Hematol 32:66–69
Meireles MC (2009) As conexões do Maranhão com a África no tráfico atlântico de escravos na segunda metade do século XVIII. Rev Outros Tempos 6(8)
Mota AS (2007) A dinâmica colonial portuguesa e as redes de poder local na capitania do Maranhão. Tese (Doutorado em História) – Programa de Pós-Graduação em História, Universidade Federal de Pernambuco 188 f
Powars DR, Elliott-Mills DD, Chan L, Niland J, Hiti AL, Opas LM, Johnson C (1991) Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med 115(8):614–620
Acknowledgments
The authors thank all the people who contributed directly to the success of this study. Thank you Luís Magno Viana dos Santos by map designed, mainly the patients with sickle cell anemia in Maranhão State, and those who were responsible for patients of this study.
Funding
This work had the financial support of Laboratory of Clinical Research structure of Clinical Research Center (CEPEC) of the University Hospital of UFMA, Federal University of Maranhão, Financier of Studies and Projects of the Ministry of Science and Technology (FINEP), Maranhão State Foundation for Research and Scientific and Technological Development (FAPEMA), and HEMOMAR (Maranhão Blood Center).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Research Ethics Committee of the University Hospital of the Federal University of Maranhão (HUUFMA), under number 111/2008. All patients and/or their caregivers signed a free and informed consent form; data collection was performed only after this form was signed.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical aproval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Research Ethics Committee of the University Hospital of the Federal University of Maranhão (HUUFMA), under number 111/2008. All patients and/or their caregivers signed a free and informed consent form; data collection was performed only after this form was signed.
Additional information
This note can be taken from the paper of A.C. ALVES and V. A. L. SILVA under my master’s orientation (R. A. GOMES OLIVEIRA) of Programa de Pós-Graduação em Ciências da Saúde da Universidade Federal do Maranhão.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alves, A.C., da Silva, V.A.L., Dos Santos, A. et al. Sickle cell anemia in the state of Maranhão: a haplotype study. Ann Hematol 99, 1225–1230 (2020). https://doi.org/10.1007/s00277-020-04048-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-020-04048-9