Abstract
Febrile neutropenia is often observed in patients with hematologic malignancies, especially in those with acute leukemia. Meropenem has potent and broad antibacterial activity against gram-positive and gram-negative bacteria, and is recommended as first-line empiric therapy for febrile neutropenia. In contrast, the safety and efficacy of doripenem in patients with febrile neutropenia and hematologic malignancies is limited. In this randomized, prospective, cooperative, open-label trial, we compared doripenem (1.0 g every 8 h) to meropenem (1.0 g every 8 h) as first-line empiric antibacterial treatment of febrile neutropenia. To evaluate efficacy and safety, 133 hospitalized patients with acute leukemia or high-risk myelodysplastic syndrome, who developed febrile neutropenia during or after chemotherapy, were randomized to each drug. Resolution of fever within 3 to 5 days without treatment modification (i.e., the primary endpoint) did not significantly differ between the doripenem and meropenem groups (60.0% vs. 45.6%, respectively; P = 0.136). However, resolution of fever within 7 days of treatment was significantly higher in the doripenem group than in the meropenem group (78.4% vs. 60.2%, respectively; P = 0.037). Similar rates of adverse events (grades 1–2) were observed in both groups. Thus, we conclude that both drugs are safe and well-tolerated for the treatment of febrile neutropenia in patients with acute leukemia or high-risk myelodysplastic syndrome, and that the clinical efficacy of doripenem is noninferior to that of meropenem. UMIN Clinical Trial Registry number: 000006124
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Infectious Diseases Society of America (IDSA) guidelines recommend that patients with febrile neutropenia (FN) who are at high risk of infection should be hospitalized for empiric administration of intravenous antibiotics [1]. Monotherapy with antipseudomonal β-lactams, such as cefepime, carbapenem, or piperacillin-tazobactam, is recommended as first-line empiric antibacterial therapy in such patients [1].
The efficacy of meropenem (MEPM) as empiric monotherapy for neutropenia in pediatric or adult patients with cancer has been assessed in randomized comparative trials against cefepime [2, 3], imipenem/cilastatin [4], ceftazidime with [5] or without amikacin [6], and piperacillin-tazobactam [7]. Because of those studies, MEPM is considered a standard antibiotic for the empiric treatment of FN in patients with hematologic malignancies [1, 8].
Doripenem (DRPM) is a relatively new carbapenem that was approved in Japan, the USA, and the European Union in 2005, 2007, and 2008, respectively. Both DRPM and MPEM possess broad-spectrum in vitro activity against many gram-positive, gram-negative, and anaerobic bacteria. DRPM is reportedly therapeutically noninferior to MEPM in adults with complicated intra-abdominal infections [9]. Some studies have shown that DRPM is a promising agent for the empiric treatment of sepsis in patients with neutropenia and hematologic malignancies [10], and a retrospective analysis [11] has demonstrated similar efficacies between MEPM and DRPM for the treatment of febrile patients with acute leukemia and myelodysplastic syndrome (MDS). However, data concerning the safety and efficacy of DRPM in such patients is limited. Thus, we conducted a trial to compare the safety and efficacy of DRPM and MEPM as first-line empiric antibacterial therapy of FN in patients with acute leukemia or MDS-refractory anemia with excess blasts (RAEB)-2.
Methods
Study design
This randomized, cooperative, open-label prospective trial included 133 FN patients with acute leukemia or MDS-RAEB-2 who received induction or consolidation chemotherapy between August 1, 2011, and December 31, 2013. The institutional review board of each hospital approved the study protocol, and written informed consent was obtained from each patient or legal guardian before enrollment. This trial was registered with the UMIN Clinical Trials Registry (UMIN-CTR #UMIN000006124).
Participants
Eligibility criteria included hospitalization for FN, ≥ 16 years of age, diagnosis of acute leukemia or MDS-RAEB-2, and no intravenous antibiotic therapy within 1 week of the study. Fever was defined as an oral temperature measurement of ≥ 38.0 °C [1] or axillary temperature of ≥ 37.5 °C [12] sustained over a 1-h period, and neutropenia was defined as a neutrophil count less than 0.5 × 109/L after chemotherapy. The exclusion criteria included hepatic or renal insufficiency, known allergy or other contraindication to the study drugs, and pregnancy or lactation.
Randomization on a 1:1 basis was performed on a centralized website for FN patients (Fig. 1).
Diagnostic evaluation
Before the start of DRPM or MERM therapy, bacterial examinations of the throat, blood, sputum (if available), and urine samples were performed; complete physical exams were conducted, after which chest radiographs were taken; and laboratory values including complete blood counts were recorded. Thereafter, bacterial cultures were collected as needed, and laboratory values (including complete blood counts) were assessed at least twice weekly. Diagnoses of catheter-related infections were confirmed by isolation of identical microorganisms from catheter materials and blood cultures. Coagulase-negative staphylococci or corynebacteria were accepted as significant pathogens only when isolated from at least two separate consecutive blood cultures. Chest CT scans were performed in cases of persistent FN in which antibiotics had been properly administered and fungal infections were suspected.
Study drug administration
DRPM or MEPM (1 g each) was administered intravenously over a 1-h period every 8 h. The study drugs were continued for at least 5 days, in the absence of toxicity, to evaluate for efficacy. Discontinuation for toxicity was at the individual investigator’s discretion. Although the criteria for discontinuation were not predefined, an independent data monitoring committee confirmed whether discontinuation of the study drug was appropriate.
If fevers persisted after the first 3–4 days of empiric therapy, co-administration with another antibiotic (usually a glycopeptide) was considered [1]. IDSA guidelines were followed for the addition of anti-MRSA agents in cases of clinically suspected serious catheter-related infections, as well as for the empirical addition of antifungal drugs in high-risk patients (i.e., those with persistent fever of unknown origin [FUO] despite treatment with 4–7 days of broad-spectrum antibiotics) [1].
Assessment criteria of efficacy and safety
The primary endpoint was defined as fever resolution (< 37 °C) within 3 to 5 days of initial treatment that was maintained for at least two consecutive days without change in therapy (i.e., “success rate”) [13]. Treatment failure was defined as no improvement or worsening of infection while receiving the initial study regimen, or the necessary addition of other antibacterial or antifungal drugs.
The secondary endpoints consisted of (1) resolution of fever by day 7 with or without treatment modification with other antibacterial or antifungal drugs (i.e., “overall response rate”); (2) resolution of fever by day 14; (3) survival rate on day 30; and (4) frequency of abnormal laboratory data and adverse events (AEs). An independent data monitoring committee assessed the AEs using Common Terminology Criteria for Adverse Events version 4.0 (CTCAE ver. 4) after each personal physician’s review and analysis of the data.
Sample size
Because no evidence has established the superiority of either study drug (DRPM or MEPM) for the treatment of FN, the sample size was determined using the efficacy of both drugs. In the Japanese population, the efficacy rates of DRPM (1.0 g every 8 h) and MEPM (1.0 g every 8 h) for FN in patients with sepsis are 69.2% and 42.0%, respectively [14, 15]. Therefore, we set the efficacy rates at 70% for DRPM and 40% for MEPM, and a power of 0.90 with 5.0% significance was used for the final analysis. The calculated total sample size (using JMP, version 8.0.2.2) was 126 patients (63 for each group).
Statistical analysis
The primary endpoint was evaluated using intention-to-treat analysis. Categorical variables were tested using the Wilcoxon rank-sum or chi-square tests. P values < 0.05 were considered statistically significant.
Results
Enrollment and patient characteristics
One hundred thirty-three patients recruited from six hospitals in Japan were equally assigned to the DRPM (n = 65) and MEPM (n = 68) groups (Fig. 1). There were no significant differences between the groups with regard to age, sex, underlying diseases, and chemotherapy treatment prior to onset of FN (Table 1). The DRPM group had one patient with MDS-RAEB-2 and the MEPM group had two patients with MDS-RAEB-2. Induction chemotherapy was performed in 43.1% (28/65) of the DRPM group and 47.1% (32/68) of the MEPM group; consolidation chemotherapy was performed in 55.4% (36/65) of the DRPM group and 51.5% (35/68) of the MEPM group. The antileukemic agents used for induction and consolidation chemotherapy were anthracycline and cytarabine. The median durations of treatment were similar (P = 0.071) between the DRPM and MPEM groups (9 days and 12 days, respectively; Table 1). No antibacterial agents were administered as prophylaxis in this study.
Duration of neutropenia and neutrophil recovery
The median durations of neutropenia (neutrophil count < 500/μL) were 12 days (range, 1 to 80 days) and 15 days (range, 1 to 106 days) in the DRPM and MEPM groups, respectively (P = 0.1719). Neutrophil recovery (neutrophil count > 500/μL within 5 days after administration of DRPM or MEPM) was observed in 36.9% (24/65) of the DRPM group, and 22.1% (15/68) of the MEPM group (P = 0.0907). Neutrophil recovery with resolution of fever within 5 days of DRPM or MEPM administration did not significantly differ between the DRPM and the MEPM groups (P = 0.065).
Type of infection and causative microorganisms
Infections were detected in 33.8% (22/65) of DRPM patients and 39.7% (27/68) of MEPM patients (Table 2). Microbiologically documented infections (MDIs) occurred in 32.3% (21/65) of the DRPM group and 36.8% (25/68) of the MEPM group. Clinically documented infections (CDIs), including radiologically confirmed pulmonary infiltration and liver abscess diagnosed by computer tomography, were found in 3.1% (2/65) of the DRPM group (pneumonia: 2 cases) and 7.4% (5/68) of the MEPM group (pneumonia: 4 cases; liver abscess: 1 case; Table 2). FUO was determined for 66.2% (43/65) of the DRPM group and 60.3% (41/68) of the MEPM group (Table 2).
No differences in types of infections were observed between the two groups. The majority of MDIs were bacterial or fungal blood stream infections (BSIs), some of which were documented venous catheter-related infections. Gram-positive bacteria were detected in 77.3% (17/21) and 69.2% (18/24) of BSIs in the DRPM and MEPM groups, respectively; gram-negative bacteria were detected in 18.2% (4/21) and 26.9% (7/24) of BSIs in the DRPM and MEPM groups, respectively; and fungi were detected in 4.5% (1/21) and 3.9% (1/24) of BSIs in the DRPM and MEPM groups, respectively (Table 3). Superinfection with gram-positive and gram-negative bacteria was observed in one of the BSIs in the DRPM group and two of the BSIs in the MEPM group (Table 3). The most common causative microorganisms were Streptococcus spp. and Staphylococcus spp., including methicillin-resistant staphylococci (Table 3).
Efficacy
The success rate (resolution of fever within 3–5 days without any modification of therapy) was higher in the DRPM group (60.0%; 39/65) than in the MEPM group (45.6%; 31/68), although the difference was not significant (P = 0.136; Table 4). Subtype analysis revealed that patients with FUO had a success rate of 69.8% (30/43) and 58.5% (24/41) in the DRPM and MEPM groups, respectively (P = 0.397); and those with MDIs had a success rate of 42.9% (9/21) and 29.2% (7/24) in the DRPM and MEPM groups, respectively (P = 0.518; Table 4). We noted that the success rates did not depend on neutrophil recovery: success rates for the DPRM group were 58.3% (14/24) and 60.9% (25/41), with and without neutrophil recovery, respectively; success rates for the MEPM group were 33.3% (5/15) and 49.0% (26/53), with and without neutrophil recovery, respectively (P = 0.233, P = 0.346; Table 5).
The most frequently added antimicrobial drugs were glycopeptides, which were added as empiric or targeted antibacterial therapy of persistent or recurrent fever in patients with MDIs caused by gram-positive bacteria, including methicillin-resistant species (Table 6). In all, 40.0% (26/65) of the DRPM group and 32.3% (22/68) of the MEPM group received glycopeptides. Antifungal medications were added as empiric or preemptive therapy of persistent or recurrent fevers believed to be caused by fungal infections; those medications were administered to 21.5% (14/65) of the DRPM group and 13.2% (9/68) of the MEPM group (Table 6).
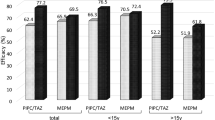
By day 7, the overall response rate was significantly better in the DRPM group (78.4%, 51/65) than in the MEPM group (60.2%, 41/68; P = 0.037). The cumulative number of afebrile cases by day 14 was 87.7% (57/65) and 83.8% (57/68) in the DRPM and MEPM groups, respectively (P = 0.523; Table 7). Survival rates 30 days after the start of administration were 98.5% (64/65) and 100% (68/68) in the DRPM and MEPM groups, respectively (P = 0.304; Table 7).
Safety
The rates of AEs did not significantly differ between the DRPM (38.5%, 25/65) and MEPM groups (41.2%, 26/68; P = 0.749; Table 8). The AEs were grades 1–2 for all but one patient. Although all patients were able to continue taking their respective study drugs until at least day 5, one patient discontinued on day 3 because of resolution of fever.
Discussion
To the best of our knowledge, this is the first prospective, randomized study to compare the safety and efficacy of DRPM to that of MEPM for the treatment of FN in patients with acute leukemia or MDS-RAEB-2.
In previous studies, the success rate of MEPM monotherapy on day 3 ranged from 35 to 87.5% for patients with FN. For clinical studies in which more than 40% of patients had acute leukemia, MEPM monotherapy has shown relatively low success rates: 56% [5], 37.0% [16], 35% [17], 44% [6]. For clinical studies that primarily included patients with malignant lymphoma and solid tumors—but not leukemia—MEPM monotherapy has shown relatively high success rates. In our study, nearly all patients (96.9%) had acute leukemia and were undergoing remission induction or consolidation therapies. We found that the success rate of MEPM treatment was 45.6% and that of DRPM treatment was 60.0%. Remarkably, the success rate did not depend on neutrophil recovery; both drugs were effective whether or not neutrophil recovery occurred within 5 days after administration of DRPM or MEPM (MEPM, 49.0%; DRPM, 60.9%). This result suggests that the clinical efficacy of DRPM was noninferior to that of MERM for the treatment of FN associated with hematological malignancies such as acute leukemia.
The distribution of infection types in our study differed from that of other studies. We observed a higher proportion of patients with FUO (63.2% vs. reported incidence of 29.3–60.9%), a lower proportion of patients with CDIs (5.3% vs. reported incidence of 10–24.7%), and a similar proportion of MDIs (34.6% vs. reported incidence of 18.1–53.6%). Sub-analysis of our patients revealed that DRPM-treated patients with FUOs and MDIs had success rates of 69.8% and 42.9%, respectively. In comparison, MEPM-treated patients with FUOs and MDIs had success rates of 58.5% and 29.2%, respectively. Our results were similar to those of previous studies in which patients with FUOs had higher response rates than those with MDIs.
The relatively low success rate in patients with MDIs was likely due to the high frequency of catheter-related infections. Those infections were primarily caused by gram-positive bacteria, some of which were predictably insensitive to the study drugs. We detected a total of 48 BSIs that were caused by 11 species of microorganisms. Thirty-five of the BSIs were caused by gram-positive bacteria (72.9%) and 11 were caused by gram-negative bacteria (22.9%). Considering the predominance of staphylococci and streptococci in patients with MDIs in our study, we recommend attention be paid to gram-positive bacteria in catheter-related infections.
Recent trends in the epidemiology of microorganisms isolated from cancer patients with bacteremia indicate a predominance of gram-negative bacteria and the global presence of antibiotic resistance in gram-negative and gram-positive bacteria [18]. An epidemiological study of bacteremia among FN patients with hematologic malignancies who had not received antibiotic prophylaxis was performed in Japan from 2006 to 2009 [19]; it demonstrated that 48.1% of the bacterial isolates are gram-negative, and 45.5% are gram-positive [19]. Nevertheless, vancomycin is not considered standard first-line empiric antibiotic therapy for FN because many randomized studies have shown no significant reduction in duration of fever and overall mortality [20].
The overall response rate on day 7 revealed that DRPM (78.4%) was significantly more effective than MERM (60.2%; P = 0.037). The reason for that is not known; however, it might be explained by the fact that DRPM is slightly more potent than MEPM against gram-positive aerobic bacteria [21].
Per our study protocol, anti-MRSA agents were added to the study drug regimen in 36.1% (48/133) of all patients as empiric or targeted antibiotic therapy for persistent or recurrent fever from MDIs, some of which were caused by gram-positive bacteria, including methicillin-resistant species. Antifungal agents were added to the study drug regimen in 17.3% (23/133) of all patients as empiric or preemptive antifungal therapy for persistent or recurrent fever believed to be caused by fungal infections. Because the frequency of addition of anti-MRSA and antifungal agents did not differ between the DRPM and MEPM groups, we do not believe that therapy modifications influenced the results. In most cases of modified treatments, chest X-rays were routinely performed on the first or second day of FN; chest CT scans were not routinely performed. All treatment modifications were considered treatment failure. Nevertheless, fevers resolved in 85.7% of all patients by day 14, and the survival rates on day 30 were similar between both groups. These data suggest that DRPM was as effective as MEPM.
The frequencies of AEs did not significantly differ between the DRPM and MEPM groups. Most of the AEs were grade 1–2 hepatic and renal dysfunctions, as was expected. None of the patients required drug discontinuation, and infection-related mortality was not observed. These results suggest that both drugs were well-tolerated by patients with high-risk FN.
In conclusion, the efficacy of monotherapy was similar between the DRPM and MEPM groups, but co-administration of anti-MRSA and/or antifungal drugs with DRPM was significantly more effective than DRPM alone. Thus, empiric therapy with DRPM or MEPM provides a safe and well-tolerated option for the treatment of FN in patients with acute leukemia and RAEB.
References
Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JAH, Wingard JR (2011) Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 52(4):e56–e93
Oguz A, Karadeniz C, Citak EC, Cil V, Eldes N (2006) Experience with cefepime versus meropenem as empiric monotherapy for neutropenia and fever in pediatric patients with solid tumors. Pediatr Hematol Oncol 23(3):245–253
Kutluk T, Kurne O, Akyüz C, Ceyhan M, Kanra G, Büyukpamukçu M, Seçmeer G (2004) Cefepime vs. meropenem as empirical therapy for neutropenic fever in children with lymphoma and solid tumours. Pediatr Blood Cancer 42(3):284–286
Shah PM, Stille W, Schaumann R, Heller A, Fuhr HG, Jung A, Köhler A, Walther F, Lips-Schulte C (1996) Empirical monotherapy with meropenem versus imipenem/cilastatin for febrile episodes in neutropenic patients. Infection 24(6):480–484
Cometta A, Calandra T, Gaya H, Zinner SH, de Bock R, Del Favero A, Bucaneve G, Crokaert F, Kern WV, Klastersky J, Langenaeken I, Micozzi A, Padmos A, Paesmans M, Viscoli C, Glauser MP (1996) Monotherapy with meropenem versus combination therapy with ceftazidime plus amikacin as empiric therapy for fever in granulocytopenic patients with cancer. The International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer and the Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto Infection Program. Antimicrob Agents Chemother 40(5):1108–1115
The Meropenem Study Group of Leuven, London and Nijmegan (1995) Equivalent efficacies of meropenem and ceftazidime as empirical monotherapy of febrile neutropenic patients. J Antimicrob Chemother 36(1):185–200
Reich G, Cornely OA, Sandherr M, Kubin T, Krause S, Einsele H, Thiel E, Bellaire T, Dörken B, Maschmeyer G (2005) Empirical antimicrobial monotherapy in patients after high-dose chemotherapy and autologous stem cell transplantation: a randomised, multicentre trial. Br J Haematol 130(2):265–270
Paul M, Yahav D, Fraser A, Leibovici L (2006) Empirical antibiotic monotherapy for febrile neutropenia: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 57(2):176–189
Lucasti C, Jasovich A, Umeh O, Jiang J, Kaniga K, Friedland I (2008) Efficacy and tolerability of IV doripenem versus meropenem in adults with complicated intra-abdominal infection: a phase III, prospective, multicenter, randomized, double-blind, noninferiority study. Clin Ther 30(5):868–883
Akiyama N, Kanamaru A, Tamura K, Tanimoto M, Ohyashiki K, Nakagawa Y, Urabe A, Masaoka T (2012) Efficacy and safety of doripenem for sepsis with neutropenia in Japanese patients with hematologic diseases. Jpn J Antibiot 65(4):251–262
Toya T, Nannya Y, Narukawa K, Ichikawa M, Kurokawa M (2012) A comparative analysis of meropenem and doripenem in febrile patients with hematologic malignancies: a single-center retrospective study. Jpn J Infect Dis 65(3):228–232
Masaoka T (1998) Management of fever of unknown origin in the neutropenic patient: the Japanese experience. Int J Hematol 68(Suppl 1):S9–S11
Feld R, Paesmans M, Freifeld AG, Klastersky J, Pizzo PA, Rolston KVI, Rubenstein E, Talcott JA, Walsh TJ (2002) Methodology for clinical trials involving patients with cancer who have febrile neutropenia: updated guidelines of the Immunocompromised Host Society/Multinational Association for Supportive Care in Cancer, with emphasis on outpatient studies. Clin Infect Dis 35(12):1463–1468
Imajo K, Ueda Y, Kawano F, Sao H, Kamimura T, Ito Y, Mugitani A, Suzuki K, Uike N, Miyamura K, Uski K, Morimatsu Y, Akiyama N, Nagai H, Ohara A, Tanimoto M, Takaki K, Chayama K, Urabe M, Nagatoshi Y, Tamura K (2012) A phase III study of the efficacy and safety of meropenem in patients with febrile neutropenia. Jpn J Infect Dis 65(4):271–287
[Finibax] (2011) Clinical phase 3 study of high dosage [CTD, common technical document]. Pharmaceuticals and Medical Devices Agency, Japan http://www.pmda.go.jp/drugs/2011/P201100084/34001800_21700AMZ00695_K100_1.pdf . Published in Japanese. Accessed 24 January 2019
de la Camara R, Figuera A, Sureda A, Hermida G, Verge G, Olalla I, Ranada JMF, Albos AD (1997) Meropenem versus ceftazidime plus amikacin in the treatment of febrile episodes in neutropenic patients: a randomized study. Haematologica 82(6):668–675
Feld R, DePauw B, Berman S, Keating A, Ho W (2000) Meropenem versus ceftazidime in the treatment of cancer patients with febrile neutropenia: a randomized, double-blind trial. J Clin Oncol 18(21):3690–3698
Montassier E, Batard E, Gastinne T, Potel G, de La Cochetière MF (2013) Recent changes in bacteremia in patients with cancer: a systematic review of epidemiology and antibiotic resistance. Eur J Clin Microbiol 32(7):841–850
Chong Y, Yakushiji H, Ito Y, Kamimura T (2011) Clinical impact of fluoroquinolone prophylaxis in neutropenic patients with hematological malignancies. Int J Infect Dis 15(4):e277–e281
Paul M, Borok S, Fraser A, Vidal L, Leibovici L (2005) Empirical antibiotics against gram-positive infections for febrile neutropenia: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 55(4):436–444
Zhanel GG, Wiebe R, Dilay L, Thomson K, Rubinstein E, Hoban DJ, Noreddin AM, Karlowsky JA (2007) Comparative review of the carbapenems. Drugs 67(7):1027–1052
Acknowledgments
The authors would like to thank all institutions and staff members that contributed to this study.
Author information
Authors and Affiliations
Contributions
YI was the principal investigator and takes primary responsibility for the paper. TO and YI designed the study. YF, NS, TM, YT, IH, and YA recruited the patients. TO, YTF, and SK reviewed and analyzed the data collected from each institute. YA and SI participated in statistical analysis. TO and YI wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oyake, T., Takemasa-Fujisawa, Y., Sugawara, N. et al. Doripenem versus meropenem as first-line empiric therapy of febrile neutropenia in patients with acute leukemia: a prospective, randomized study. Ann Hematol 98, 1209–1216 (2019). https://doi.org/10.1007/s00277-019-03634-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-019-03634-w