Abstract
Thalassemia patients have a high cell turnover rate due to chronic hemolysis and ineffective erythropoiesis; therefore, hyperuricemia is anticipated. This study aimed to identify the prevalence of hyperuricemia, gout and nephrolithiasis, conditions associated with serum uric acid (SUA), and urine uric acid excretion (UUA) in thalassemia patients. This was a cross-sectional study in patients aged 15 years or older at Chiang Mai University Hospital. All patients had blood and 24-h urine collection test. We enrolled 112 thalassemia patients in which 67.0% were female, 64.3% had beta thalassemia/Hb E, 76.8% were transfusion dependent, and 59.8% were post splenectomy. The median age was 29 (16–58) years. Mean SUA was 6.7 ± 2.0 mg/dl and hyperuricemia (SUA > 6.8 mg/dl) was found in 47 cases (45.2%). Intact spleen (ORs 4.3, 95%CI 1.55–12.50, p = 0.01) and lower FEuric (ORs 2.08, 95%CI 1.35–3.33, p < 0.01) were associated with hyperuricemia significantly. Seven (6.3%) had gouty arthritis and nine (8%) had microscopic hematuria, one case being confirmed nephrolithiasis. The mean UUA excretion was 981.3 ± 335.0 mg/day and UUA hyperexcretion (> 700 mg/24 h) was found in 83.3%. UUA hyperexcretion patients had renal hyperfiltration 46%, glomerular dysfunction 84%, and tubular dysfunction 7.7%. From our study, hyperuricemia was found in approximately 40% of thalassemia patients but gouty arthritis occurred only in few patients (6%). This may be explained by urinary uric hyperexcretion which is found in over 80%. The significant risk factors for hyperuricemia were intact spleen and lower fraction excretion of uric acid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thalassemia is a hereditary disease characterized by chronic hemolysis and ineffective erythropoiesis resulting from defects in the globin component in the hemoglobin in red blood cells.
Thalassemia is common problem in Thailand as the prevalence is high at 30% [1] compared to the global figure of only 5.2% [2]. The commonest thalassemia types in Thailand are both beta and alpha, including hemoglobin E and hemoglobin Constant Spring [3, 4]. In general, patients with thalassemia have a high cell turnover rate due to chronic hemolysis and ineffective erythropoiesis; therefore, hyperuricemia is anticipated. A study in beta thalassemia major children demonstrated the presence of a higher serum uric acid level (SUA) than seen in a healthy control group [5]. Nevertheless, there is limited data regarding SUA and prevalence of hyperuricemia in adult thalassemia patients.
Common conditions associated with the complication of hyperuricemia are gout and nephrolithiasis. Although hyperuricemia is expected in thalassemia patients, but evidence of gouty complications in thalassemia patients have only been documented in case reports [6, 7].
Thus, the objectives of this study are to determine the prevalence of hyperuricemia and its associated complications to as certain factors associated with a high SUA and to study the correlation between SUA, urine uric acid (UUA), and its complications in thalassemia patients.
Patients and method
This study is a cross-sectional study. All thalassemia patients, age 15 years or older, who attended the Adult Hematology Clinic of Chiang Mai University Hospital between November 2016 and October 2017 were enrolled onto the study. The diagnosis and type of thalassemia were confirmed by hematologist review and based on hemoglobin analysis using a high-performance liquid column chromatography (HPLC) method. The patients with other hematologic disease, malignancy, and active infection were chronic alcohol drinkers and users of medication that interfered with SUA levels including uric acid-lowering drugs (e.g., allopurinol, febuxostat, probenecid, benzbromarone, and sulfinpyrazone), diuretics, anti-tuberculous drugs (particularly pyrazinamide and ethambutol), cyclosporine, levodopa, and nicotinic acid that were excluded from the analysis of SUA and UUA due to possible interference with the laboratory tests. Patients taking low-dose aspirins (< 325 mg/day) for thromboprophylaxis after splenectomy were accepted for this study.
According to prevalence of allopurinol use in thalassemia intermedia that was 25% from previous study [8], the sample size was calculated using a one-sample proportion test for a power of 80% and alpha error of 0.05. The estimated sample size was 103 patients to detect a significant result regarding hyperuricemia.
The medical records that included demographic characteristics, thalassemia type, transfusion status, and current medication were reviewed. The laboratory investigations included complete blood count, reticulocyte count, serum creatinine, electrolyte, SUA, and urinalysis. A 24-h urine collection enabled urine chemistry tests including creatinine, electrolytes, uric acid, protein, microalbumin, and urine neutrophil gelatinase-associated lipocalin (uNGAL). The uNGAL was measured using an ARCHITECT urine NGAL assay kit. Other investigations, such as MRI of the liver, the heart (to determine tissue iron deposition), and echocardiogram (to determine cardiac function), all performed within 12 months, according to clinical indication that was also collected for analysis. A renal ultrasonography was requested additionally for patients with significant microscopic hematuria (three or more red blood cells per high-power field in collected urine specimen [9]) to determine renal calculi.
Hyperuricemia was defined as a SUA level of more than 6.8 mg/dl [10]. Hyperexcretion of uric acid was defined as more than 700 mg/day of UUA excretion while on a normal, unrestricted purine diet [11, 12]. The 24-h urine sample was collected from all enrolled patients, but the chemical analysis of the urine was done only in patients with adequate urine collection which was defined as more than 15 mg/kg/day of urine creatinine excretion in females and 20 mg/kg/day in males [13]. The flow algorithm depicting patient analysis is shown in Fig. 1.
The estimated glomerular filtration rate (eGFR) was calculated using the Cockcroft-Gault formula ((140 − age) × weight / (72 × serum creatinine) × 0.85 (if female)) [14]. Patients with eGFR < 60 ml/min were excluded from the analysis as this might interfere with SUA. Renal hyperfiltration was defined as eGFR > 135 ml/min [15]. Glomerular dysfunction in this study was defined as the presence of either proteinuria (> 150 mg/day) or microalbuminuria (> 30 mg/day) [16]. In addition, at least one piece of evidence from glucosuria, fraction excretion of phosphate > 20% or high uNGAL was defined as a marker of renal tubular dysfunction [17].
The history taking, physical examination, medical record review, and laboratory tests were performed in the same day. Informed consent was obtained from all individual participants included in the study. The study was approved by the Ethics Committee of the Faculty of Medicine, Chiang Mai University, study code MED-2559-04246 and performed in accordance with guidance from the declaration of Helsinki. This study was supported by a research grant from the Faculty of Medicine, Chiang Mai University.
Statistical analysis
The SPSS statistics software (version 23, International business machines corporation, United States) was used for statistical analysis. Results of categorical variables were expressed as proportion or percentage, and continuous variables were expressed as mean ± standard deviation (SD) or median (range) depending on their distribution. The Chi-square or Fisher’s exact test was used to compare categorical variables and a Student’s t test or Mann-Whitney U test was used to compare continuous data, where appropriate. Variables that were calculated as significant in the univariate analysis were entered into the multivariate analyses. Multiple logistic regression analysis was used to identify independent associated factors. Odd ratios (ORs) and 95% confidence intervals (CI) were calculated for all associations. A p value of less than 0.05 was considered as statistically significant.
Results
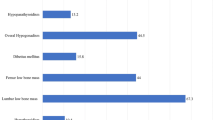
One hundred and twenty-three thalassemia patients were invited onto the study and 11 patients refused to participate due to their career commitments or routine daily activities in which a 24-h urine collection was presumably impossible. A total of 112 patients participated in the study. The median age of patients was 29 (range 16–58) years with the number of females predominating, 75 (67.0%). The most common thalassemia type was beta thalassemia/hemoglobin E disease, followed by homozygous beta thalassemia [72 (64.3%) and 30 (26.8%), respectively]. The patients were classed as being transfusion-dependent thalassemia (TDT) in 76.8% and post splenectomy in 59.8% of cases. The mean pre-transfusion hemoglobin was 7.1 ± 1.0 g/dl in TDT patients and 7.6 ± 1.2 g/dl in non-transfusion dependent (NTDT). The mean serum creatinine was 0.5 ± 0.2 mg/dl, and eGFR 122.7 ± 37.0 ml/min with hyperfiltration was found in 42 (40.4%) cases. The demographic data is shown in Table 1. Eight patients who had chronic kidney disease or were receiving allopurinol were excluded from the analysis of uric acid due to possible interference of the drug with SUA and UUA. Out of the 104 patients, the mean SUA was 6.7 ± 2.0 mg/dl and hyperuricemia was found in 47 out of 104 cases (45.2%). Hyperuricemia was identified as significant in patients: with an intact spleen (51.1% vs. 28.1%, p = 0.01); higher nRBC (222.7 ± 155.8 vs. 152.5 ± 117.9, p = 0.05); higher serum creatinine (0.6 ± 0.2 vs. 0.5 ± 0.1 mg/dl, p = 0.03), and lower fraction excretion of uric (FEuric) (7.9 ± 2.6 vs. 10.3 ± 3.6%, p = 0.01) compared with normal uric acid level patients. On the other hand, hyperuricemia was found to show a significant negative correlation with deferasirox use [2 (4.2%) vs. 11 (19.3%), p = 0.02)] and aspirin use [21 (44.7%) vs. 41 (71.9%), p = 0.01] compared with normouricemia patients (Table 2). The following factors: age, sex, BMI, thalassemia type by HPLC, transfusion status, hemoglobin, WBC, platelet, iron status, FEsodium, FEpotassium, FEphospate, and PHT showed no significant different when the groups were compared. The factors associated with hyperuricemia according to the results of the multivariate analysis were an intact spleen and a lower FEuric as shown in Table 3.
The 24-h urine collection was done in all enrolled patients, but adequate urine collection was achieved only in 60 patients. Mean UUA excretion was 981.3 ± 335.0 mg/day. Fifty (83.3%) of the patients who had adequate urine collections had hyperexcretion of UUA. Twenty-seven (54.0%) of UUA hyperexcretion patients had normal SUA levels. Factors associated with high UUA excretion in normouricemia patients were a higher eGFR (138.9 ± 29.8 vs. 94.1 ± 25.1 ml/min, p = 0.01) and a higher proteinuria level (268.2 ± 125.0 vs. 146.5 ± 50.7 mg/day) compared to normal UUA excretion normouricemia patients.
Mean uNGAL was 21.0 ± 8.2 mcg/l. A high uNGAL level, classed as more than 37 mcg/l, showed a significant correlation with lower eGFR (89.9 ± 63.4 vs. 127.9 ± 32.3 ml/min, p = 0.04), higher FEphosphate (13.9 ± 4.9 vs. 10.1 ± 3.3%, p = 0.04), and higher serum ferritin (2659.1 ± 1340.0 vs. 1434.2 ± 1041.8 mcg/l, p = 0.03).
A total of 50 cases of UUA hyperexcretion and renal hyperfiltration was documented in 23 cases (46%), glomerular dysfunction was documented in 42 cases (84%) (42 cases with proteinuria and 15 cases with microalbuminuria), and renal tubular dysfunction was documented in 4 cases (7.7%) with a uNGAL of more than 37 mcg/l.
Complications of hyperuricemia, gouty arthritis, and tophi were documented in seven cases (6.3%) and two cases, respectively. The median age of gouty arthritis onset was 30 years (range 20–44 years). Details of the clinical features of patients who had gout are shown in Table 4. Microscopic hematuria was detected in nine (8%) cases, in which one case had a calyceal stone and one case a renal cyst detected from the renal ultrasound. The patient with renal calculi was a female, 47 years old with beta thalassemia/Hb E, was transfusion dependent, and had an intact spleen. Her serum creatinine was 0.5 mg/dl, eGFR was 81.25 ml/min, SUA was 7.5 mg/day, UUA excretion was 1003.6 mg/24 h, and urine pH was 5.5.
Discussion
This study demonstrated that the prevalence of hyperuricemia in this cohort of thalassemia patients was 45.19% and of gouty arthritis was 6.3%. The associated factors of high SUA from the multivariate analysis were an intact spleen and lower FEuric. Hyperexcretion of uric acid was found in 83.3% of cases of which 23% had renal hyperfiltration, 84% with evidence of glomerular dysfunction, and 7.7% with renal tubular dysfunction. Microscopic hematuria was detected in 8% of cases which included one patient with a calyceal stone.
The prevalence of hyperuricemia is 10.6% in the general Thai populations [18]. A study on sickle cell anemia, which is another type of chronic hemolytic hemoglobinopathy, found that the prevalence of hyperuricemia was 9.2% [19]. A retrospective study into thalassemia intermedia revealed that 25% of patients were prescribed allopurinol but there was no certainty as regards the prevalence of hyperuricemia [8]. The prevalence of hyperuricemia in thalassemia patients from this study is higher than in the general population and the sickle cell anemia population. The pathophysiology of sickle cell disease is documented as being episodic hemolysis and vaso-occlusion precipitated by deoxygenation or stress [20] whereas thalassemia disease results not only in chronic hemolysis but also in ineffective erythropoiesis [21] so the degree of red blood cell turnover rate in thalassemia might be higher than that seen in sickle cell anemia, something possibly associated with higher endogenous uric production and higher SUA. In addition, the mean pre-transfusion hemoglobin in this study was 7.1 ± 1.0 g/dl in TDT patients and 7.6 ± 1.2 g/dl in NTDT, which indicated the inadequate transfusion in population of TDT according to guidelines which keep pre-transfusion hemoglobin of 9.0–9.5 g/dl and severe cases in NTDT populations, respectively. These data accord also with the high prevalence of renal hyperfiltration reported. Therefore, such a drive in ineffective erythropoiesis could be responsible for the high level of serum uric acid and prevalence of hyperuricemia in this study.
From the univariate analysis, hyperuricemia was identified as being significantly associated with patients with an intact spleen, higher nRBC, higher serum creatinine, lower FEuric, and as having a negative correlation with deferasirox and aspirin use.
Ricchi et al. revealed that a splenectomy was a risk factor for hyperuricemia which was paradoxical to the findings from our study [8]. They hypothesized that a splenectomy caused an increase in the circulating erythrocyte turnover. Additionally, that study included only thalassemia intermedia patients so splenectomy could have different implications in the more severe cases. However, the spleen is the hematopoietic organ in the reticuloendothelial system which is involved in extramedullary hematopoiesis and clearance of deformed circulating RBC so the existence of the spleen in a dyserythropoiesis disease such as thalassemia may contribute to the high cell turnover rate due to chronic extravascular hemolysis and extramedullary hematopoiesis, supporting endogenous overproduction of uric acid in this population but our study showed that a significant factor for a higher prevalence of hyperuricemia in thalassemic patients was with when the spleen was intact. This may be explained by the indications for a splenectomy in our center that are not quite the same as those generally recommended [22], as we are extremely concerned about the long-term complications of splenectomy (increased risk of thrombosis, pulmonary hypertension, and infection [23]). Therefore, not all severe thalassemia patients had undergone a splenectomy. Moreover, a quarter of our study population was NTDT, a demographic which usually do not undergo a splenectomy.
Hyperuricemia is not only associated with overproduction of uric acid but is also associated with inefficient renal excretion of uric acid presenting as lower FEuric. FEuric is the percentage of the uric acid filtered by the kidney which is excreted in the urine. Although this study showed that most of the patients had a high 24-h UUA excretion, the high UUA could be from a high filtered load of uric acid resulting from the overproduction. UUA excretion was quantitative whereas FEuric was the qualitative measure of renal handling. This finding was similar to that of the previous study which showed evidence of inefficient renal excretion of uric acid in most patients with gout, including patients with apparent overexcretion of uric acid [11].
This study demonstrated lower SUA in patients who used deferasirox, a finding that was similar to two previous studies in children with thalassemia [23, 24]. Deferasirox increases UUA excretion from renal tubular dysfunction, and low SUA has been found in over 90% of patients who used deferasirox for 6 years [22, 23]. In addition, the use of this medication was also associated with increased risk of nephrolithiasis among thalassemia patients [24, 25].
In terms of low-dose aspirin, there was study which demonstrated a decrease of UUA excretion in the elderly who were taking aspirin [24] but some studies showed no effect of low-dose aspirin with SUA and UUA in healthy young adults [25] and in gouty arthritis patients [26]. Therefore, the effect of low-dose aspirin with SUA/UUA is controversial. However, this study did not show any difference in the use of aspirin between UUA hyperexcreters and normoexcreters confirming that there was no effect as a result of low-dose aspirin on UUA excretion. All splenectomized thalassemia patients in this study were prescribed aspirin for thromboprophylaxis. The finding that aspirin use was significantly lower in patients with hyperuricemia than those who were normouricemia could be explained by aspirin being a dependent factor associated with a splenectomy which was the factor associated with hyperuricemia from the multivariate analysis.
A previous study found that serum uric acid level was associated with the thalassemia type which a higher incidence of hyperuricemia in thalassemia intermedia than in thalassemia major, explained by the higher cellular turnover secondary to the use of hydroxyurea as a fetal hemoglobin inducer in over 90% of thalassemia intermedia children in that study [27]. On the contrary, we could not found a correlation between thalassemia type and SUA. This may be explained by the different populations that there is no hydroxyurea usage in thalassemia intermedia patients in our study. A study in community populations in China revealed that lower hemoglobin was associated with hyperuricemia due to an increase in erythropoietic response, confirming that there was a trend in anemia patients such as thalassemia sufferers to have higher SUA than in healthy population. Moreover, in the same study, it was found that a higher WBC count was also associated with hyperuricemia due to both WBC and uric acid being markers of the inflammatory process and there was no differential in the count of nRBC (representing a high erythroid turnover) from other nucleated cells which could be counted as WBC [28]. There was a low variation in the baseline hemoglobin of this study so we could not demonstrate a correlation between degrees of anemia with SUA. In the case of WBC, this study separated the nRBC from WBC and we found that the factor associated with hyperuricemia was the nRBC not WBC.
In terms of UUA excretion, several different reference ranges have been suggested for hyperexcretion of 24 h UUA as it can vary from 700 to 1000 mg/day [11, 29] Since all thalassemia patients are routinely advised to avoid a high iron diet, especially red meat, organ meat, seafood, and alcohol [30], all of them also included in a high purine diet [31], the low body weight and low purine diet intake of thalassemia patients, the cut off point for UUA in this study was 700 mg/day. This study demonstrated the prevalence of UUA hyperexcretion in 83.3% and 38.3% of individuals at the cut point of 700 and 1000 mg/day of UUA, respectively. A previous study documented a high UUA excretion (defined as higher UUA excretion than normal control) in 38% of beta thalassemia major patients [32] and 20% of beta thalassemia minor children (Uricosuria defined as > 0.53 mg/dl on 24-h urine collection) [33]. However, the uricosuria definition, age group, and severity of thalassemia were different in each study so the prevalence might not directly compare.
NGAL is a protein which is expressed and secreted by immune cells, hepatocytes, and renal tubular cells. The expression of uNGAL rises 1000-fold in response to renal tubular injury [17]. Nowadays, it is used as a biomarker for early acute kidney injury especially in the case of tubulopathy [34]. According to a previous study, the uNGAL cut off level to predict renal function in different situations was highly variable ranging approximately from 10 to 200 mcg/l [35, 36]. Our study demonstrated that a uNGAL level greater than 37 mcg/l was significantly associated with lower eGFR and higher excretion of phosphate confirming the reliability of this new marker in renal function monitoring in thalassemia patients, showing a correlation with a previous study in beta thalassemia [37]. Some published data supports that the hyperexcretion of UUA was a result of tubular dysfunction [33, 38]. Furthermore, tubular dysfunction could be attributed to hemosiderin deposition in proximal tubules [39]. This study confirmed the significant correlation between renal tubular dysfunction by uNGAL and high ferritin level. Although there is a high prevalence of UUA hyperexcretion, renal tubular dysfuction was detected in 7.7% of cases so tubular dysfunction from renal hemosiderin deposition might not be the only cause of hyperexcretion of UUA in thalassemia patients.
Some studies have shown a relationship between uricosuria and renal hyperfiltration [40, 41]. There were 40% of patients (thalassemia intermedia and major) who showed renal hyperfiltration in one study in which none of the patients had a decreased eGFR and also found that female gender and previous history of splenectomy were independent predictors of renal hyperfiltration [38]. Similarly, this study showed 40.4% of renal hyper filtration in thalassemia patients. The mechanism of renal hyperfiltration has not been well investigated, but has been observed most frequently in patients who were not regularly transfused, suggesting that renal hyperfiltration could be a consequence of chronic anemia or chronic hypoxia. Chronic hypoxia/anemia causing hyperdynamic circulation results in an increase in renal plasma flow and a decrease in glomerular vascular resistance [42]. Furthermore, long standing glomerular capillary stretching as a result of hyperfiltration and subsequent endothelial and epithelial injury were associated with glomerular dysfunction [43] so this study and others prior to this also found evidence of proteinuria or albuminuria in more than half of thalassemia patients [44, 45]. This study found that the hyperexcretion of UUA in thalassemia patients was mainly due to glomerular dysfunction and hyperfiltration rather than renal tubular dysfunction.
The prevalence of gout in the general population is approximately 1% in China [46] and 0.16% in Thailand [47]. Therefore, the high prevalence of gout in these thalassemia patients could not be explained by the racial factor, but by the disease itself. Data concerning the prevalence of gouty arthritis in thalassemia patients is limited. Chronic tophaceous gout, a long standing complication of gouty, has rarely been reported in thalassemia patients [6, 7]. From the characteristics of all seven gouty cases in this study, there was a trend to develop gout among those who had a very high SUA and a decreased eGFR. Therefore, physician should be aware of the development of gouty arthritis in these patients.
Another major complication of hyperuricemia is renal calculi. This study demonstrated 8% of microscopic hematuria of which one case had a calyceal stone. The prevalence of hematuria showed a correlation with another study into all variants of beta thalassemia [48]. The prevalence of renal calculi in this study was lower than the range of 4.9 to 12.5% which has been previously reported [49, 50]. This lower prevalence might have been related to the use of uricosuric agents and the method that was used to determine renal calculi. In the study by Ricchi et al. [8], they found a prevalence of 12% (determined by abdominal ultrasonography), and 25% of their patients used uricosuric drugs for the treatment of hyperuricemia. It is well recognized that uricosuric agents increase uric acid excretion through the kidney and can cause renal calculi, particularly those with hyperexcretion of uric acid, such as thalassemia patients. Our patients did not receive uricosuric agent for the treatment of hyperuricemia; therefore, this could explain the low prevalence of renal calculi in this study. However, as only cases with significant microscopic hematuria underwent an ultrasonographic study to confirm the presence of renal calculi, the low prevalence of renal calculi in this study might be underestimated as patients who were asymptomatic or did not have significant hematuria were not given an ultrasound to detect the calculi. Lastly, the mean age of the patients in this study was lower than those reported in previous studies and whether age also influences the presence of renal calculi was not clear. Hyperuricosuria, low urinary volume, and persistently low urinary pH are risk factors for uric acid nephrolithiasis [51] a condition also found in the case with a renal stone in this study with a UUA of 1003 mg/day with a urine pH 5.5.
The limitations of this study were as follows: first, this is a cross-sectional study which could not represent the variation in SUA and UUA over time; second, this study recruited patients attending the hematology clinic so asymptomatic thalassemia minor patients were not included in this study; finally, there was no age and sex matched healthy control population with which to compare; nevertheless, there are several studies in children which demonstrated clearly higher serum uric acid levels than in a normal healthy control group [5]. The novel renal biomarkers such as N-acetyl-β-glucosaminidase (NAG), β2-microglobulin (β2M), or cystatin-C could be used in further studies to identify detailed renal function and particular mechanisms involved in uric acid handling.
In conclusion, hyperuricemia was found in approximately 40% of thalassemia patients but gouty arthritis only occurred in 6%. This may be explained by urinary hyperexcretion of uric acid which was found in over 80% of thalassemia patients. Although majority of the patients had hyperexcretion of uric acid, the prevalence of renal calculi in this population was very low. The significant risk factors for hyperuricemia were an intact spleen and a lower excretory fraction of uric acid. Various renal abnormalities such as hyperfiltration and glomerular and tubular dysfunction were also detected in most cases. Since thalassemia patients now have an increasing lifespan due to more effective medical care, there is a corresponding increase in the likelihood of the occurrence of gouty complications, renal calculi, or renal dysfunction in long-term follow-ups.
References
Wasi P, Pootrakul S, Pootrakul P, Pravatmuang P, Winichagoon P, Fucharoen S (1980) Thalassemia in Thailand. Ann N Y Acad Sci 344:352–363
Bernadette Modell MD (2008) Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ 86(6):480–487
Panich V, Pornpatkul M, Sriroongrueng W (1992) The problem of thalassemia in Thailand. Southeast Asian J Trop Med Public Health 23(Suppl 2):1–6
Fucharoen S (2013) Anemia, thalassemia and hemogloboinopathies in Northeast Thailand, Lao PDR and Vietnam. Srinagarind med J 25
Abdelmotaleb GAM, Abdelmonaem E, Ahmed E, Aboelsoued A et al (2017) Comparative study between measurements of serum cholesterol, uric acid and glucose in children with β-thalassemia by laboratory and bedside methods. Int J Adv Res [internet] Int J Adv Res 5(6):963–973. https://doi.org/10.21474/ijar01/4498
Mishra V, Sorabjee J, Mirgh S, Mishra M (2016) Tophaceous gout in thalassemia intermedia: a rare association. Oxf Med Case Rep 2016(5):105–106. https://doi.org/10.1093/omcr/omw030
Kumar V, Gruber B (2003) Tophaceous gout in a patient with thalassemia. J Clin Rheumatol : Pract Rep Rheumatic Musculoskelet Dis 9(6):380–384. https://doi.org/10.1097/01.rhu.0000099746.74211.78
Ricchi P, Ammirabile M, Costantini S, Di Matola T, Spasiano A, Genna ML, Cinque P, Prossomariti L (2012) Splenectomy is a risk factor for developing hyperuricemia and nephrolithiasis in patients with thalassemia intermedia: a retrospective study. Blood Cells Mol Dis 49(3–4):133–135. https://doi.org/10.1016/j.bcmd.2012.05.012
Davis R, Jones JS, Barocas DA, Castle EP, Lang EK, Leveillee RJ, Messing EM, Miller SD, Peterson AC, Turk TM, Weitzel W (2012) Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol 188(6 Suppl):2473–2481. https://doi.org/10.1016/j.juro.2012.09.078
Neogi T (2011) Clinical practice. Gout N Engl J Med 364(5):443–452. https://doi.org/10.1056/NEJMcp1001124
Perez-Ruiz F, Calabozo M, Erauskin GG, Ruibal A, Herrero-Beites AM (2002) Renal underexcretion of uric acid is present in patients with apparent high urinary uric acid output. Arthritis Rheum 47(6):610–613. https://doi.org/10.1002/art.10792
Emmerson BT (1996) The Management of Gout. N Engl J Med 334(7):445–451. https://doi.org/10.1056/nejm199602153340707
Yang C-Y, Chen F-A, Chen C-F, Liu W-S, Shih C-J, Ou S-M, Yang W-C, Lin C-C, Yang A-H (2015) Diagnostic accuracy of urine protein/creatinine ratio is influenced by urine concentration. PLoS One 10(9):e0137460. https://doi.org/10.1371/journal.pone.0137460
KDIGO (2012) Clinical practice guideline for the evaluation and Management of Chronic Kidney Disease (2013). Off J Int Soc Nephrol 3(1)
Cachat F, Combescure C, Cauderay M, Girardin E, Chehade H (2015) A systematic review of glomerular hyperfiltration assessment and definition in the medical literature. Clin J Am Soc Nephrol: CJASN 10(3):382–389. https://doi.org/10.2215/cjn.03080314
Gowda S, Desai PB, Kulkarni SS, Hull VV, Math AAK, Vernekar SN (2010) Markers of renal function tests. N Am J Med Sci 2(4):170–173
Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J (2007) Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 18(2):407–413. https://doi.org/10.1681/asn.2006080882
Lohsoonthorn V, Dhanamun B, Williams MA (2006) Prevalence of hyperuricemia and its relationship with metabolic syndrome in Thai adults receiving annual health exams. Arch Med Res 37(7):883–889. https://doi.org/10.1016/j.arcmed.2006.03.008
Arlet JB, Ribeil JA, Chatellier G, Pouchot J, de Montalembert M, Prie D, Courbebaisse M (2012) Hyperuricemia in sickle cell disease in France. Rev Med Interne / fondee par la Societe nationale francaise de medecine interne 33(1):13–17. https://doi.org/10.1016/j.revmed.2011.07.002
Melo TRFD, Ercolin LDR, Chelucci RC, Melchior ACB, Lanaro C, Chin CM, Santos JLD (2015) Sickle Cell Disease – Current Treatment and New Therapeutical Approaches. In: Munshi A (ed) Inherited Hemoglobin Disorders. InTech, Rijeka, p Ch. 09. doi:https://doi.org/10.5772/60515
Rachmilewitz EA, Giardina PJ (2011) How I treat thalassemia. Blood 118(13):3479–3488
Piga A, Serra M, Longo F, Forni G, Quarta G, Cappellini MD, Galanello R (2011) Changing patterns of splenectomy in transfusion-dependent thalassemia patients. Am J Hematol 86(9):808–810. https://doi.org/10.1002/ajh.22102
Inthawong K, Charoenkwan P, Silvilairat S, Tantiworawit A, Phrommintikul A, Choeyprasert W, Natesirinilkul R, Siwasomboon C, Visrutaratna P, Srichairatanakool S, Chattipakorn N, Sanguansermsri T (2015) Pulmonary hypertension in non-transfusion-dependent thalassemia: correlation with clinical parameters, liver iron concentration, and non-transferrin-bound iron. Hematology (Amsterdam, Netherlands) 20(10):610–617. https://doi.org/10.1179/1607845415y.0000000014
Caspi D, Lubart E, Graff E, Habot B, Yaron M, Segal R (2000) The effect of mini-dose aspirin on renal function and uric acid handling in elderly patients. Arthritis Rheum 43:13–108. https://doi.org/10.1002/1529-0131(200001)43:1<103::AID-ANR13>3.0.CO;2-C
Louthrenoo W, Kasitanon N, Wichainun R, Sukitawut W (2003) Effect of Minidose Aspirin on Renal Function and Renal Uric Acid Handling in Healthy Young Adults, vol 8. doi:https://doi.org/10.1097/00124743-200212000-00003,
Hyo Jin C, Yun Jong L, Jeong Jin P, Jung Chan L, Eun Young L, Eun Bong L, Yeong Wook S (2006) The effect of low dose aspirin on serum and urinary uric acid level in gouty arthritis patients. J Korean Rheum Assoc 13(3):203–208
Ali D, Mehran K, Moghaddam AG (2008) Comparative evaluation of renal findings in Beta-thalassemia major and intermedia. Saudi J Kidney Dis Transpl : Off Publ Saudi Center Organ Transplant, Saudi Arabia 19(2):206–209
Su P, Hong L, Zhao Y, Sun H, Li L (2016) The association between hyperuricemia and hematological indicators in a Chinese adult population. Medicine 95(7):e2822. https://doi.org/10.1097/md.0000000000002822
Moriwaki Y, Yamamoto T, Takahashi S, Yamakita J, Tsutsumi Z, Hada T (2001) Spot urine uric acid to creatinine ratio used in the estimation of uric acid excretion in primary gout. J Rheumatol 28(6):1306–1310
Molazem Z, Noormohammadi R, Dokouhaki R, Zakerinia M, Bagheri Z (2016) The effects of nutrition, exercise, and a praying program on reducing Iron overload in patients with Beta-thalassemia major: a randomized clinical trial. Iran J Pediatr 26(5):e3869. https://doi.org/10.5812/ijp.3869
Zhang Y, Chen C, Choi H, Chaisson C, Hunter D, Niu J, Neogi T (2012) Purine-rich foods intake and recurrent gout attacks. Ann Rheum Dis 71(9):1448–1453. https://doi.org/10.1136/annrheumdis-2011-201215
Bekhit OE, El Dash HH, Ahmed MS (2017) Early detection of kidney dysfunction in Egyptian patients with beta-thalassemia major. Egypt Pediatr Assoc Gazette 65(3):85–89. https://doi.org/10.1016/j.epag.2017.02.002
Sadeghi-Bojd S, Hashemi M, Naderi M, Shikhani S (2011) Kidney function tests in children with beta-thalassemia minor in Zahedan, southeast of Iran. Iran J Kidney Dis 5(3):201–203
Holzscheiter L, Beck C, Rutz S, Manuilova E, Domke I, Guder Walter G, Hofmann W (2014) NGAL, L-FABP, and KIM-1 in comparison to established markers of renal dysfunction. Clin Chem Lab Med 52. https://doi.org/10.1515/cclm-2013-0693
Thanakitcharu P, Jirajan B (2014) Determination of urinary neutrophil gelatinase-associated lipocalin (NGAL) cut-off level for early detection of acute kidney injury in Thai adult patients undergoing open cardiac surgery. J Med Assoc Thailand = Chotmaihet thangphaet 97(Suppl 11):S48–S55
Rostami Z, Nikpoor M, Einollahi B (2013) Urinary neutrophil gelatinase associated Lipocalin (NGAL) for early diagnosis of acute kidney injury in renal transplant recipients. Nephro-urol Monthly 5(2):745–752. https://doi.org/10.5812/numonthly.9385
Sen V, Ece A, Uluca U, Soker M, Gunes A, Kaplan I, Tan I, Yel S, Mete N, Sahin C (2015) Urinary early kidney injury molecules in children with beta-thalassemia major. Ren Fail 37(4):607–613. https://doi.org/10.3109/0886022x.2015.1007871
Deveci B, Kurtoglu A, Kurtoglu E, Salim O, Toptas T (2016) Documentation of renal glomerular and tubular impairment and glomerular hyperfiltration in multitransfused patients with beta thalassemia. Ann Hematol 95(3):375–381. https://doi.org/10.1007/s00277-015-2561-2
Smolkin V, Halevy R, Levin C, Mines M, Sakran W, Ilia K, Koren A (2008) Renal function in children with β-thalassemia major and thalassemia intermedia. Pediatr Nephrol 23(10):1847–1851. https://doi.org/10.1007/s00467-008-0897-8
Bjornstad P, Roncal C, Harra T, Pyle L, Lanaspa MA, Bishop FK, Snell-Bergeon JK, Johnson RJ, Wadwa RP, Maahs DM (2016) Hyperfiltration and uricosuria in adolescents with type 1 diabetes. Pediatric Nephrology (Berlin, Germany) 31(5):787–793. https://doi.org/10.1007/s00467-015-3299-8
Cheung KL, Lafayette RA (2013) Renal physiology of pregnancy. Adv Chronic Kidney Dis 20(3):209–214. https://doi.org/10.1053/j.ackd.2013.01.012
Helal I, Brosnahan G, Gitomer B, Schrier R (2012) Glomerular hyperfiltration: Definitions, mechanisms and clinical implications, vol 8. doi:https://doi.org/10.1038/nrneph.2012.19
Musallam KM, Taher AT (2012) Mechanisms of renal disease in β-thalassemia. J Am Soc Nephrol 23(8):1299–1302. https://doi.org/10.1681/asn.2011111070
Quinn CT, Johnson VL, Kim HY, Trachtenberg F, Vogiatzi MG, Kwiatkowski JL, Neufeld EJ, Fung E, Oliveri N, Kirby M, Giardina PJ (2011) Renal dysfunction in patients with thalassaemia. Br J Haematol 153(1):111–117. https://doi.org/10.1111/j.1365-2141.2010.08477.x
Sumboonnanonda A, Malasit P, Tanphaichitr VS, Ong-ajyooth S, Petrarat S, Vongjirad A (2003) Renal tubular dysfunction in alpha-thalassemia. Pediatr Nephrol 18(3):257–260. https://doi.org/10.1007/s00467-003-1067-7
Liu R, Han C, Wu D, Xia X, Gu J, Guan H, Shan Z, Teng W (2015) Prevalence of hyperuricemia and gout in mainland China from 2000 to 2014: a systematic review and meta-analysis. Biomed Res Int 2015:762820–762812. https://doi.org/10.1155/2015/762820
ชัยอำนวย พ ระบาดวิทยาของโรครูมาติค. Thai arthritis foundation. https://www.thaiarthritis.org/people23.php. Accessed 27 Sept 2018
Nickavar A, Qmarsi A, Ansari S, Zarei E (2017) Kidney function in patients with different variants of Beta-thalassemia. Iran J Kidney Dis 11(2):132–137
Efthimia V, Neokleous N, Agapidou A, Economou M, Vetsiou E, Teli A, Perifanis V (2013) Nephrolithiasis in beta thalassemia major patients treated with deferasirox: an advent or an adverse event? A single Greek center experience. Ann Hematol 92(2):263–265. https://doi.org/10.1007/s00277-012-1558-3
Ricchi PAM, Costantini S, Spasiano A, Di Matola T, Cinque P et al (2013) Nephrolithiasis in patients exposed to deferasirox and desferioxamine: probably an age-linked event with different effects on some renal parameters. Ann Hematol 93(3):525–527. https://doi.org/10.1007/s00277-013-1833-y
Ngo TC, Assimos DG (2007) Uric acid nephrolithiasis: recent Progress and future directions. Rev Urol 9(1):17–27
Acknowledgements
I would like to sincerely thank Ms. Antika Wongthani, Head of Analytical & Statistical data unit, Research Institute for Health Sciences, Chiang Mai University for suggestion in statistics of this study.
Funding
This study was supported by a research grant from the Faculty of Medicine, Chiang Mai University. Grant number: FUND-25591019-08194.
Author information
Authors and Affiliations
Contributions
J.C. designed the research, collected, summarized, analyzed clinical data, and wrote the paper; A.T. designed the research, obtained researched grant, analyzed data, wrote the paper, and corresponding author; T.R., E.R.,C.C., L.N., P.C. wrote, revised, and approved the final manuscript. W.L designed the research, wrote, revised manuscript, approved the final manuscript, and gave critical comment.
Corresponding author
Ethics declarations
This study was approved by the ethical research committee, Faculty of Medicine, Chiang Mai University. Study code: MED-2559-04246 and have been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chaloemwong, J., Tantiworawit, A., Rattanathammethee, T. et al. Hyperuricemia, urine uric excretion, and associated complications in thalassemia patients. Ann Hematol 98, 1101–1110 (2019). https://doi.org/10.1007/s00277-019-03630-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-019-03630-0