Abstract
Skeletal muscle atrophy and loss of adipose tissue, referred to as sarcopenia and adipopenia, respectively, are often observed in cancer patients. We investigated the impact of sarcopenia and adipopenia on clinical outcomes in 90 adult patients with newly diagnosed acute myeloid leukemia (AML) who received induction chemotherapy. Computed tomography (CT) before treatment revealed sarcopenia in 39 patients (43%) and adipopenia in 35 patients (39%). We analyzed the treatment efficacy of induction chemotherapy and survival outcomes. Three-year overall survival (OS) was 35% in the sarcopenic group and 67% in the non-sarcopenic group (P < 0.001). Three-year OS was 33% in the adipopenic group and 67% in the non-adipopenic group (P < 0.005). Multivariate analysis showed an association between sarcopenia and lower OS (hazard ratio, 2.27; 95% confidence interval, 1.11–4.79; P < 0.05), with other prognostic factors of performance status > 2 (P < 0.05) and adverse cytogenetic risk (P < 0.05). In elderly patients over 60 years old, 3-year OS was 0% for the sarcopenic group and 49% for the non-sarcopenic group (P < 0.0005). These results indicate the prognostic values of sarcopenia in adult patients with AML.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of patients with acute myeloid leukemia (AML) who receive allogeneic hematopoietic stem cell transplantation (HSCT) has been increasing, due to the development of reduced intensity conditioning (RIC) and non-myeloablative regimens complementing improvements in supportive care [1]. However, AML treatment remains a significant challenge in terms of comorbidities and frailty [2]. Before treating elderly patients, we should consider whether intensive curative therapy including allogeneic HSCT is feasible or whether low-intensity therapy would be better to improve survival and quality of life [3]. Nevertheless, standard tools have yet to be developed to assess the status of individual patient function and thus predict treatment efficacy and the prognosis of AML.
Sarcopenia, defined as a decrease in muscle mass and strength, is often observed in cancer patients, particularly elderly patients [4]. Age-related sarcopenia is differentiated from secondary sarcopenia resulting from diseases such as cancer. Sarcopenia has been demonstrated to be associated with the risks of morbidity and mortality for some kinds of cancer [4, 5]. The metabolic activity of cancer cells and systemic inflammation may lead to a loss of skeletal muscle [6]. We have previously reported sarcopenia as an independent prognostic factor in patients with diffuse large B cell lymphoma (DLBCL) [7]. Adipose tissue is another important component in the body, acting as a reserve of energy. Volumes of skeletal muscle and adipose tissue are easily measurable using computed tomography (CT) [8]. Takeoka et al. reported low subcutaneous adipose tissue, referred to as adipopenia, before treatment was a predictor of poor survival outcome in patients with multiple myeloma [9]. However, the clinical impacts of sarcopenia and adipopenia in adult AML have not been studied and remain unclear. The aim of this retrospective study was to reveal the impacts of sarcopenia and adipopenia on clinical outcomes in patients with AML. The term “sarcopenia” was used in the sense of reduced muscle mass as assessed by CT in this study.
Patients and methods
Patients
We retrospectively analyzed 90 patients (age ≥ 18 years) with de novo AML confirmed in accordance with WHO criteria. All patients received chemotherapy at Gifu University Hospital between December 2004 and October 2016. Cytogenetic risks were grouped by standard criteria and classified according to the Medical Research Council (MRC) classification [10]. All follow-up data were updated on April 1, 2017. This retrospective study was approved by our institutional review board. This study was conducted in accordance with the Helsinki guidelines.
Treatment strategies
Sixty-five patients underwent standard intensive chemotherapy consisting of anthracycline and cytarabine (Ara-C). In brief, induction therapy comprised either 12 mg/m2 of idarubicin (IDA) on days 1–3 and 100 mg/m2 of cytosine Ara-C on days 1–7, or 50 mg/m2 of daunorubicin on days 1–5 and 100 mg/m2 of Ara-C on days 1–7, followed by consolidation therapy. All patients with acute promyelocytic leukemia (APL) (n = 15) received induction therapy with regimens using all-trans retinoic acid. Consolidation chemotherapy for 11 patients with core binding factor (CBF)-AML comprised high-dose Ara-C, and 40 patients underwent three or four courses of standard consolidation chemotherapy based on anthracycline and Ara-C. Twenty-eight patients received HSCT. No hypomethylating or histone-deacetylating agents were administered to any patients. Twenty-five patients received low-dose chemotherapy [Ara-C combined with the VP-16 regimen (AV regimen [11])] or AV with granulocyte-colony stimulating factor (G-CSF) (AVG regimen [12]). Patients who received palliative care alone were excluded. Complete remission (CR) was defined according to International Working Group criteria [13].
Definitions of sarcopenia and adipopenia
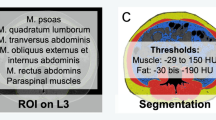
Skeletal muscle mass and fat tissues were measured using stored images from CT performed prior to any treatment, as previously described [7]. Skeletal muscles and visceral and subcutaneous fat areas were assessed from a single axial slice at the third lumbar (L3) level [14]. Hounsfield unit (HU)-based analysis of images was performed using dedicated SliceOMatic software (version 4.3; TomoVision, Montreal, Canada) to segment fat and lean tissue and quantify the cross-sectional area of each tissue type by totaling the given pixels of a tissue and multiplying that sum by the absolute unit pixel surface area (Supplemental Fig. 1). The following parameters were assessed: skeletal muscle mass including the mass of the psoas, quadratus lumborum, transversus abdominis, external and internal obliques, rectus abdominis, and erector spinae muscles; subcutaneous fat mass including the fat present in the subcutaneous tissue; and intramuscular fat contained in intercostal muscles and visceral fat mass. The fat present in the psoas was not considered for the assessment of adiposity. CT attenuation thresholds were − 29 HU to 150 HU for skeletal muscles and − 190 HU to − 30 HU and − 50 HU to − 150 HU for subcutaneous and visceral adipose tissues, respectively. Adipose tissue was taken as the sum of subcutaneous and visceral adipose tissues. This value was normalized for stature to calculate the lumbar L3 skeletal muscle index (SMI) and lumbar L3 adipose tissue index (ATI).
Due to the lack of consensus in definitions for sarcopenia and adipopenia using measurements from CT, we defined sex-specific cutoffs for our population. Receiver operator curve (ROC) analysis was used to identify cutoffs for SMI and ATI associated with mortality ascertained by optimum stratification that would best predict patient prognosis. Optimum stratification solved the threshold value of SMI that best separated patients with sarcopenia from those without sarcopenia with respect to mortality [15].
Statistical analysis
Overall survival (OS) was measured from the start of chemotherapy until death from any cause, with data censored as of the date of last contact. Disease-free survival (DFS) for patients who achieved complete response (CR) was measured from the date of CR until the date of AML relapse or death from any cause and was censored as of final follow-up. Event-free survival (EFS) was measured from the date of diagnosis to the date of induction treatment failure, relapse from CR, or death from any cause. Survival curves were estimated using the Kaplan-Meier method and were compared using the log-rank test. After multivariate analysis using a Cox proportional hazards regression model with a stepwise method, the hazard ratio (HR) was calculated. The Cox model was tested for the assumption of proportionality and checked for interactions between the significant covariates retained in the final model. Relationships between clinical, biological, and imaging parameters were determined using a non-parametric Mann-Whitney U test or chi-squared test, as appropriate. Differences between the results of comparative tests were considered statistically significant for values of P < 0.05. All statistical analyses were performed using JMP version 13 software (SAS Institute, Cary, NC).
Results
Patient baseline characteristics
Clinical characteristics of the 90 patients examined in this study are summarized in Table 1. The study population comprised 51 males and 39 females. Median age at diagnosis was 59 years (range, 18–84 years). Thirty patients were classified as showing favorable cytogenetic risk, while 46 showed intermediate risk and 12 showed adverse risk. Cytogenetic risk was unknown in two patients. Sixty-five patients (72%) received intensive chemotherapy, and 25 patients (28%) received reduced intensity chemotherapy as induction therapy. Median SMI was 42.5 cm2/m2 (range, 26.8–61.8 cm2/m2) and median ATI was 73.9 cm2/m2 (range, 2.1–188.9 cm2/m2). SMI was significantly higher in males than in females (P < 0.0001), whereas no difference in ATI was seen between males and females (P = 0.96). The cutoff SMI used to identify sarcopenia was defined as < 48.4 cm2/m2 in males and < 33.5 cm2/m2 in females. The cutoff ATI used to identify adipopenia was defined as < 63.5 cm2/m2 in males and < 46.0 cm2/m2 in females. Thirty-nine patients (43%) showed sarcopenia, and 35 patients (39%) showed adipopenia. According to performance status (PS), 39% of patients with PS ≤ 1 showed sarcopenia, compared to 67% of patients with PS ≥ 2 (P < 0.05). Fat mass was significantly lower in patients < 60 years old than in elderly patients (Table 1). No correlation was evident between the value of serum albumin and either SMI or ATI. The presence or absence of comorbidity before treatment was unrelated to sarcopenia (data not shown).
Response and outcomes
Of the 90 patients, 62 (78%) achieved CR within 2 cycles of induction chemotherapy. CR rate was significantly lower in patients with adverse cytogenetics than in those with favorable cytogenetics. CR rate was significantly lower in patients receiving less-intensive chemotherapy than in those with intensive chemotherapy. The CR rate was significantly lower in patients with body mass index (BMI) < 25 kg/m2 than in those with BMI ≥ 25 kg/m2. Patients with sarcopenia or adipopenia tended to show lower CR rates than patients without these conditions, but the difference was not significant (Table 2). Median follow-up was 13.8 months (range, 0.5–146.5 months). Three-year OS, EFS, and DFS in all patients were 53%, 35%, and 52%, respectively (Table 2). For sarcopenia and non-sarcopenia groups, 3-year OS was 35% and 67% (P < 0.001) and 3-year DFS was 25% and 58% (P < 0.0001), respectively (Fig. 1). For adipopenia and non-adipopenia groups, 3-year OS was 33% and 67% (P < 0.005), and 3-year DFS was 31% and 64% (P < 0.05), respectively (Fig. 2). Multivariate analysis showed an association between sarcopenia and lower OS (hazard ratio, 2.27; 95% confidence interval, 1.11–4.79; P < 0.05), with other prognostic factors of worse PS (≥ 2) (P < 0.05) and adverse cytogenetic risk (P < 0.05) (Table 3). Sarcopenia was also significantly associated with lower DFS (P < 0.0001) and lower EFS (P < 0.001). Patients with both sarcopenia and adipopenia exhibited the worst prognosis (Supplemental Fig. 2).
Survival analysis in elderly patients
Next, we investigated the impact of sarcopenia in elderly patients (> 60 years old), who need careful assessment to decide treatment strategies. A total of 44 patients were > 60 years old (median age, 67 years; range, 60–84 years). Clinical characteristics are summarized in Table 4. Thirty-four patients (77%) showed PS 0 or 1, and 48% received intensive chemotherapy for induction. Reasons for receiving less-intense chemotherapy were age (n = 10), comorbidities (n = 7), and hypoplastic bone marrow (n = 4). The cutoff value for SMI used to identify sarcopenia was defined as < 42 cm2/m2 in males and < 32 cm2/m2 in females according to ROC analysis of elderly patients. Nine patients (20%) were defined as showing sarcopenia. Sarcopenia was seen in 12% of patients with PS ≤ 1 and 50% of patients with PS ≥ 2 (P < 0.05). Three-year OS was 0% for the sarcopenic group and 49% for the non-sarcopenic group (P < 0.0005), 3-year DFS was 0% and 61% (P < 0.0001), and 3-year EFS was 0% and 46% (P < 0.005), respectively (Fig. 3). Causes of death in sarcopenia patients were leukemia (n = 8) and infection (n = 1). Causes of death in non-sarcopenia patients were leukemia (n = 9), pulmonary hemorrhage (n = 1), acute graft-versus-host disease (n = 1), and pancreas cancer (n = 1).
Discussion
This study indicates the clinical values of sarcopenia and adipopenia assessed by CT in adult patients with AML. In this series of 90 patients with AML who received induction chemotherapy, sarcopenia and adipopenia were not associated with efficacy but were significantly associated with poor survival. This may suggest that the prognostic impacts of sarcopenia and adipopenia are more related to the physiological condition than to the leukemia itself. Sarcopenia has been demonstrated to be associated with adverse events of chemotherapy leading to dose reductions, delays, or termination of treatment [4]. Suzuki et al. recently reported that muscle loss was observed in all childhood patients with acute lymphoblastic leukemia (ALL) after induction therapy, and sarcopenia was significantly associated with severe adverse events during induction therapy [16]. Patients with sarcopenia may thus be unable to obtain the complete therapeutic effects of chemotherapy, potentially explaining why sarcopenia is a poor prognostic factor for AML patients.
Ando et al. recently reported higher BMI as associated with better CR rate and OS in adult patients with AML [17]. They suggested that obese patients have a large amount of adipose tissue, which influences the metabolism of daunorubicin and could improve treatment response [17]. We also analyzed the impact of BMI and adipopenia, but neither was identified as a significant prognostic factor in our multivariate analysis. The median age of patients in our study was 12 years more than in their study, which may have affected the impact. Sarcopenia thus appears more useful as a factor for predicting survival than BMI in elderly patients.
Which patients will benefit most from intensive chemotherapy remained unclear for this heterogeneous AML population. Although patient comorbidities are commonly cited as one reason why elderly patients are not candidates for intensive chemotherapy and HSCT, most prognostic factors focus on disease-specific characteristics such as cytogenetic pattern [2]. Some tools in the Comprehensive Geriatric Assessment are based on activities of daily living, comorbidities and cognitive abilities [18, 19]. This assessment has recently been proposed as a useful method for choosing the correct intensity of treatment in elderly patients with DLBCL [20]. We investigated the impact of sarcopenia in elderly patients over 60 years old and surprisingly found that all patients with sarcopenia died within 1 year. They received induction chemotherapy, which means they had not been judged as unfit for chemotherapy by a physician. Our data suggest that induction chemotherapy may only exacerbate patient prognosis and quality of life for elderly patients with sarcopenia. On the other hand, treatment with intensive chemotherapy can be expected to contribute to survival for non-sarcopenia patients, even over 60 years old. Sarcopenia reflect many clinical conditions, such as frailty, low nutritional status, active catabolism, and systemic inflammation [6]. Sarcopenia could thus offer a very useful tool for detecting frailty in elderly patients with AML, who would thus be unfit for chemotherapy.
Improved understanding of AML etiology has resulted in investigations of targeted therapy such as hypomethylating drugs, histone-deacetylating drugs, inhibitor of FLT3 mutation, and inhibitor of IDH2 mutation [21, 22]. These therapies could be suitable for elderly patients with sarcopenia, given the reduced frequency of adverse events compared with chemotherapy. Even though patients exercise with a physical therapist during hospitalization, improving sarcopenia is not easy in AML patients, because AML treatment requires urgent, long-term hospitalization, and sometimes severe infections develop. Increasing muscle mass in contrast to strength may be challenging during AML induction therapy.
Despite the important findings in this study, several limitations should be discussed. First, our data were obtained from retrospective investigation of a single, small center. A larger prospective study is needed to confirm the impact of sarcopenia and adipopenia on clinical outcomes in patients with AML. Second, appropriate examinations to assess muscle function were not performed due to the retrospective nature of the analysis. Some recently published criteria for sarcopenia have recommended using the presence of not only low muscle mass but also low muscle function for the diagnosis of sarcopenia [23]. However, these criteria are sometimes not easy to apply to patients with AML, who usually suffer from severe infections or are in an immune-compromised state. On the other hand, CT is an accurate method for measuring muscle and adipose tissues using stored images in cancer patients. Since CT is generally performed before the treatment of leukemia and software is now commercially available, evaluations of muscle and adipose mass can be easily performed for patients with AML at any institution. Third, the main limitation to routine clinical usage is the lack of well-defined cutoff values for both sarcopenia and adipopenia. A large control population is needed to define cutoff values.
Our data illustrate a marked clinical impact of sarcopenia and adipopenia in patients with AML. Evaluation of skeletal muscle and adipose tissue depletion by CT offers a useful objective tool for predicting AML patient outcomes. Moreover, in elderly patients, the presence of sarcopenia before treatment may offer a useful factor for avoiding intensive therapeutic strategies for AML.
References
Muffly L, Pasquini MC, Martens M, Brazauskas R, Zhu X, Adekola K, Aljurf M, Ballen KK, Bajel A, Baron F, Battiwalla M, Beitinjaneh A, Cahn JY, Carabasi M, Chen YB, Chhabra S, Ciurea S, Copelan E, D’Souza A, Edwards J, Foran J, Freytes CO, Fung HC, Gale RP, Giralt S, Hashmi SK, Hildebrandt GC, Ho V, Jakubowski A, Lazarus H, Luskin MR, Martino R, Maziarz R, McCarthy P, Nishihori T, Olin R, Olsson RF, Pawarode A, Peres E, Rezvani AR, Rizzieri D, Savani BN, Schouten HC, Sabloff M, Seftel M, Seo S, Sorror ML, Szer J, Wirk BM, Wood WA, Artz A (2017) Increasing use of allogeneic hematopoietic cell transplantation in patients age 70 years and older in the United States. Blood 130(9):1156–1164. https://doi.org/10.1182/blood-2017-03-772368
Finn L, Dalovisio A, Foran J (2017) Older patients with acute myeloid leukemia : treatment challenges and future directions. 398–404. https://doi.org/10.1043/TOJ-17-0035
Appelbaum FR, Gundacker H, Head DR et al (2015) Age and acute myeloid leukemia. Blood 107:3481–3486. https://doi.org/10.1182/blood-2005-09-3724.Supported
Pamoukdjian F, Bouillet T, Lévy V, et al (2017) Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: a systematic review. Clin Nutr
Imai K, Takai K, Watanabe S, et al (2017) Sarcopenia impairs prognosis of patients with hepatocellular carcinoma: the role of liver functional reserve and tumor-related factors in loss of skeletal muscle volume. Nutrients 9. https://doi.org/10.3390/nu9101054
Argilés JM, Busquets S, Stemmler B, López-Soriano FJ (2015) Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol 22:100–106
Nakamura N, Hara T, Shibata Y, Matsumoto T, Nakamura H, Ninomiya S, Kito Y, Kitagawa J, Kanemura N, Goto N, Shiraki M, Miyazaki T, Takeuchi T, Shimizu M, Tsurumi H (2015) Sarcopenia is an independent prognostic factor in male patients with diffuse large B-cell lymphoma. Ann Hematol 94:2043–2053. https://doi.org/10.1007/s00277-015-2499-4
Go S-I, Park MJ, Song H-N, Kim HG, Kang MH, Kang JH, Kim HR, Lee GW (2017) A comparison of pectoralis versus lumbar skeletal muscle indices for defining sarcopenia in diffuse large B-cell lymphoma—two are better than one. Oncotarget 8:47007–47019. https://doi.org/10.18632/oncotarget.16552
Takeoka Y, Sakatoku K, Miura A, Yamamura R, Araki T, Seura H, Okamura T, Koh H, Nakamae H, Hino M, Ohta K (2016) Prognostic effect of low subcutaneous adipose tissue on survival outcome in patients with multiple myeloma. Clin Lymphoma Myeloma Leuk 16:434–441. https://doi.org/10.1016/j.clml.2016.04.010
Grimwade D, Walker H, Oliver F et al (1998) The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. Blood 92:2322–2333. https://doi.org/10.1182/blood-2012-05-431486
Tsurumi H, Kanemura N, Hara T, Kasahara S, Yamada T, Sawada M, Oyama M, Moriwaki H (2007) Therapeutic strategy of untreated de novo acute myeloid leukemia in the elderly: the efficacy of continuous drip infusion with low dose cytarabine and etoposide. J Cancer Res Clin Oncol 133:547–553. https://doi.org/10.1007/s00432-007-0203-3
Kanemura N, Tsurumi H, Kasahara S, Hara T, Yamada T, Sawada M, Goto N, Kitagawa JI, Shimizu M, Oyama M, Moriwaki H (2008) Continuous drip infusion of low dose cytarabine and etoposide with granulocyte colony-stimulating factor for elderly patients with acute myeloid leukaemia ineligible for intensive chemotherapy. Hematol Oncol 26:33–38. https://doi.org/10.1002/hon.834
Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Löwenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD, International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia (2003) Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 21:4642–4649. https://doi.org/10.1200/JCO.2003.04.036
Shen W (2004) Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 97:2333–2338. https://doi.org/10.1152/japplphysiol.00744.2004
Cerda J, Cifuentes L (2012) Using ROC curves in clinical investigation: theoretical and practical issues. Rev Chil Infectol 29:138–141. https://doi.org/10.4067/S0716-10182012000200003
Suzuki D, Kobayashi R, Sano H, Hori D, Kobayashi K (2017) Sarcopenia after induction therapy in childhood acute lymphoblastic leukemia: its clinical significance. Int J Hematol 107:1–4. https://doi.org/10.1007/s12185-017-2388-9
Ando T, Yamazaki E, Ogusa E et al (2017) Body mass index is a prognostic factor in adult patients with acute myeloid leukemia. Int J Hematol 105:623–630. https://doi.org/10.1007/s12185-017-2183-7
Ellis G, Whitehead MA, Robinson D, O’Neill D, Langhorne P (2011) Comprehensive geriatric assessment for older adults admitted to hospital: meta-analysis of randomised controlled trials. Br J Sociol 343:d6553. https://doi.org/10.1136/bmj.d6553
Overcash JA, Beckstead J, Extermann M, Cobb S (2005) The abbreviated comprehensive geriatric assessment (aCGA): a retrospective analysis. Crit Rev Oncol Hematol 54:129–136
Francesco Merli, Stefano Luminari, Giuseppe Rossi, et al (2010) Outcome of elderly frail patients with diffuse large B-cell lymphoma (DLBCL) prospectively identified by comprehensive geriatric assessment (CGA). Results from a study of the intergruppo italiano linfomi (IIL). Leuk Lymphoma 55(1):38–43. https://doi.org/10.3109/10428194.2013.788176
Sekeres MA, Othus M, List AF, et al (2017) Randomized phase II study of azacitidine alone or in combination with lenalidomide or with vorinostat in higher-risk myelodysplastic syndromes and chronic myelomonocytic leukemia: North American Intergroup Study SWOG s1117. In: Journal of Clinical Oncology. p 2745–2753
Perl AE, Altman JK, Cortes J, Smith C, Litzow M, Baer MR, Claxton D, Erba HP, Gill S, Goldberg S, Jurcic JG, Larson RA, Liu C, Ritchie E, Schiller G, Spira AI, Strickland SA, Tibes R, Ustun C, Wang ES, Stuart R, Röllig C, Neubauer A, Martinelli G, Bahceci E, Levis M (2017) Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1–2 study. Lancet Oncol 18:1061–1075. https://doi.org/10.1016/S1470-2045(17)30416-3
Heymsfield SB, Gonzalez MC, Lu J, et al (2015) Skeletal muscle mass and quality: evolution of modern measurement concepts in the context of sarcopenia. In: Proceedings of the Nutrition Society. p 355–366
Acknowledgements
H.T. and M.S. designed this research. N.N., S.N., T.M., and J.K. performed the research. M.S. and T.H. analyzed the data. S.N., N.N., and H.T. wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendment or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Supplementary Figure 1
(PDF 434 kb)
Supplementary Figure 2
(PDF 252 kb)
Rights and permissions
About this article
Cite this article
Nakamura, N., Ninomiya, S., Matsumoto, T. et al. Prognostic impact of skeletal muscle assessed by computed tomography in patients with acute myeloid leukemia. Ann Hematol 98, 351–359 (2019). https://doi.org/10.1007/s00277-018-3508-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-018-3508-1