Abstract
We describe the first multicenter prospective study to assess the efficacy, safety, and immune reconstitution of a novel sequential transplant approach in 24 patients with primary induction failure/relapsed acute myeloid leukemia (AML). The sequential regimen consisted of cladribine 5 mg/m2/day and cytarabine 2 g/m2/day for 5 days and mitoxantrone 7 mg/m2/day for 3 days, followed by myeloablative allogeneic hematopoietic stem cell transplantation (allo-HSCT) using intravenous busulfan (3.2 mg/kg/day) for 4 days and cyclophosphamide (60 mg/kg/day) for 2 days. Patients in CR without acute graft-versus-host disease at day + 90 received prophylactic donor lymphocyte infusion (pDLI). At the time of transplantation, a marrow blast infiltration > 20% or any level of circulating blasts was found in 62.5% of patients. The cumulative incidence of relapse at 2 years was 29.8%. Overall survival (OS) was 74.5% at 1 year and 56.5% at 2 years. Leukemia-free survival (LFS) at 1 and 2 years was 62.5 and 50.5%, respectively. Multivariate analysis demonstrated that haploidentical related donor, pDLI, and experiencing chronic graft-versus-host disease (cGVHD) were protective from relapse. Total T cells and T cell subsets in peripheral blood recovered at 3 months post-HSCT. The expressions of immune checkpoints (cytotoxic T lymphocyte antigen 4 and programmed death 1) were extremely low in T cells over the first 1 year post-transplantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although advances in chemotherapy have improved the prognosis of patients with acute myeloid leukemia (AML), approximately 30–35% of patients with newly diagnosed AML are refractory to first induction chemotherapy [1, 2]. For those patients receiving salvage chemotherapy, the chance to achieve a complete remission (CR) is 10–20% at best and overall survival (OS) at 1 year is less than 10% with a median survival of 4 months [3].
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is considered the most effective salvage option with curative potential available for refractory AML. However, allo-HSCT when a patient is not in CR is of uncertain benefit and the long-term outcomes remain dismal. The 5-year OS rate hovers round 19–22% in patients receiving allo-HSCT with standard myeloablative conditioning (MAC) regimens either based on total body irradiation (TBI) or busulfan treatment [4,5,6]. The intensity of the preparative regimen has been shown to directly influence relapse incidence and leukemia-free survival (LFS) after allo-HSCT, especially for advanced disease, because it needs to sufficiently eradicate the underlying leukemic cells to allow donor-cell engraftment and a graft-versus-leukemia (GVL) effect to occur. Therefore, more recent trials addressing patients with active or advanced disease receiving allo-HSCT focused on developing bridging strategies to increase dose intensity with reduced toxicity. Results from the most commonly employed regimen using sequential chemotherapy including fludarabine, cytarabine, and amsacrine (FLAMSA regimen) followed by reduced-intensity conditioning (RIC) allo-HSCT in refractory AML achieved promising results with long-term LFS of 25.6–40% [3, 7,8,9]. However, further work with regard to better bridging strategies to improve outcomes in such patients is certainly warranted and necessary. On the other hand, little is known about whether the sequential transplant strategies delay immune reconstitution post-HSCT.
Cladribine, a purine analog, has a direct cytotoxic effect against both dividing and resting leukemic cells based on proposed mechanisms including ribonucleotide reductase inhibition, incorporation of its metabolite into the DNA of proliferating cells, inhibition of DNA repair, pro-apoptotic effects, and epigenetic alteration via adenosine deaminase inhibition [10]. Furthermore, cladribine has been demonstrated to increase cellular uptake of cytarabine (AraC) and accumulation of AraCTP in leukemic blasts by 50–65% [11, 12]. Published studies have confirmed that in adult patients with newly diagnosed AML, the addition of cladribine to the standard two-drug induction regimen (DA) consisting of daunorubicin and AraC would increase the efficacy of the induction regimen to achieve approximately 70% of CR [13, 14], but no advantageous effect was seen with the addition of fludarabine to DA compared with DA alone [14]. Perhaps the most interesting attribute of cladribine is that the established CR rates for combination regimens consisting of cladribine, AraC, filgrastim, and mitoxantrone in relapsed/refractory AML patients were approximately 50% [15, 16]. Following these rationales, we developed a novel sequential transplant approach to treat refractory AML with a combination of re-induction chemotherapy consisting of cladribine, AraC, and mitoxantrone (CLAM regimen), immediately followed by busulfan-based MAC for allo-HSCT and prophylactic donor lymphocyte infusion (pDLI) to reinforce the GVL effect. We also first designed a multicenter prospective study to assess the efficacy, safety, and influence on immune reconstitution of this treatment option.
Patients and methods
Patients
We conducted a phase 2, prospective, multicenter trial involving patients recruited at HSCT centers of Guangzhou General Hospital of Guangzhou Military Command, the First Affiliated Hospital of Zhejiang University School of Medicine, Nanfang Hospital of Southern Medical University, and the Second Affiliated Hospital of Guangzhou University of Chinese Medicine from September 2013 through February 2017. All eligible patients were included if they fulfilled at least one of the following criteria defining refractory AML: (1) primary induction failure (PIF) defined as [17, 18] (i) bone marrow blast percentage above 25% or in case of initial blast percentage below 50% less than a 50% reduction in the blast percentage after the first cycle of induction therapy and (ii) in cases of partial response after first induction therapy at blood count recovery persistence of > 5% blasts in the bone marrow after second induction therapy, (2) first early relapse after a remission duration of fewer than 6 months, (3) relapse refractory to at least one course of salvage combination chemotherapy containing high-dose AraC, and (4) second or subsequent relapse. Additional inclusion criteria were age (14 to 55 years) and the availability of a suitable donor. Exclusion criteria were M3 subtype AML, Karnofsky performance score below 60%, and significant dysfunctions in vital organs. All the patients gave their written informed consent. The protocol was approved by the ethics review committee of each institution and registered at the Chinese Clinical Trial Registry (www.chictr.org) (Identifier: ChiCTR-ONRC-13003482).
Re-induction chemotherapy and myeloablative conditioning regimen
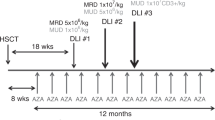
As shown in Fig. 1, for re-induction, patients received cladribine 5 mg/m2/day and high-dose cytarabine 2 g/m2/day on days − 12 to − 8 and mitoxantrone 7 mg/m2/day from days − 12 to − 10 (CLAM regimen). The myeloablative conditioning regimen used involved busulfan (Bu; 3.2 mg/kg/day IV on days − 7 to − 4) and cyclophosphamide (Cy; 60 mg/kg/day IV on days − 3 to − 2).
HLA typing and donor selection
High-resolution DNA typing for HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1 was performed in all patients and donors. The order of donor selection has been described previously [19]. In brief, if a fully HLA-matched sibling donor (MSD) was available, patients were assigned treatment with MSD-HSCT. If an MSD was unavailable, a suitably matched unrelated donor (URD) was used as the alternative, where a suitable match involved matching more than eight of 10 HLA-A, HLA-B, HLA-C, HLA-DRB1, and DQB1 allele loci (≥ 8/10) and at least five of six matching HLA-A, HLA-B, and HLA-DRB1 antigen loci. If an MSD or suitably matched URD was unavailable within the timeframe appropriate for the patient’s malignancy and clinical circumstances, patients were allowed treatment with a haploidentical related donor (HRD) HSCT.
GVHD prophylaxis
Graft-versus-host disease (GVHD) prophylaxis was based on cyclosporin A (CSA) and methotrexate (MTX) as previously described [19]. CSA was scheduled to be given intravenously at 2.5 mg/kg/day from day − 7, with a target blood level of 200–300 ng/mL. MTX was given at 15 mg/m2 on day + 1 and 10 mg/m2 on days + 3 and + 6. For patients receiving URD- or HRD-HSCTs, low-dose mycophenolate mofetil (MMF) was added. MMF was initiated orally at 500 mg/day on day + 1 and withdrawn on day + 100. Rabbit anti-thymocyte globulin (ATG, Thymoglobulin, Genzyme, Cambridge, MA, USA) was also administered to patients receiving URD-HSCTs (7.5 mg/kg total dose). For patients receiving HRD-HSCT, anti-T lymphocyte globulin (ATG-F, Fresenius, Bad Homburg, Germany) (20 mg/kg total dose) was added.
Prophylactic donor lymphocyte infusion and CSA withdrawal
Patients received one course of pDLI if they were in CR without evidence of acute GVHD (aGVHD) at day + 90. DLIs used G-CSF-mobilized blood cells and the dose was 3–4 × 107 CD3+ cells/kg. In the absence of GVHD, the dosage of CSA was tapered rapidly in a stepwise fashion (i.e., total dose reduced by 20%/week) during 4 weeks post-pDLTs.
Minimal residual disease monitoring
Bone marrow samples were analyzed at 1, 2, 3, 4, 5, 6, 9, and 12 months after transplantation and at 6-month intervals thereafter for the monitoring of minimal residual disease (MRD). We used two strategies to test for MRD in bone marrow samples: (1) aberrant leukemia-associated immune phenotypes (LAIPs) detected by multiparameter flow cytometry (MFC), (2) Taqman-based real-time fluorescent quantitative PCR (RT-PCR) detecting leukemia-specific targets (such as gene fusions, gene mutations, overexpressed genes), including WT1, AML1/ETO, CBFβ/MYH11, MLL gene fusions, FLT3, HOX11, and EVI1. MFC-positive status was defined as > 0.01% of cells with a LAIP phenotype in bone marrow samples. The transcript level of leukemia-specific gene fusions ≥ 0.001 was defined as PCR-positive. In accordance with the LeukemiaNet guidelines and the detection of WT1 copies > 250/104 copies of the control gene Abelson (Abl) in bone marrow was defined as WT1 overexpression [20]. MRD-positive was defined as patients with two consecutive MFC-positive or PCR-positive results.
T cell recovery and co-stimulatory molecule expression
We prospectively quantified the percentages of the total T cells and T cell subsets in fresh peripheral blood samples collected after allo-HSCT. Furthermore, it is well established that T cell alloreactivity is determined in part by the balance between the co-stimulatory and co-inhibitory pathways, which serve to keep the immune system in check. CD28 is the primary co-stimulatory molecule and is constitutively expressed on the majority of T cells. CD28 transduces a signal that enhances the activation and proliferation of T cells. Cytotoxic T lymphocyte antigen 4 (CTLA-4), a structural homolog of CD28, is a key factor in regulating and maintaining self-tolerance, providing a negative signal to T cell responses. Programmed death 1 (PD-1; CD279), a member of the B7:CD28 superfamily, is an inhibitory receptor that attenuates T cell receptor signaling by recruitment of phosphatases. Recently, the PD1/PDL1 pathway has emerged as a central player in immune regulation, and allogeneic effector T cell responses are susceptible to PD1 pathway modulation, as evidenced in animal models of GVHD [21,22,23]. Therefore, we first investigated the expression of co-stimulatory molecules including CD28, CTLA-4, and PD-1 on recovered allogeneic T cells.
Peripheral blood samples were obtained using heparin anti-coagulation tubes and stained without further separation to minimize selective loss shortly after collection, using directly conjugated monoclonal antibodies (mAbs) conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), allophycocyanin (APC), peridinin chlorophyll protein (Per-CP), and PE Cy7 recognizing CD3, CD4, CD8, CD45RA, CD45RO, CD25, FoxP3, CD28, CTLA-4 (CD152), and PD-1 (CD279).
Statistical analysis
The sample size was calculated on the basis of expected 2-year OS rate of 50% in refractory AML patients receiving our novel sequential transplant regimen and 15% of the null hypothesis rate referenced by the published studies evaluating agents for allo-HSCT therapy in refractory AML. We estimated that a sample size of 25 patients would provide at least 80% power to reject the null hypothesis that OS rate is not higher than 15%, with a type I error level of 5% (two-sided).
The primary endpoint of the study was OS defined as time to death from any cause. The secondary endpoints included LFS, relapse, non-relapse mortality (NRM), incidences of acute and chronic GVHD, as well as an evaluation of immune reconstitution. Relapse was defined as recurrence of BM blasts > 5%, reappearance of blasts in the peripheral blood, or development of extramedullary disease infiltrates at any site. Patients who were MRD-positive were not considered as having relapsed for LFS determination. LFS was defined as the time interval to the first event (relapse or death). Acute and chronic GVHD, incidence of serious, life-threatening, or fatal infection, NRM, and relapse were described using cumulative incidence, with relapse as the competing event for NRM and death as the competing event for all other outcomes. OS and LFS were estimated using the Kaplan-Meier method.
Results
Patients, disease, and transplantation characteristics
A total of 24 consecutive patients met the study eligibility criteria. The characteristics of the patients, AML disease, and transplantation are shown in Table 1. The median age of patients was 27.5 (range, 8–45). Only one patient aged < 14 years was included in the trial after approval of the protocol steering committee. Nineteen patients (79.2%) had de novo AML, three patients (12.5%) had AML secondary to myelodysplastic syndrome (MDS), and two patients (8.3%) had blast phase chronic myeloid leukemia (CML) refractory to tyrosine kinase inhibitors (imatinib, dasatinib, and nilotinib) combined with chemotherapy. AML cytogenetic risk groups at diagnosis, classified according to the European Leukemia Net [24], were intermediate in 11 patients (45.8%) and adverse in 12 (50%); one patient had favorable cytogenetics at diagnosis but experienced relapse.
At the time of transplantation, 18 patients (75%) had PIF and six patients had relapsed leukemia that was refractory to salvage chemotherapy (three patients with early relapse, two patients with relapse > 180 days, and one patient with third relapse). Besides the two patients who had < 5% blasts in the bone marrow but with persistent extramedullary disease, the median percentages of leukemic blasts in bone marrow and peripheral blood (PB) before the start of CLAM re-induction chemotherapy were 21.25% (range, 6–90%) and 3% (0–92%), respectively. A marrow blast infiltration > 20% or any level of PB blasts was found in 62.5% of patients. In four patients (16.7%), the Karnofsky score was < 90%, whereas in 20 patients (83.3%), it was ≥ 90%. Ten patients (41.7%) underwent MSD-HSCTs, one patient (4.2%) underwent URD-HSCT, and 13 patients (54.2%) underwent HRD-HSCTs.
The endpoint of the final follow-up for all of the surviving patients was December 1, 2017. The median follow-up for surviving patients was 21 months (range 9.5–40).
Engraftment and chimerism
The median infused MNC count and CD34+ cell count were 11.45 × 108/kg (range 4.86–18.35 × 108/kg) and 6.78 × 106/kg (range 2.27–18.46 × 106/kg), respectively. All 24 patients engrafted with absolute neutrophil counts exceeding 0.5 × 109/L in a median time of 12 days (range 8–16). The median time to platelet engraftment was 14.5 days (range 8–28). Following myeloid recovery, all patients achieved sustained, full donor chimerism by day + 30 after HSCT.
Donor lymphocyte infusion
Apart from three patients with unavailability or unwillingness of their donors and two patients experiencing aGVHD, 19 patients (79.2%) received DLI in our study. Of these 19 patients, 15 patients (78.9%) fulfilled the criteria for pDLI and received pDLI at a median of 3 months (range 2–4.7 months) post-HSCT. Full donor chimerism was detected in all 15 patients at the time of pDLI. Patients received only one transfusion, and the median dose of infused CD3+ cells was 3.34 (range, 1–9.35) × 107/kg. The remaining four patients received targeted therapeutic DLI plus chemotherapy as intervention for positive MRD (n = 1) and hematologic relapse (n = 3). Antileukemia chemotherapy before therapeutic DLI included decitabine (20 mg/m2 per day for 5 days), aclarubicin (10 mg/m2 per day for 5 days), and Ara-C (100 mg/m2 per day for 5 days). All four patients had mixed chimerism and received the first therapeutic DLI at 17.5, 5, 2, and 2 months post-HSCT, respectively. The median dose of the first CD3+ cell infusion was 4.4 (range, 3.24–9.33) × 107/kg. One patient received one transfusion, and three received two transfusions with escalating doses.
Acute and chronic graft-versus-host disease
All 24 patients achieved successful engraftment and survived at least 30 days. Consequently, all patients were included in the analysis of aGVHD. Only four patients experienced aGVHD, and the cumulative incidence of grades II–IV aGVHD at 100 days post-HSCT was 16.7%. In four patients, one had grade III aGVHD at day + 45, two had grade IV disease at days + 20 and + 41 after transplantation, and only one patient developed grade II aGVHD after receiving DLI and withdrawal of CSA.
The cumulative incidences of chronic GVHD (cGVHD) and severe cGVHD at 1 year were 33 and 18.6%, respectively.
Toxicities and infectious complications
Five episodes of bacteremia were detected in three patients (two patients reported two episodes), causing symptoms of sepsis or septic shock in two cases. Two patients developed bacteremia before myeloid recovery, and one patient experienced refractory septic shock caused by Pseudomonas aeruginosa during treatment of cGVHD. Seven patients experienced pneumonia, caused by Aspergillus spp. in six patients, and unknown etiology in one patient, and of these seven, three patients developed pneumonia before myeloid recovery, and four patients 6 months post-HSCT.
Non-hematologic side effects not related to GVHD or infections were classified according to World Health Organization criteria. Seventeen patients (70.8%) developed none or maximum grade I toxicity. In contrast, nine grade III–IV adverse events were reported in seven patients (Table 2). No deaths were caused.
Disease response and outcomes
At day + 30, all 24 patients were alive and the CR rate was 100% (including two patients with extramedullary disease). After a median follow-up of 21 months (range, 9.5–40 months), disease relapse occurred in seven patients at a median of 4 months (range, 1.8–13.6 months) post-HSCT. The cumulative incidence of relapse at 2 years post-HSCT was 29.8% (Fig. 2a). As of December 1, 2017, nine patients had died at a median time of 8 months (range 1.7–23.3 months) after HSCT, of whom five patients died after relapsing, two patients died of invasive pulmonary fungal infections, and two patients died of severe aGVHD. The cumulative incidences of NRM at 1 and 2 years were 12.5 and 20.6%, respectively (Fig. 2b). Besides two patients who achieved the second CR (CR2) and were still alive after relapse, 13 patients were alive with persistent MRD-negative tests detected by MFC and RQ-PCR. The Kaplan-Meier estimates of OS at 1 and 2 years were 74.5 and 56.5%, respectively (Fig. 2c). The respective LFS rates were 62.5 and 50.5% (Fig. 2d).
Immune reconstitution and expression of immune checkpoints on recovered T cells
To explore whether our intensified sequential transplant approach delayed immune reconstitution post-HSCT, we evaluated changes in T cell populations of CD4+ T cells, CD8+ T cells, CD4+ regulatory T cells (Tregs, CD4+CD25+FoxP3+), and cytotoxic T cells (CTLs, CD3+CD4+CD25+), as well as expression of pivotal immune checkpoints, CTLA-4 (CD152) and PD-1 (CD279), on recovered T cells using peripheral blood samples obtained post-transplantation. The outcomes of immune reconstitution are shown in Supplementary Table 1. Except for persistent CD4+ T lymphopenia, the percentages of total T cells and CD8+ T cells, Tregs and CTL cell subsets in peripheral blood were all recovered by around 3 months post-HSCT. The ratio of CD4+/CD8+ T cells was significantly inverted up to 1 year post-transplantation.
The expression of immune checkpoints (CTLA-4 and PD-1) was extremely low (persistently < 10%) on both CD4+- and CD8+-T cells throughout the first year post-transplantation (Supplementary Table 1).
Univariate and multivariate analyses for leukemia relapse and LFS post-HSCT
Kaplan-Meier analysis for leukemia relapse and LFS was performed according to major known risk factors including patient age, donor type, AML diagnosis, WBC count at diagnosis, cytogenetics risk group at diagnosis, number of chemotherapy cycles pre-HSCT, disease status at HSCT, blasts in bone marrow at HSCT, blasts in PB at HSCT, CD34+ counts in the graft, pDLI, aGVHD, and cGVHD. Multivariate Cox regression models using a forward stepwise procedure with the likelihood ratio criterion (inclusion/exclusion criteria: p < 0.05/p > 0.1 respectively) were applied to analyze the effects of these known clinical and biological factors on HSCT outcomes. All variables in the univariate analysis with a P value at or below 0.2 were included in the multivariate analysis.
Multivariate Cox regression analysis revealed that patients receiving stem cells from an HRD (P = 0.034, RR = 15.108), receiving pDLI (P = 0.011, RR = 23.315), and experiencing cGVHD (P = 0.04, RR = 23.12) were protective from relapse. Acute GVHD had an adverse effect on LFS (P = 0.001, RR = 15.059). In contrast, cGVHD had a beneficial effect on LFS (P = 0.016, RR = 6.961) (Table 3).
Discussion
Primary induction failure and relapse remain among the most challenging scenarios in the management of AML. Although allo-HSCT is considered to be the best treatment option for these patients, disappointing outcomes have been reported in several retrospective trials, including in a large patient population with active AML at allotransplant. To date, the largest retrospective analysis conducted by the Center for International Blood and Marrow Transplant Research (CIBMTR) including 1673 AML patients with active disease at the time of allo-HSCT, who received TBI- or busulfan-based MAC, found a 3-year OS rate of 19% [5]. Recently published outcome data obtained from the European Group for Blood and Marrow Transplantation (EBMT) on 1041 patients with primary refractory AML allotransplanted with HLA-matched sibling donors or unrelated donors showed that 2-year LFS and OS were approximately 25 and 30%, respectively [25]. Data from a similar cohort of 523 AML patients from 20 Italian HSCT centers, who were allotransplanted with active disease receiving MAC or RIC allo-HSCT from an HLA-identical sibling, or matched unrelated cord blood, or a haplo/mismatched unrelated donor, reported that 3-year OS and LFS were 16 and 21%, and the 3-year relapse rate was 56% in patients receiving MAC and 60% in RIC, respectively [26].
The past few years have seen growing improvement and increasing interest in sequential transplant regimens by combining intensive chemotherapy before conditioning for allo-HSCT to minimize the leukemic burden as a “bridge to transplantation” with DLI post-HSCT to accelerate the GVL effect. Since 2005, Schmid et al. developed the concept of the FLAMSA-RIC protocol, sequential use of intensive chemotherapy based on Flu/Ara-c/amsacrine, 4-Gy TBI-based RIC allo-HSCT, and prophylactic DLI. The results achieved with the FLAMSA-RIC strategy in refractory AML are among the most promising published so far. Schmid et al. first used the FLAMSA-RIC sequential transplant regimen in 75 patients with high-risk AML and MDS and achieved a 2-year OS of 42% and LFS of 40% [27]. They further used the same treatment in 103 patients with refractory AML and reported improved survival, with 2-year OS and LFS of 40 and 37%, respectively [3]. Pfeiffer et al. uniformly used the FLAMSA-RIC protocol in 141 adult AML patients who had received an allo-HSCT either in PIF or beyond first CR and achieved a 4-year OS of 44 ± 5% [9]. The latest retrospective multicenter analysis released by the Acute Leukaemia Working Party of the EBMT Group Registry on 267 patients with refractory/relapsed AML treated according to the FLAMSA-RIC protocol showed that the 3-year OS, LFS, and relapse rate were 30.4, 25.6 and 48.5%, respectively [28].
However, the results remain unsatisfactory in terms of relapse incidence and long-term leukemia control, and the high incidence of severe toxicities, related to amsacrine (mainly cardiotoxicity) and TBI. Several studies focused on improving the sequential approach by modifying chemotherapeutic strategies and use of MAC and/or non-TBI-based conditioning regimens. Liu et al. recently reported that using the strategy of Flu/Ara-C salvage chemotherapy and TBI/CY/VP-16 MAC allo-HSCT followed by prophylactic DLI in 153 refractory patients with advanced acute leukemia, of whom 50.3% had acute lymphoblastic leukemia, 37.3% had AML, and 12.4% had acute biphenotypic leukemia, 5-year OS and LFS were 51.1 and 27.3%, respectively [29]. Mohty et al. developed a new sequential transplant option in primary refractory AML through avoidance of amsacrine and the use of clofarabine and intravenous Bu to replace Flu and TBI, respectively. Data from their multicenter prospective phase 2 study showed that 2-year OS and LFS were 38 and 29%, respectively [30].
Our novel sequential approach used in PIF/refractory relapsed AML patients could yield a 100% rate of achieving CR in all 24 evaluable patients at the time of neutrophil reconstitution and improved 2-year OS and LFS (56.5 and 50.5%) with a very low incidence of 2-year NRM (20.6%), acceptable incidences of aGVHD (16.7%) and cGVHD (33%). Several factors may be related to our beneficial outcomes of the sequential transplant approach. First, cladribine-based re-induction chemotherapy and Bu-based MAC reduced the leukemia burden at the time of transplantation. Although there is no standard salvage chemotherapy regimen for all patients with refractory/early relapsed AML, high-dose cytarabine in combination with mitoxantrone is a common regimen used in refractory/relapsed AML [31]. Adding purine analogs including fludarabine, clofarabine, and cladribine to salvage chemotherapy is also practiced in this situation with an improved CR rate [32,33,34]. To our knowledge, our study is the first prospective clinical trial to use cladribine, high-dose AraC, and mitoxantrone (CLAM regimen) as re-induction chemotherapy in the setting of a sequential allo-HSCT approach and we achieved CR of 100% by day 30 post-HSCT, while rates of 90 and 75% have been reported in Flu- or clofarabine-based sequential transplant regimens, respectively [9, 30]. On the other hand, intravenous Bu-based MAC also contributed to the higher CR rate. That the TBI-containing regimen in patients with AML has no distinct advantage in leukemia control but has a higher rate of NRM over the intravenous Bu-containing conditioning regimen has been widely recognized [35, 36].
Secondly, earlier pDLI while patients are still under immunosuppressive therapy might be expected to result in acceleration of a GVL effect without increasing the risk of GVHD. In our study, 78.9% of patients received pDLI at a median of 3 months (range 2–4.7 months) post-HSCT. Further multivariate analysis also revealed that pDLI was the favorable factor in reducing relapse, which is consistent with a study published by Liu et al. on sequential transplant options in refractory acute leukemia [29]. On the other hand, very few patients receiving pDLI in a published study on Flu- or clofarabine-based sequential transplant regimens, and 16.5%, 13 and 25% receiving pDLI in studies conducted by Schmid et al. [3], EBMT [28] and Mohty et al. [30], respectively, may contribute to inferior OS and LFS.
Thirdly, in our study, > 50% of refractory AML patients received an HRD-HSCT compared with the majority of those patients receiving an MSD or URD in the above-mentioned published studies on sequential transplant options. The priority use of HRD-HSCT, either with or without an in vitro T cell depletion approach, in high-risk patients, has been well established and can achieve a stronger GVL effect than MSD or URD in high-risk acute leukemia patients [19, 37,38,39,40]. In our multivariate analysis, HRD was identified as a protective factor from relapse.
Although patient age was not identified to have significant effect on transplant outcomes in our univariate and multivariate analyses, our patients were younger than patients in above-mentioned studies. Median age of patients was 27.5 in our cohort, 52.3 in Schmid’s study [27], and 47 in Mohty’s study [30]. There are also other aspects that might have contributed to our beneficial results including high percentage of patients with PIF, improvement in supportive care over time and so on.
Finally, our intensified sequential transplant regimen yielded perfect efficacy to decrease the leukemia burden while retaining substantial antileukemic activity after allo-HSCT. Our regimen did not aggravate the delay in immune recovery or increase the risk of infection. Although the ratio of CD4+/CD8+ T cells was significantly inverted up to 1 year after transplantation, the percentages of total T cells and CD8+ T cells, Tregs, and CTL cell subsets in the PB all recovered around 3 months post-HSCT, which is comparable to data reported by studies on immune reconstitution post allo-HSCT using standard myeloablative conditioning [41, 42]. This observation was strengthened by analysis of the expression of immune checkpoints (CTLA-4 and PD-1) on recovered T cells post-HSCT, showing that they were extremely low (persistently < 10%) on both CD4+- and CD8+-T cells throughout the first year post-transplantation.
In conclusion, our data provide preliminary data to illustrate the feasibility and efficacy of our novel sequential transplant approach to treat primary refractory/relapsed AML by using the combination of a CLAM chemotherapy regimen for re-induction, followed by Bu-based MAC allo-HSCT and early pDLI. We must acknowledge some limitations of our study including the small number of patients resulting in too large a 95% confidence interval (CI) in multivariate analysis and the short follow-up period. Further evaluation is also needed to be validated in a phase 3 randomized trial including a large number and more homogeneous of patient population and longer follow-up.
References
Juliusson G, Antunovic P, Derolf A, Lehmann S, Mollgard L, Stockelberg D, Tidefelt U, Wahlin A, Hoglund M (2009) Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood 113(18):4179–4187. https://doi.org/10.1182/blood-2008-07-172007
Buchner T, Schlenk RF, Schaich M, Dohner K, Krahl R, Krauter J, Heil G, Krug U, Sauerland MC, Heinecke A, Spath D, Kramer M, Scholl S, Berdel WE, Hiddemann W, Hoelzer D, Hehlmann R, Hasford J, Hoffmann VS, Dohner H, Ehninger G, Ganser A, Niederwieser DW, Pfirrmann M (2012) Acute myeloid leukemia (AML): different treatment strategies versus a common standard arm—combined prospective analysis by the German AML Intergroup. J Clin Oncol 30(29):3604–3610. https://doi.org/10.1200/JCO.2012.42.2907
Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D, Harsdorf SV, Scheid C, Holtick U, Greinix H, Keil F, Schneider B, Sandherr M, Bug G, Tischer J, Ledderose G, Hallek M, Hiddemann W, Kolb HJ (2006) Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood 108(3):1092–1099. https://doi.org/10.1182/blood-2005-10-4165
Michallet M, Thomas X, Vernant JP, Kuentz M, Socie G, Esperou-Bourdeau H, Milpied N, Blaise D, Rio B, Reiffers J, Jouet JP, Cahn JY, Bourhis JH, Lioure B, Leporrier M, Sotto JJ, Souillet G, Sutton L, Bordigoni P, Dreyfus F, Tilly H, Gratecos N, Attal M, Leprise PY, Demeocq F, Michel G, Buzyn A, Delmas-Marsalet B, Bernaudin F, Ifrah N, Sadoun A, Guyotat D, Cavazzana-Cavo M, Caillot D, De Revel T, Vannier JP, Baruchel A, Fegueux N, Tanguy ML, Thiebaut A, Belhabri A, Archimbaud E (2000) Long-term outcome after allogeneic hematopoietic stem cell transplantation for advanced stage acute myeloblastic leukemia: a retrospective study of 379 patients reported to the Societe Francaise de Greffe de Moelle (SFGM). Bone Marrow Transplant 26(11):1157–1163. https://doi.org/10.1038/sj.bmt.1702690
Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM, Kamble R, Copelan E, de Lima M, Gupta V, Keating A, Lazarus HM, Litzow MR, Marks DI, Maziarz RT, Rizzieri DA, Schiller G, Schultz KR, Tallman MS, Weisdorf D (2010) Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol 28(23):3730–3738. https://doi.org/10.1200/JCO.2010.28.8852
Craddock C, Labopin M, Pillai S, Finke J, Bunjes D, Greinix H, Ehninger G, Steckel NK, Zander AR, Schwerdtfeger R, Buchholz S, Kolb HJ, Volin L, Fauser A, Polge E, Schmid C, Mohty M, Rocha V (2011) Factors predicting outcome after unrelated donor stem cell transplantation in primary refractory acute myeloid leukaemia. Leukemia 25(5):808–813. https://doi.org/10.1038/leu.2011.13
Ringden O, Labopin M, Schmid C, Sadeghi B, Polge E, Tischer J, Ganser A, Michallet M, Kanz L, Schwerdtfeger R, Nagler A, Mohty M (2016) Sequential chemotherapy followed by reduced-intensity conditioning and allogeneic haematopoietic stem cell transplantation in adult patients with relapse or refractory acute myeloid leukaemia: a survey from the Acute Leukaemia Working Party of EBMT. Br J Haematol 176:431–439. https://doi.org/10.1111/bjh.14428
Holtick U, Shimabukuro-Vornhagen A, Chakupurakal G, Theurich S, Leitzke S, Burst A, Hallek M, von Bergwelt-Baildon M, Scheid C, Chemnitz JM (2016) FLAMSA reduced-intensity conditioning is equally effective in AML patients with primary induction failure as well as in first or second complete remission. Eur J Haematol 96(5):475–482. https://doi.org/10.1111/ejh.12615
Pfeiffer T, Schleuning M, Mayer J, Haude KH, Tischer J, Buchholz S, Bunjes D, Bug G, Holler E, Meyer RG, Greinix H, Scheid C, Christopeit M, Schnittger S, Braess J, Schlimok G, Spiekermann K, Ganser A, Kolb HJ, Schmid C (2013) Influence of molecular subgroups on outcome of acute myeloid leukemia with normal karyotype in 141 patients undergoing salvage allogeneic stem cell transplantation in primary induction failure or beyond first relapse. Haematologica 98(4):518–525. https://doi.org/10.3324/haematol.2012.070235
Freyer CW, Gupta N, Wetzler M, Wang ES (2015) Revisiting the role of cladribine in acute myeloid leukemia: an improvement on past accomplishments or more old news? Am J Hematol 90(1):62–72. https://doi.org/10.1002/ajh.23862
Gandhi V, Estey E, Keating MJ, Chucrallah A, Plunkett W (1996) Chlorodeoxyadenosine and arabinosylcytosine in patients with acute myelogenous leukemia: pharmacokinetic, pharmacodynamic, and molecular interactions. Blood 87(1):256–264
Kornblau SM, Gandhi V, Andreeff HM, Beran M, Kantarjian HM, Koller CA, O'Brien S, Plunkett W, Estey E (1996) Clinical and laboratory studies of 2-chlorodeoxyadenosine +/− cytosine arabinoside for relapsed or refractory acute myelogenous leukemia in adults. Leukemia 10(10):1563–1569
Holowiecki J, Grosicki S, Robak T, Kyrcz-Krzemien S, Giebel S, Hellmann A, Skotnicki A, Jedrzejczak WW, Konopka L, Kuliczkowski K, Zdziarska B, Dmoszynska A, Marianska B, Pluta A, Zawilska K, Komarnicki M, Kloczko J, Sulek K, Haus O, Stella-Holowiecka B, Baran W, Jakubas B, Paluszewska M, Wierzbowska A, Kielbinski M, Jagoda K (2004) Addition of cladribine to daunorubicin and cytarabine increases complete remission rate after a single course of induction treatment in acute myeloid leukemia. Multicenter, phase III study. Leukemia 18(5):989–997. https://doi.org/10.1038/sj.leu.2403336
Holowiecki J, Grosicki S, Giebel S, Robak T, Kyrcz-Krzemien S, Kuliczkowski K, Skotnicki AB, Hellmann A, Sulek K, Dmoszynska A, Kloczko J, Jedrzejczak WW, Zdziarska B, Warzocha K, Zawilska K, Komarnicki M, Kielbinski M, Piatkowska-Jakubas B, Wierzbowska A, Wach M, Haus O (2012) Cladribine, but not fludarabine, added to daunorubicin and cytarabine during induction prolongs survival of patients with acute myeloid leukemia: a multicenter, randomized phase III study. J Clin Oncol 30(20):2441–2448. https://doi.org/10.1200/JCO.2011.37.1286
Wrzesien-Kus A, Robak T, Wierzbowska A, Lech-Maranda E, Pluta A, Wawrzyniak E, Krawczynska A, Kuliczkowski K, Mazur G, Kiebinski M, Dmoszynska A, Wach M, Hellmann A, Baran W, Holowiecki J, Kyrcz-Krzemien S, Grosicki S (2005) A multicenter, open, noncomparative, phase II study of the combination of cladribine (2-chlorodeoxyadenosine), cytarabine, granulocyte colony-stimulating factor and mitoxantrone as induction therapy in refractory acute myeloid leukemia: a report of the Polish Adult Leukemia Group. Ann Hematol 84(9):557–564. https://doi.org/10.1007/s00277-005-1046-0
Wierzbowska A, Robak T, Pluta A, Wawrzyniak E, Cebula B, Holowiecki J, Kyrcz-Krzemien S, Grosicki S, Giebel S, Skotnicki AB, Piatkowska-Jakubas B, Kuliczkowski K, Kielbinski M, Zawilska K, Kloczko J, Wrzesien-Kus A (2008) Cladribine combined with high doses of arabinoside cytosine, mitoxantrone, and G-CSF (CLAG-M) is a highly effective salvage regimen in patients with refractory and relapsed acute myeloid leukemia of the poor risk: a final report of the Polish Adult Leukemia Group. Eur J Haematol 80(2):115–126. https://doi.org/10.1111/j.1600-0609.2007.00988.x
Ferguson P, Hills RK, Grech A, Betteridge S, Kjeldsen L, Dennis M, Vyas P, Goldstone AH, Milligan D, Clark RE, Russell NH, Craddock C (2016) An operational definition of primary refractory acute myeloid leukemia allowing early identification of patients who may benefit from allogeneic stem cell transplantation. Haematologica 101(11):1351–1358. https://doi.org/10.3324/haematol.2016.148825
Wattad M, Weber D, Dohner K, Krauter J, Gaidzik VI, Paschka P, Heuser M, Thol F, Kindler T, Lubbert M, Salih HR, Kundgen A, Horst HA, Brossart P, Gotze K, Nachbaur D, Kohne CH, Ringhoffer M, Wulf G, Held G, Salwender H, Benner A, Ganser A, Dohner H, Schlenk RF (2017) Impact of salvage regimens on response and overall survival in acute myeloid leukemia with induction failure. Leukemia 31(6):1306–1313. https://doi.org/10.1038/leu.2017.23
Luo Y, Xiao H, Lai X, Shi J, Tan Y, He J, Xie W, Zheng W, Zhu Y, Ye X, Yu X, Cai Z, Lin M, Huang H (2014) T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood 124(17):2735–2743. https://doi.org/10.1182/blood-2014-04-571570
Cilloni D, Renneville A, Hermitte F, Hills RK, Daly S, Jovanovic JV, Gottardi E, Fava M, Schnittger S, Weiss T, Izzo B, Nomdedeu J, van der Heijden A, van der Reijden BA, Jansen JH, van der Velden VH, Ommen H, Preudhomme C, Saglio G, Grimwade D (2009) Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J Clin Oncol 27(31):5195–5201. https://doi.org/10.1200/JCO.2009.22.4865
Blazar BR, Carreno BM, Panoskaltsis-Mortari A, Carter L, Iwai Y, Yagita H, Nishimura H, Taylor PA (2003) Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol 171(3):1272–1277
Amarnath S, Mangus CW, Wang JC, Wei F, He A, Kapoor V, Foley JE, Massey PR, Felizardo TC, Riley JL, Levine BL, June CH, Medin JA, Fowler DH (2011) The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med 3(111):111ra120. https://doi.org/10.1126/scitranslmed.3003130
Saha A, Aoyama K, Taylor PA, Koehn BH, Veenstra RG, Panoskaltsis-Mortari A, Munn DH, Murphy WJ, Azuma M, Yagita H, Fife BT, Sayegh MH, Najafian N, Socie G, Ahmed R, Freeman GJ, Sharpe AH, Blazar BR (2013) Host programmed death ligand 1 is dominant over programmed death ligand 2 expression in regulating graft-versus-host disease lethality. Blood 122(17):3062–3073. https://doi.org/10.1182/blood-2013-05-500801
Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson RA, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz MA, Sierra J, Tallman MS, Lowenberg B, Bloomfield CD (2010) Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 115(3):453–474. https://doi.org/10.1182/blood-2009-07-235358
Brissot E, Labopin M, Stelljes M, Ehninger G, Schwerdtfeger R, Finke J, Kolb HJ, Ganser A, Schafer-Eckart K, Zander AR, Bunjes D, Mielke S, Bethge WA, Milpied N, Kalhs P, Blau IW, Kroger N, Vitek A, Gramatzki M, Holler E, Schmid C, Esteve J, Mohty M, Nagler A (2017) Comparison of matched sibling donors versus unrelated donors in allogeneic stem cell transplantation for primary refractory acute myeloid leukemia: a study on behalf of the Acute Leukemia Working Party of the EBMT. J Hematol Oncol 10(1):130. https://doi.org/10.1186/s13045-017-0498-8
Todisco E, Ciceri F, Oldani E, Boschini C, Mico C, Vanlint MT, Donnini I, Patriarca F, Alessandrino PE, Bonifazi F, Arcese W, Barberi W, Marenco P, Terruzzi E, Cortelazzo S, Santarone S, Proia A, Corradini P, Tagliaferri E, Falcioni S, Irrera G, Dallanegra L, Castagna L, Santoro A, Camboni A, Sacchi N, Bosi A, Bacigalupo A, Rambaldi A (2013) The CIBMTR score predicts survival of AML patients undergoing allogeneic transplantation with active disease after a myeloablative or reduced intensity conditioning: a retrospective analysis of the Gruppo Italiano Trapianto Di Midollo Osseo. Leukemia 27(10):2086–2091. https://doi.org/10.1038/leu.2013.208
Schmid C, Schleuning M, Ledderose G, Tischer J, Kolb HJ (2005) Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol 23(24):5675–5687. https://doi.org/10.1200/JCO.2005.07.061
Ringden O, Labopin M, Schmid C, Sadeghi B, Polge E, Tischer J, Ganser A, Michallet M, Kanz L, Schwerdtfeger R, Nagler A, Mohty M (2017) Sequential chemotherapy followed by reduced-intensity conditioning and allogeneic haematopoietic stem cell transplantation in adult patients with relapse or refractory acute myeloid leukaemia: a survey from the Acute Leukaemia Working Party of EBMT. Br J Haematol 176(3):431–439. https://doi.org/10.1111/bjh.14428
Xuan L, Fan Z, Zhang Y, Zhou H, Huang F, Dai M, Nie D, Lin D, Xu N, Guo X, Jiang Q, Sun J, Xiao Y, Liu Q (2016) Sequential intensified conditioning followed by prophylactic DLI could reduce relapse of refractory acute leukemia after allo-HSCT. Oncotarget 7(22):32579–32591. https://doi.org/10.18632/oncotarget.8691
Mohty M, Malard F, Blaise D, Milpied N, Socie G, Huynh A, Reman O, Yakoub-Agha I, Furst S, Guillaume T, Tabrizi R, Vigouroux S, Peterlin P, El-Cheikh J, Moreau P, Labopin M, Chevallier P (2017) Sequential regimen of clofarabine, cytosine arabinoside and reduced-intensity conditioned transplantation for primary refractory acute myeloid leukemia. Haematologica 102(1):184–191. https://doi.org/10.3324/haematol.2016.150326
Kern W, Aul C, Maschmeyer G, Schonrock-Nabulsi R, Ludwig WD, Bartholomaus A, Bettelheim P, Wormann B, Buchner T, Hiddemann W (1998) Superiority of high-dose over intermediate-dose cytosine arabinoside in the treatment of patients with high-risk acute myeloid leukemia: results of an age-adjusted prospective randomized comparison. Leukemia 12(7):1049–1055
Faderl S, Wetzler M, Rizzieri D, Schiller G, Jagasia M, Stuart R, Ganguly S, Avigan D, Craig M, Collins R, Maris M, Kovacsovics T, Goldberg S, Seiter K, Hari P, Greiner J, Vey N, Recher C, Ravandi F, Wang ES, Vasconcelles M, Huebner D, Kantarjian HM (2012) Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I Trial. J Clin Oncol 30(20):2492–2499. https://doi.org/10.1200/JCO.2011.37.9743
Thol F, Schlenk RF, Heuser M, Ganser A (2015) How I treat refractory and early relapsed acute myeloid leukemia. Blood 126(3):319–327. https://doi.org/10.1182/blood-2014-10-551911
Middeke JM, Herbst R, Parmentier S, Bug G, Hanel M, Stuhler G, Schafer-Eckart K, Rosler W, Klein S, Bethge W, Bitz U, Buttner B, Knoth H, Alakel N, Schaich M, Morgner A, Kramer M, Sockel K, von Bonin M, Stolzel F, Platzbecker U, Rollig C, Thiede C, Ehninger G, Bornhauser M, Schetelig J (2016) Clofarabine salvage therapy before allogeneic hematopoietic stem cell transplantation in patients with relapsed or refractory AML: results of the BRIDGE trial. Leukemia 30(2):261–267. https://doi.org/10.1038/leu.2015.226
Uberti JP, Agovi MA, Tarima S, Haagenson M, Gandham S, Anasetti C, Baker KS, Bolwell BJ, Bornhauser M, Chan KW, Copelan E, Davies SM, Finke J, Hale GA, Kollman C, McCarthy PL, Ratanatharathorn V, Ringden O, Weisdorf DJ, Rizzo JD (2011) Comparative analysis of BU and CY versus CY and TBI in full intensity unrelated marrow donor transplantation for AML, CML and myelodysplasia. Bone Marrow Transplant 46(1):34–43. https://doi.org/10.1038/bmt.2010.81
Nagler A, Rocha V, Labopin M, Unal A, Ben Othman T, Campos A, Volin L, Poire X, Aljurf M, Masszi T, Socie G, Sengelov H, Michallet M, Passweg J, Veelken H, Yakoub-Agha I, Shimoni A, Mohty M (2013) Allogeneic hematopoietic stem-cell transplantation for acute myeloid leukemia in remission: comparison of intravenous busulfan plus cyclophosphamide (Cy) versus total-body irradiation plus Cy as conditioning regimen—a report from the acute leukemia working party of the European group for blood and marrow transplantation. J Clin Oncol 31(28):3549–3556. https://doi.org/10.1200/JCO.2013.48.8114
Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S, Felicini R, Falcinelli F, Velardi A, Ruggeri L, Aloisi T, Saab JP, Santucci A, Perruccio K, Martelli MP, Mecucci C, Reisner Y, Martelli MF (2005) Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol 23(15):3447–3454. https://doi.org/10.1200/JCO.2005.09.117
Ciceri F, Labopin M, Aversa F, Rowe JM, Bunjes D, Lewalle P, Nagler A, Di Bartolomeo P, Lacerda JF, Lupo Stanghellini MT, Polge E, Frassoni F, Martelli MF, Rocha V (2008) A survey of fully haploidentical hematopoietic stem cell transplantation in adults with high-risk acute leukemia: a risk factor analysis of outcomes for patients in remission at transplantation. Blood 112(9):3574–3581. https://doi.org/10.1182/blood-2008-02-140095
Wang Y, Liu DH, Xu LP, Liu KY, Chen H, Chen YH, Han W, Shi HX, Huang XJ (2011) Superior graft-versus-leukemia effect associated with transplantation of haploidentical compared with HLA-identical sibling donor grafts for high-risk acute leukemia: an historic comparison. Biol Blood Marrow Transplant 17(6):821–830. https://doi.org/10.1016/j.bbmt.2010.08.023
Srour SA, Milton DR, Bashey A, Karduss-Urueta A, Al Malki MM, Romee R, Solomon S, Nademanee A, Brown S, Slade M, Perez R, Rondon G, Forman SJ, Champlin RE, Kebriaei P, Ciurea SO (2017) Haploidentical transplantation with post-transplantation cyclophosphamide for high-risk acute lymphoblastic leukemia. Biol Blood Marrow Transplant 23(2):318–324. https://doi.org/10.1016/j.bbmt.2016.11.008
Chang YJ, Zhao XY, Huo MR, Xu LP, Liu DH, Liu KY, Huang XJ (2012) Immune reconstitution following unmanipulated HLA-mismatched/haploidentical transplantation compared with HLA-identical sibling transplantation. J Clin Immunol 32(2):268–280. https://doi.org/10.1007/s10875-011-9630-7
Chang YJ, Xu LP, Wang Y, Zhang XH, Chen H, Chen YH, Wang FR, Han W, Sun YQ, Yan CH, Tang FF, Mo XD, Liu KY, Huang XJ (2016) Controlled, randomized, open-label trial of risk-stratified corticosteroid prevention of acute graft-versus-host disease after haploidentical transplantation. J Clin Oncol 34(16):1855–1863. https://doi.org/10.1200/JCO.2015.63.8817
Acknowledgements
We thank all transplant center physicians who participated in the study.
Funding
This work was funded in part by the National Natural Science Foundation of China (81470309).
Author information
Authors and Affiliations
Contributions
Conception and design: Haowen Xiao.
Collection, analysis, and interpretation of the data: Haowen Xiao, Li Li, Yuanbin Wu, Jiulong Wu, and Fen Huang.
Drafting the article: Haowen Xiao.
Provision of study materials or patients: Haowen Xiao, Li Li, Yan Pang, Yuanbin Wu, Zujun Jiang, Zenghui Liu, Jiulong Wu, Yang Xiao, Fen Huang, Qifa Liu, Hang Zhang, Yi Luo, and He Huang.
Obtaining of funding: Haowen Xiao.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Table 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Xiao, H., Li, L., Pang, Y. et al. Sequential treatment combining cladribine-based re-induction, myeloablative allogeneic HSCT, and prophylactic donor lymphocyte infusion: a promising treatment for refractory acute myeloid leukemia. Ann Hematol 97, 2479–2490 (2018). https://doi.org/10.1007/s00277-018-3453-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-018-3453-z