Abstract
Patients with acute myeloid leukemia (AML) who progress after exposure to hypomethylating agents (HMA) have a dismal prognosis. We hypothesized that the addition of venetoclax, a BCL-2 inhibitor, to AML patients who previously failed HMA might overcome resistance. Adult patients (≥ 18 years) with AML were eligible if leukemia relapsed after, or was refractory to HMA. In general, in addition to venetoclax, patients continued HMA or other low-intensity therapies. Patients who previously underwent allogeneic hematopoietic cell transplantation (HCT) were also eligible. Data were analyzed in November 2018. Twenty-three patients were treated between October 2016 and October 2018 and were eligible for this study. Median age was 76 years and 6 patients had leukemia that relapsed post allogeneic HCT. None of the patients experienced tumor lysis syndrome and toxicities were as expected and manageable. Febrile neutropenia was the most common toxicity (78% of patients). Median hospitalization time was 13 days. Forty-three percent of the patients achieved CR/CRi. Overall survival (OS) was 74% at 6 months and median OS in patients who achieved remission was 10.8 months. Higher number of blasts in both bone marrow and peripheral blood was associated with lower chances of CR, while higher WBC, LDH, and bone marrow or peripheral blasts were associated with increased mortality rate. The addition of venetoclax to patients with HMA-refractory AML may result in a substantial anti-leukemic activity, specifically in those achieving complete remission. This should be further tested in a well-designed prospective trial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukemia (AML) is primarily a disease of the elderly for which prognosis remains poor [1, 2]. In those patients who are not eligible for induction chemotherapy, hypomethylating agents (HMA), i.e., decitabine or azacytidine, have been shown to be beneficial [1, 3, 4]. While objective response to HMA occurs in only 30–40% of the patients, the median time of response is approximately 12 months [5]. In those patients who are primary refractory to or have progressed after given HMA, and are not eligible to hematopoietic cell transplantation, the prognosis is dismal [6].
Venetoclax is a BCL-2 inhibitor that has shown a remarkable activity in patients with AML and has been recently approved by the regulatory authorities in the USA and in some European countries as first-line treatment when combined with either low-dose cytarabine or HMA [7]. However, a significant portion of patients with AML have already been exposed to HMA at the time of their leukemia occurrence, either as part of the first-line treatment for AML or when progressing from an antecedent hematologic disease.
In this national historical prospective study, we focused on this group of patients, with HMA-refractory AML, and hypothesized that the addition of venetoclax may be beneficial.
Methods
Patients
The electronic charts of AML patients diagnosed between January 2016 and January 2018 in 3 centers were systematically reviewed.
Eligibility criteria for this analysis included patients with de novo or secondary leukemia (except for acute promyelocytic leukemia) who received HMA (either decitabine or azacytidine) at any time during their course of therapy and either failed to achieve remission within 4 cycles, had a response and later progressed while still receiving HMA, or had a refractory disease during HMA therapy after at least 2 cycles of HMA. Patients who received HMA for progression after allogeneic hematopoietic cell transplantation (HCT) and did not respond were also included. All patients were given venetoclax.
Baseline cytogenetic analysis and molecular data were collected at diagnosis. AML type was classified according to the WHO 2016 classification and disease risk was scored according to the European leukemia network (ELN) 2017 criteria [8].
The protocol was approved by the Institutional Review Board in accordance with the Declaration of Helsinki.
Treatment schedules and supportive care
Prior HMA included either I.V decitabine at a dose of 20 mg/msq for 5 consecutive days every 28 days, or S.C azacytidine at a dose ranging between 37.5 and 75 mg/msq given for 5–7 consecutive days every 28 days.
Venetoclax was started at a daily dose of 100 mg and ramped up every 2 days to a maximal dose of 400 mg. Cycle 1 of venetocolax was always given in addition to HMA (azacitidine, n = 16 or decitabine, n = 4) or low-dose cytarabine (n = 3). Dose reduction of either HMA/low-dose cytarabine or venetecolax was optional in subsequent cycles.
Hospitalization was not required and most patients were given the protocol as outpatients, with daily assessment of blood count, electrolytes, and renal function.
Supportive care was given according to the physician’s discretion and included ciprofloxacin (500 mg twice daily), fluconazole (400 mg daily), and G-CSF (5 μg/kg/day). In patients that were given azoles, the maximal dose of venetoclax was reduced to 200 mg daily.
Patients treated for a relapse post allogeneic HCT were basically off immune-suppression prior to the initiation of the protocol. All patients were given in addition to venetoclax, donor lymphocyte infusion every 4–6 weeks. Dose of infusion was escalated from 5 × 107 CD3-positive cells/kg to 108 CD3-positive cells/kg in cases of a sibling donor, and from 107 CD3-positive cells/kg to 5 × 107 CD3-positive cells/kg in cases of unrelated donors. In patients who developed persistent neutropenia (neutrophil count below 500/μL), the HMA dose was either reduced or completely eliminated.
Toxicity assessment
Patients were evaluated for tumor lysis syndrome and hematologic toxicity at least once a week. In addition, patients were also evaluated for the incidence of significant major bleeding (any gastrointestinal or life-threatening bleeding), the number of clinical and microbiology documented infections (CDI, MDI, respectively), and the number and the total days of hospitalization. Toxicity profile was graded according to CTCAE v4.0. Acute and chronic GVHD were assessed according to the MAGIC and the NIH criteria, respectively.
Efficacy assessment
The primary endpoint of this study was overall survival (OS), defined as the time from initiation of first course of venetoclax to last follow-up or death. Refractory disease was defined as a stable or an increase in the number of marrow blast cells after completion of second course of treatment, and relapse as the reappearance of blasts after the achievement of remission. Secondary end points were the rate of CR and CRi and toxicity profile of the regimen. CR/CRi rates were evaluated according to the standard criteria for hematological CR [8]. Bone marrow aspiration was performed according to physician’s discretion and center’s policy, but in general was performed between the 2nd and the 4th cycles of treatment. Only patients that underwent at least one bone marrow aspiration for the evaluation of response were included in the analysis of response assessment.
Statistical analysis
Data were analyzed as of November 2018. Overall survival was estimated using the Kaplan-Meier method. Death was treated as a competing risk in the analyses of relapse/progression. Cox regression was used for univariate analyses of risk factors for all time-to-event end points. For each analysis, hazard ratios (HR) and 95% confidence intervals (95% CI) are given together with p values for comparisons with the reference category. All p values are derived from likelihood ratio statistics and are two sided. Data were analyzed using the SPSS version 24.0.
Results
Patients
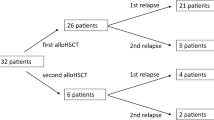
Between October 2016 and October 2018, we treated 23 patients with AML who were refractory to HMA (azacitidine, n = 21; decitabine, n = 2). Table 1 depicts patients’ characteristics. Ten patients (44%) had myelodysplasia-related changes and 5 patients (22%) had therapy-related AML. Majority of patients had intermediate-risk (n = 11, 48%) or high-risk (n = 10, 43%) ELN score. The median number of HMA courses prior to venetoclax was 6 (range, 2–25). In 18 patients, HMA were the last line of therapy given before venetoclax was added while in 5 patients, HMA were given as part of a more distant line of therapy and were reused with venetoclax as part of a salvage attempt. Median age was 76 (range, 41–92) years and median follow-up was 5.3 (range, 2.3–16.1) months. Six patients (26%) underwent allogeneic HCT with documented disease progression prior to venetoclax. In addition to venetoclax, patients were treated with low-dose cytarabine (n = 4), azacytidine (n = 13), azacytidine and DLI (n = 5), and only DLI (n = 1).
Administration of venetoclax and documented side effects
Most patients (21 of 23) were treated as outpatients with every other day visit at the outpatient clinic. Ramp-up protocol was mainly based on rapid doubling of the dose (every 1–2 days); thus, by day 6, all patients have been treated with the maximal dose. None of the patients experienced laboratory or clinical tumor lysis.
Patients were given different final doses of venetoclax (100 mg, n = 3; 200 mg, n = 9; 400 mg, n = 11). After adjustment for concomitant medications (mainly azoles), only 2 patients were given less than the recommended 400-mg dose (both received 200 mg). In the case of hematologic toxicity, the dose was decreased or withheld, or the HMA was discontinued. Of note, in 7 out of the 11 (64%) patients that survived more than 4 months after the initiation of venetoclax, the dose of venetoclax was reduced and maintained at the same dose thereafter.
Eighteen patients (78%) developed at least one infectious episode. None experienced a major bleeding episode. Sixteen patients (70%) were admitted at least once during treatment and median duration of hospitalization among all patients was 13 (range, 0–52) days throughout the study period.
Response to venetoclax
Ten patients (43%) achieved CR (n = 5)/CRi (n = 5). Median time to CR/CRi was 62 (range, 28–102) days. Among them, only 3 patients with a molecular marker were evaluated for minimal residual disease (MRD). Of those, 1 patient with an NPM1-mut AML achieved a negative MRD state.
For the subgroup of patients that relapsed after allogeneic HCT (n = 6), CR was achieved in 4 patients (67%) and the median OS was 12.4 months. Four patients were given DLI in addition to venetoclax. Of them, one patient developed overlap GVHD, 3 weeks after the infusion and continued venetoclax treatment for 6 additional months. One patient proceeded to a second allogeneic HCT. This patient is currently alive in continued CR, 16 months after the original relapse.
The following factors were evaluated as predictors for response—age (continuous variable), LDH (continuous variable), percentage of blasts in bone marrow (continuous variable), percentage of circulatory blasts (continuous variable), cytogenetics risk group, number of treatment lines prior to venetoclax, number of courses of HMA prior to venetocalx, prior HCT, and the dose of venetoclax. Only the percentage of bone marrow blasts and circulatory blasts was found to predict remission inversely (OR 0.45, 95% CI 0.23–0.51, p = .041 and OR 0.51, 95% CI 0.39–0.65, p = .009, respectively). The dose of venetocalx did not correlate with the achievement of remission state.
Survival analyses
At data analysis, there were 17 deaths and 6 patients are still alive. The incidences of survival at 6 and 12 months were 74% and 25%, respectively. Median OS was 5.6 (95% 4.9–6.2) months, Fig. 1a. OS in patients who undergone allogeneic HCT was 6.5 months (95% 0–17.3), with no statistical difference when compared with patients who did not undergo HCT (p = .96, log-rank test). In the group of patients who achieved CR (n = 10), the median OS was better when compared with the group of patients who did not achieve CR (n = 13) (10.8 months, 95% CI 6.2–15.4 vs. 2.8 months, 95% CI 0.9–4.8, p < .001) and the 6-month projected OS was 80% vs. 12% (Fig. 1b). Median OS for patients achieving CR was longer when compared with patients achieving CRi (12.5 months, 95% CI 9.7–15.3 vs. 5.6 months, 95% CI 5.4–5.8, p = .07).
The following factors were evaluated as predictors for mortality: age (continuous variable), cytogenetics risk group, ELN risk stratification, WHO classification, LDH (continuous variable), percentage of blasts in bone marrow (continuous variable), percentage of circulatory blasts (continuous variable), number of treatment lines prior to venetoclax, number of courses of HMA prior to venetocalx, prior HCT, and the maximal given dose of venetoclax.
WBC count prior to the initiation of venetoclax, high LDH levels, and a higher percentage of blasts in both bone marrow and peripheral blood were all associated with increased mortality rate (Table 2).
Discussion
HMA failure in patients with AML defines a particular high-risk group of patients with dismal outcome and limited salvage options [9,10,11]. We showed in this difficult-to-treat patient cohort that the addition of venetoclax to low-intensity therapy resulted in significant response rates with a CR/CRi rate of 42% with a median time to response of less than 2 months. We were also able to identify factors that are associated with response and survival, most of which are correlated with leukemia burden. The toxicity was manageable, expected, and mainly related to infectious complications. The dose intensity of venetoclax was maintained in most patients throughout the treatment period.
In the upfront setting, venetoclax in combination with low-intensity therapies such as low-dose cytarabine and HMA demonstrated significant activity in older patients and in those not eligible for intensive chemotherapy [7]. In this group of patients, the overall CR/CRi rate was 74% and the median time to response was within 2 cycles of therapy [7, 12]. Venetoclax in combination with low-dose cytarabine was also shown to be safe and active with a CR/CRi rate of 54% [13]. The results of large randomized, controlled phase 3 trials comparing venetoclax or placebo with either azacitidine (NCT02993523) or low-dose cytarabine (NCT03069352) are eagerly awaited for, but several regulatory agencies including the Food and Drug Administration (FDA) have already approved these combinations for the first-line setting in patient ineligible for intensive induction based on comorbidity or age (https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm626499.htm, accessed 01/07/2019).
The safety and efficacy of low-intensity therapy in combination with venetoclax in the context of relapsed or refractory (R/R) AML are less clear. As with most therapies in advanced AML, the reported response rates for venetoclax-based combination therapies are lower than those reported in the upfront setting. Pollyea et al. demonstrated that the combination of azacytidine and venetoclax in untreated patients with AML targets leukemia stem cells by disrupting crucial energy metabolism pathways and decreasing oxidative phosphorylation, an effect that was lost in the R/R setting [12, 14].
DiNardo et al. reported on 43 patients with R/R AML and related myeloid malignancies that received venetoclax in combination with low-intensity therapies. This cohort included older (median age 68 years) and heavily pretreated patients (median number of prior treatment lines = 3), and most patients (77%) had previous exposure to HMA. The overall response in this cohort was rather low (21%) with 12% of the patients attaining a CR/CRi [15]. Better overall responses were reported by Aldoss et al. who reported on 33 adult patients with R/R AML (median age 62 years), 60% of whom had previous exposure to HMA. The overall response rate was 64% and best response was achieved after a median of 2 cycles [16].
Our results are similar, showing a CR/CRi of 43%, even though the patients in our cohort were older (median age 76 years), with one-quarter of patients treated for post-transplant relapse.
The use of venetoclax in the post-transplantation relapse setting is increasingly described [17, 18]. Among the 6 patients in our study, CR/CRi was achieved in 67% of the patients. The combination of DLI with venetoclax seems to be safe and potentially effective and may represent an attractive therapeutic approach in this setting. Whether venetoclax affects graft-versus-leukemia properties needs to be proven and further insights into the pathobiology of this potential effect are needed.
Median OS was 10.8 months for the responding patients in our cohort, yet 1-year OS was 25% which is inferior to the 53% reported by Aldoss et al. This was probably because of the older age in our cohort and the fact all of our patients were failures of previous HMA treatment (as compared with only 61% in the cohort report by Aldoss et al.) [16]. Febrile neutropenia was common in our cohort, similar to the other studies including patients with refractory AML, yet none of our patients developed TLS and median hospitalization period during the study period was 13 days, suggesting that this treatment does not significantly compromise patients’ quality of life. The relative safe profile and the fact that patients can remain in the outpatient setting should be considered when discussing with these patients treatment goals and options.
Our study has several limitations. The cohort of patients analyzed is relatively small and rather heterogeneous. Nonetheless, this is one of the first reports on the use of venetoclax as a sensitizing agent to salvage patients with AML that failed HMA, a common clinical scenario. Furthermore, the combination of DLI with venetoclax is a novel salvage approach and might have potential utility in the treatment of the high-risk post-transplant relapse setting.
To conclude, this study shows the feasibility of adding venetoclax to patients relapsing post-HMA treatment. Validation of these results in a prospective trial and addition of other potential target therapies are needed.
References
Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, Kumar R, Cavenagh J, Schuh AC, Candoni A, Recher C, Sandhu I, Bernal del Castillo T, Al-Ali HK, Martinelli G, Falantes J, Noppeney R, Stone RM, Minden MD, McIntyre H, Songer S, Lucy LM, Beach CL, Dohner H (2015) International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 126:291–299
Buchner T, Berdel WE, Haferlach C, Haferlach T, Schnittger S, Muller-Tidow C, Braess J, Spiekermann K, Kienast J, Staib P, Gruneisen A, Kern W, Reichle A, Maschmeyer G, Aul C, Lengfelder E, Sauerland MC, Heinecke A, Wormann B, Hiddemann W (2009) Age-related risk profile and chemotherapy dose response in acute myeloid leukemia: a study by the German Acute Myeloid Leukemia Cooperative Group. J Clin Oncol 27:61–69
Ram R, Gatt M, Merkel D, Helman I, Inbar T, Nagler A, Avivi I, Ofran Y (2017) Second line azacitidine for elderly or infirmed patients with acute myeloid leukemia (AML) not eligible for allogeneic hematopoietic cell transplantation-a retrospective national multicenter study. Ann Hematol 96:575–579
Seymour JF, Dohner H, Butrym A, Wierzbowska A, Selleslag D, Jang JH, Kumar R, Cavenagh J, Schuh AC, Candoni A, Recher C, Sandhu I, Del Castillo TB, Al-Ali HK, Falantes J, Stone RM, Minden MD, Weaver J, Songer S, Beach CL, Dombret H (2017) Azacitidine improves clinical outcomes in older patients with acute myeloid leukaemia with myelodysplasia-related changes compared with conventional care regimens. BMC Cancer 17:852
Maurillo L, Venditti A, Spagnoli A, Gaidano G, Ferrero D, Oliva E, Lunghi M, D’Arco AM, Levis A, Pastore D, Di Renzo N, Santagostino A, Pavone V, Buccisano F, Musto P (2012) Azacitidine for the treatment of patients with acute myeloid leukemia: report of 82 patients enrolled in an Italian Compassionate Program. Cancer 118:1014–1022
Niscola P, Tendas A, Cupelli L, Giovannini M, Piccioni D, Scaramucci L, Dentamaro T, Del Poeta G, de Fabritiis P (2015) Dismal outcome of acute myeloid leukemia secondary to myelodysplastic syndrome and chronic myelomonocytic leukemia after azacitidine failure in a daily-life setting. Acta Haematol 133:64–66
DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, Frankfurt O, Konopleva M, Wei AH, Kantarjian HM, Xu T, Hong WJ, Chyla B, Potluri J, Pollyea DA, Letai A (2019) Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 133:7–17
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF, Wei AH, Lowenberg B, Bloomfield CD (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129:424–447
Prebet T, Gore SD, Esterni B, Gardin C, Itzykson R, Thepot S, Dreyfus F, Rauzy OB, Recher C, Ades L, Quesnel B, Beach CL, Fenaux P, Vey N (2011) Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. J Clin Oncol 29:3322–3327
Jabbour E, Garcia-Manero G, Batty N, Shan J, O’Brien S, Cortes J, Ravandi F, Issa JP, Kantarjian H (2010) Outcome of patients with myelodysplastic syndrome after failure of decitabine therapy. Cancer 116:3830–3834
Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, Gau JP, Chou WC, Buckstein R, Cermak J, Kuo CY, Oriol A, Ravandi F, Faderl S, Delaunay J, Lysak D, Minden M, Arthur C (2012) Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol 30:2670–2677
Pollyea DA, Jordan CT (2019) Why are hypomethylating agents or low-dose cytarabine and venetoclax so effective? Curr Opin Hematol 26:71–76
Wei A, Strickland SA, Hou J. Venetoclax with low-dose cytarabine induces rapid, deep, and durable responses in previously untreated older adults with AML ineligible for intensive chemotherapy. American society of hematology conference 2018;Abstract #284
Pollyea DA, Stevens BM, Jones CL, Winters A, Pei S, Minhajuddin M, D’Alessandro A, Culp-Hill R, Riemondy KA, Gillen AE, Hesselberth JR, Abbott D, Schatz D, Gutman JA, Purev E, Smith C, Jordan CT (2018) Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia. Nat Med 24:1859–1866
DiNardo CD, Rausch CR, Benton C, Kadia T, Jain N, Pemmaraju N, Daver N, Covert W, Marx KR, Mace M, Jabbour E, Cortes J, Garcia-Manero G, Ravandi F, Bhalla KN, Kantarjian H, Konopleva M (2017) Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol 93:401–407
Aldoss I, Yang D, Aribi A, Ali H, Sandhu K, Al Malki MM, Mei M, Salhotra A, Khaled S, Nakamura R, Snyder D, O’Donnell M, Stein AS, Forman SJ, Marcucci G, Pullarkat V (2018) Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Haematologica 103:e404–e407
Andreani G, Dragani M, Serra A, Nicoli P, De Gobbi M, Cilloni D (2019) Venetoclax plus decitabine induced complete remission with molecular response in acute myeloid leukemia relapsed after hematopoietic stem cell transplantation. Am J Hematol 94:E48–E50
Moukalled NM, El Darsa H, Haibe Y, Massoud R, Kanj SS, Mahfouz R, Bazarbachi A, El-Cheikh J (2019) Feasibility of venetoclax-based combinations for adult patients with acute myeloid leukemia relapsing after allogenic stem cell transplantation. Bone Marrow Transplant 54(4):620–624
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RR and OW served on an advisory board for AbbVie. OA, TZ, RG, PR, YB, and IR declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ram, R., Amit, O., Zuckerman, T. et al. Venetoclax in patients with acute myeloid leukemia refractory to hypomethylating agents—a multicenter historical prospective study. Ann Hematol 98, 1927–1932 (2019). https://doi.org/10.1007/s00277-019-03719-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-019-03719-6