Abstract
The clinical consequences of the infectious events in patients receiving azacitidine are poorly documented. Likewise, the role of primary antimicrobial prophylaxis is unknown. In this retrospective, single-center study, we compare the impact of prophylaxis on the incidence of infection and morbidity in all consecutive higher-risk myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) patients, during the first 4 azacitidine cycles. Seventy-six patients, corresponding to 283 azacitidine cycles, were studied. There were infectious events in 43% of the patients. Development of infections led to more hospital admissions, increased red blood cells and platelet requirements, and a delay in subsequent cycles. Median overall survival was comparable between patients with or without infections. In the multivariate analysis, a neutrophil count below 0.5 × 109/L (OR 12.5 [2.6–50]) and antimicrobial prophylaxis (OR 0.1 [0.02–04]) were independent factors for the development of infection. We conclude that infectious events have a significant impact in the early clinical course of azacitidine-treated patients by increasing hospital admissions and transfusion requirements. Antimicrobial prophylaxis may prevent infections, leading to a decreased need for supportive care in these patients with poor outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Azacitidine is currently considered the standard of care for patients with higher-risk myelodysplastic syndrome (MDS) who are not candidates for allogeneic stem cell transplantation in relation to their age or comorbidities. Based on a recent randomized trial [1], this drug has been also approved for treatment of patients with acute myeloid leukemia (AML) with more than 30% blasts in bone marrow and age above 65 years. Additionally, it can also be used as a debulking therapy before allogeneic stem cell transplantation [2]. Compared to intensive chemotherapy, this drug offers a better safety profile [3, 4], mainly due to a lower risk of infectious complications, especially after the first cycles of treatment. However, data regarding infection rates and their impact are scarce and have yielded heterogeneous results.
Antimicrobial prophylaxis is only indicated for patients undergoing intensive chemotherapy and/or allogeneic stem cell transplantation, as it is the only case where it has been unequivocally demonstrated to improve survival [5]. However, this level of evidence has not been determined for higher-risk MDS patients treated with the hypomethylating agents because of several reasons: the number of published studies is low and its nature is highly variable, most of them are retrospective studies whose data are limited to the clinical characteristics of the infectious events; the overall incidence of infection in them varies greatly; likewise, the mortality attributable to infection in these patients is unknown, since the figures are often biased due to the inclusion and mixing of patients with different outcomes. Consequently, there is no clinical evidence to establish a pattern of antimicrobial prophylaxis in this subgroup [6] and therefore, clinical practice varies according to the centers.
The main objective of this work is to evaluate the incidence, morbidity, and mortality of infectious events in patients with MDS or AML who receive azacitidine as first line of treatment, focusing on the impact of antimicrobial prophylaxis. Considering that more than 80% of the infections occur in the first cycles [7], we have performed a retrospective analysis of the first 4 azacitidine cycles with multivariate techniques to clarify the role of prophylactic antimicrobials.
Patients and methods
The present study was evaluated and approved by the Regional Ethics Committee. Due to its observational nature, the need for informed consent was waived.
Patients
All consecutive patients with a diagnosis of higher-risk MDS (defined as those with IPSS score equal or above 1.5) [8] who received azacitidine as first-line treatment at the Hematology Department at the University Central Hospital of Asturias (Oviedo, Spain), from December 2007 to October2016, were included in the study. Patients with MDS belonging to low- or intermediate-1 IPSS categories and neutrophil counts below 0.5 × 109/L or platelet counts below 30 × 109/L in peripheral blood were also included, as these patients show survival rates similar to higher-risk patients [9, 10]. Regarding leukemia patients, those with AML and more than 30% blasts in bone marrow aspirates, not candidates for transplantation, who received azacitidine in the setting of the AZA-AML-001 trial (along 2010) [1], or after approval of the drug for this indication, were also included. Comorbidity was measured using the Hematopoietic Cell Transplantation Comorbidity Index [11].
Azacitidine was administered in the outpatient setting at a dose of 75 mg/m2 for 7 days. Demographical and clinical data from these patients, including disease-related variables, infectious episodes, and follow-up, were collected and analyzed. Patients were followed weekly and peripheral blood counts, adherence to therapy, and adverse events systematically registered. Evaluation of response was performed after 6 cycles, as recommended [6]. Therefore, all deaths occurred before this evaluation were considered as related to treatment.
Antimicrobial prophylaxis and infectious events
The management of infections in these patients in our center evolved during the time of the study. In the first years after approval of azacitidine, the standard approach for infection prevention and management followed the ECIL guidelines for prophylaxis in acute leukemia [12]. Primary antimicrobial prophylaxis with ciprofloxacin and an extended-spectrum azole (posaconazole, voriconazole, or itraconazole) was indicated in all patients receiving azacitidine with a basal neutrophil count below 0.5 × 109/L. Prophylaxis was started from the first day of azacitidine cycle until neutrophil count was above 0.5 × 109/L. Later on, in 2013, as several groups reported low infection and infection-related mortality rates after treatment with azacitidine, we stopped routine administration of primary prophylaxis. Routine administration of granulocytic colony-stimulating factor was avoided.
Febrile episodes were defined as an increase in axillary temperature above 38 °C recorded twice, or 38.3 °C recorded once. The routine management of these episodes included complete physical exam, blood, urine and suspicious site cultures, chest x-rays, and other imaging techniques according to the suspected diagnosis. Only one case of fever was considered from non-infectious origin based on the presence of constitutional symptoms, negative cultures, no radiological findings and good response to steroids, and not included in the present analysis. The physician in charge of the patient decided hospital admissions. Empirical treatment according to the current ECIL guidelines and the local flora was administered in all patients.
Statistical analysis
Data are presented as mean ± standard deviation or as median (range). Univariate comparisons were done using the chi-square test (categorical variables), Fisher’s exact test (for contingency tables with frequencies below 5) or Wilcoxon test (for continuous variables). Variables with a p value in the comparison lower than 0.1 were introduced in a logistic regression, mixed-effects model to take into account the interindividual variability. A propensity score reflecting the probability of receiving prophylaxis, according to age, gender, blast count, and the WHO category, was calculated and introduced in the multivariate model to adjust for the putative baseline differences and to avoid indication bias. As prophylaxis in the subsequent cycles may not be an independent event, the analyses were repeated considering prophylaxis in the first cycle as an intent to treat. Odds ratios (OR) and their 95% confidence intervals were calculated and a p value lower than 0.05 was considered significant.
Overall survival was calculated from the day of diagnosis and from the beginning of azacitidine treatment until date of last follow-up or death from any cause. As evaluation of response to azacitidine was performed after 6 cycles, all deaths that occurred before this evaluation and within 42 days of each cycle initiation were considered as related to treatment. Probability of survival was estimated using the Kaplan-Meier method and the differences between survival curves evaluated using a log-rank test. Statistical analyses were performed using the R statistical package (version 3.2.1).
Results
Seventy-six patients with a diagnosis of MDS or AML were ncluded during the study period. Azacitidine was administered according to the approved EMA schedule (7 consecutive days) in 65 patients (85%). Due to logistic reasons, 11 patients (15%) followed a 5-2-2 scheme (5 days of treatment, followed by a weekend stop and two additional treatment days). Sixty-four patients (84%) completed the 4 azacitidine cycles, 4 (5%) patients received 3 cycles, 7 (10%) patients received 2 cycles, and 1 (1%) patient received a single cycle. Therefore, 283 cycles were available for analysis.
Median age of the sample was 70 (63–77) years. The majority of the MDS patients were included in categories with excess of blasts according to the WHO classification (RAEB-1 28%, RAEB-2 18%, AML 37%). As expected, 77% of the patients had an intermediate-high or high IPSS score. Sixty-three percent of the patients had at least one concomitant disease, with a comorbidity index above 3 in 10% of the cases. The first dose of azacitidine was administered 28 (14–77) days after diagnosis. The main clinical characteristics of the sample are detailed in Table 1.
Infectious episodes
There were 59 infectious events, corresponding to 21% of the 283 cycles of treatment, and affecting 33 (43%) patients. Thirteen patients had only one episode, fifteen patients had 2, four patients had 3, and only one patient had 4 infectious events. Along the whole episodes, 20 (34%) were classified as fever of unknown origin. In the remaining cases in which a focus was identified, the most frequent diagnoses were respiratory (18 cases, including 10 pneumonias) and urinary tract infections (8 episodes). In 15 episodes (25%), a pathogen was identified. The most commonly isolated pathogens were Pseudomonas aeruginosa (five isolations, one from urine and the other 4 from respiratory samples) and Escherichia coli (4 isolates, all of them from urine). There were two cases of invasive fungal infections: a proven Candida krusei candidemia and a probable pulmonary aspergillosis (positive galactomannan antigen, radiological lesion and neutropenia).
Univariate analyses showed that a higher IPSS score and comorbidity index, lower neutrophil counts at the start of each cycle and a longer duration of neutropenia during the cycle were factors related to infectious events. Table 2 shows the results of these univariate comparisons.
Impact of infectious events
The outcomes after an infectious episode were evaluated in four different dimensions: need for hospital admission, transfusional requirements, treatment-related mortality, and overall survival. There were 32 hospital admissions during cycles with an infectious event, compared to 0 in those without infection (p < 0.001). Median length of stay was 12 days.
Development of an infection was associated with a delay in the delivery of the next azacitidine cycle. The interval between cycles in patients without infection was 28 days (28–29) vs 31 (28–35) in those patients who suffered an infectious event (p < 0.001). The percentage of cycles with delay was also higher after an infection (Table 2). Infections were the main cause of delay between cycles 1 and 2, whereas cytopenias were more frequent than infection beyond cycle 2 (Fig. 1). Regarding transfusional requirements, infections were related to higher red blood cells and platelet transfusions (Table 2).
Overall, 12 (16%) patients died with infection. Eight of them (11% of the entire population) died from infection during the first 4 cycles of azacitidine without evaluation of the response. Among the remaining four patients, one died from infection after cycle 5 of azacitidine, three with infection in the context of progressive disease after cycles 7, 11, and 20, respectively. Infection was not associated to higher treatment-related mortality (9 out of 33 [27%] compared to 4 out of 43 [9%] in patients with and without infection, respectively, p = 0.08).
In 15 patients, azacitidine was used as a way to reduce disease burden before an allogeneic stem cell transplant. Among them, two patients died before SCT because of infectious complications after 2 and 5 cycles. Three died with infection in the context of progressive disease before an allogeneic stem cell transplant could be performed.
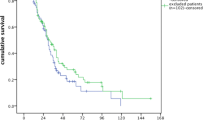
With a follow-up of 485 days (337–769), median overall survival of the whole sample was 554 days (493–1030). The median overall survival from the beginning of azacitidine treatment was 480 (381–609) days, with no differences between patients with infections (median survival 330 [274–660] days) and those without (487 [428–624] days, log-rank p = 0.243, Fig. 2). Causes of death were progression (39 patients, 75%), infection (9 patients, 17%) or related to other treatments once azacitidine was discontinued (4 patients, 8%).
Impact of prophylaxis
Antimicrobial prophylaxis was administered in 117 cycles (41%). There were significant differences between the cycles with and without prophylaxis. As shown in Table 3, cycles with prophylaxis showed lower neutrophil counts and more severe disease characteristics. The majority of patients (75%) received a combination therapy with quinolones and antifungals. Among the remaining, 5% received only quinolones and 20% received only antifungals. No patient developed clinically significant adverse events related to antimicrobial prophylaxis.
Globally, prophylaxis did not decrease the incidence of infection (17 vs 24%, p = 0.22). However, when only cycles starting with a neutrophil count below 0.5 × 109/L were analyzed, the incidence of infection was significantly lower (16 vs 51%, p < 0.001). Based on these results, a multivariate logistic regression, mixed-effects model was calculated. A propensity score was added to the model to take into account the baseline differences, as described. In this analysis, antimicrobial prophylaxis decreased the risk of infection, whereas a neutrophil count below 0.5 × 109/L at the start of the cycle increased this risk. Moreover, there was a significant interaction between these two variables, suggesting that the beneficial effects of prophylaxis were more pronounced in neutropenic patients. These results are shown in Table 4. Addition of duration of neutropenia to this model did not change the overall results.
When the analyses were repeated, considering the indication of prophylaxis in the first cycle as “intent to treat,” the direction of the results did not change. Risk factors for infection were neutropenia (OR 9.6 [2.63–34.7], p < 0.001) and comorbidity index (OR 1.62 [1.02–2.56], p = 0.003). Prophylaxis decreased the risk of infection (OR 0.13 [0.03–0.56], p = 0.006), with a significant interaction with neutropenia (OR 16.7 [2.5–109.8], p = 0.003).
Discussion
In this work, we have analyzed the incidence and risk factors related to the development of infections during the first 4 treatment cycles with azacitidine in a cohort of patients with higher-risk MDS and AML. Our results show that during this early period, there is a high incidence of infections, mainly of bacterial etiology, and that fragile patients, this is, those with severe comorbidities, neutropenia or diseases with a poor prognosis, are at special risk. These results are in line with recently published evidence [13]. In addition, we describe how antimicrobial prophylaxis may decrease the incidence and consequences of these infections, with a stronger effect in patients with neutropenia at the start of the cycle.
Comorbidities are a major determinant of the outcome in acute myeloid leukemia. Several groups have reported a relationship between a comorbidity index of 3 or above and early mortality rates around 25–30% [14, 15]. In line with this, comorbidities are a risk factor for ICU admission after intensive chemotherapy, the most common cause of these admissions being respiratory failure [16], mainly due to infection [17]. The impact of comorbidities on azacitidine-treated patients has been less studied, but general performance indexes such as the ECOG score have been used to identify patients with a decreased risk of survival [18]. Based on our results showing that comorbidities are also a major determinant of infections, a systematic evaluation of concomitant diseases should be performed before treatment with hypomethylating drugs in order to identify the most fragile patients to adopt prophylactic strategies.
There are some discrepancies on the association between neutropenia an infection in azacitidine-treated patients. This can be due to several reasons. First, studies may have included heterogeneous populations, with newly diagnosed, relapsed, and refractory cases [19]. It is widely known that these last cases have the highest risk of infection among the patients with acute leukemia [20]. The second reason might be that the risk of infection is not constant among the successive cycles. It has been reported that infection rates below 10% can be doubled if only the first treatment cycles are considered [7, 19]. Moreover, response to azacitidine treatment usually appears after 3–4 cycles, thus modifying the inherent risk of infection by decreasing it in responders and increasing it in refractory patients [13]. By this reasons, some disease-related factors such as cytogenetics or chemorefractoriness have been related to infections more consistently than neutropenia itself. To avoid these factors, our study was limited to the first 4 cycles and only in first-line treatments.
Infections may have a large impact on the outcomes of these patients. Only those patients with infections required a hospital admission during the study period. Moreover, up to 70% of the infected patients showed more than one episode, requiring subsequent hospitalizations. It is recognized that hospital admissions for febrile neutropenia are related to a worse quality of life [21] and higher costs of care [22]. Although our study does not allow to extract firm conclusions on this issue, data from the AZA-MDS-001 trial show that quality of life improves only after 4 cycles of treatment [23]. As previously discussed, this time point coincides with the onset of the response to the drug and a reduction in the infection rates. Overall, this suggests a strong relationship between infections and quality of life in this population. Infections may also have an impact on subsequent treatments, particularly if stem cell transplantation is planned. Azacitidine is being increasingly used as a way to reduce disease burden before an allogeneic stem cell transplant [2]. In our study, 13% of the patients in which a SCT was planned died as a consequence of infection.
Our study shows 17% mortality in the first 4 cycles, in line with data from larger cohorts of unselected patients [7, 24]. Interestingly, mortality was threefold higher in patients with infections. However, this difference did not reach our significance criteria, or the differences in overall survival. Probably, the sample size results in a low statistical power to detect differences in mortality. In addition, it is plausible that deaths beyond this point are more related to disease progression. In fact, progression is the main cause of death in our sample. Although hypomethylating therapies have been a significant advance in MDS and AML, overall survival in unselected cohorts is still disappointing, being about 13 and 10 months, respectively [1, 25].
Finally, we focused on the role of antimicrobial prophylaxis. Our data suggest that this measure is effective to decrease infection rates, especially in patients with severe neutropenia. This finding is in line with the previous knowledge on other hematological diseases. By reducing infection rates, prophylaxis may help to achieve secondary benefits such as a decrease in transfusion requirements, absence in delays in the subsequent cycles, or reduction in death rates before transplantation. In diseases with a limited life expectancy, such MDS and AML in elderly patients, supportive care is as important as the antineoplastic treatment. Actually, the main goals of any treatment should be improving patients’ quality of life and keeping them out of hospital, not only lengthening their only survival [4]. In this scenario, other supportive treatments such as antimicrobial prophylaxis must be considered as a valuable strategy to achieve this objective, avoiding hospital admissions and infection-related adverse events.
Our study has several limitations. First, the rate of identification of a causative organism is low. However, there is a large variability in the reported rates, ranging from 32 [19] to 84% [7]. As we aimed on febrile episodes rather than documented infections, this should not have influenced the results. Second, the most important limitation of the study derives from its retrospective, single-center nature. In spite of the statistical approach, we cannot completely discard the presence of indication bias or other hidden factors. However, our results provide new data and should prompt the design of additional prospective, randomized trials.
In conclusion, we have shown that infections are common in patients with MDS or AML during the first 4 cycles of azacitidine. These infectious events have a significant impact in the clinical course, requiring more hospital admissions and transfusion of blood products. Patients with neutropenia or comorbidities are at special risk of these complications. Antimicrobial prophylaxis may prevent infections, especially in these fragile patients. How infections affect quality of life should be evaluated in prospective studies to identify the impact not only in survival, but also in patient-centered outcomes.
References
Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, Kumar R, Cavenagh J, Schuh AC, Candoni A, Recher C, Sandhu I, Bernal del Castillo T, Al-Ali HK, Martinelli G, Falantes J, Noppeney R, Stone RM, Minden MD, McIntyre H, Songer S, Lucy LM, Beach CL, Dohner H (2015) International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 126:291–299
Damaj G, Duhamel A, Robin M, Beguin Y, Michallet M, Mohty M, Vigouroux S, Bories P, Garnier A, El Cheikh J, Bulabois CE, Huynh A, Bay JO, Legrand F, Deconinck E, Fegueux N, Clement L, Dauriac C, Maillard N, Cornillon J, Ades L, Guillerm G, Schmidt-Tanguy A, Marjanovic Z, Park S, Rubio MT, Marolleau JP, Garnier F, Fenaux I, Yakoub-Agha I (2012) Impact of azacitidine before allogeneic stem-cell transplantation for myelodysplastic syndromes: a study by the Societe Francaise de Greffe de Moelle et de Therapie-Cellulaire and the Groupe-francophone des Myelodysplasies. J Clin Oncol 30:4533–4540
Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, Schoch R, Gattermann N, Sanz G, List A, Gore SD, Seymour JF, Bennett JM, Byrd J, Backstrom J, Zimmerman L, McKenzie D, Beach C, Silverman LR, International Vidaza High-Risk MDSSSG (2009) Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 10:223–232
Seymour JF, Dohner H, Minden MD, Stone R, Gambini D, Dougherty D, Beach CL, Weaver J, Dombret H (2017) Incidence rates of treatment-emergent adverse events and related hospitalization are reduced with azacitidine compared with conventional care regimens in older patients with acute myeloid leukemia. Leuk Lymphoma 58:1412–1423
Gafter-Gvili A, Fraser A, Paul M, Leibovici L (2005) Meta-analysis: antibiotic prophylaxis reduces mortality in neutropenic patients. Ann Intern Med 142:979–995
Fenaux P, Bowen D, Gattermann N, Hellstrom-Lindberg E, Hofmann WK, Pfeilstocker M, Sanz G, Santini V (2010) Practical use of azacitidine in higher-risk myelodysplastic syndromes: an expert panel opinion. Leuk Res 34:1410–1416
Merkel D, Filanovsky K, Gafter-Gvili A, Vidal L, Aviv A, Gatt ME, Silbershatz I, Herishanu Y, Arad A, Tadmor T, Dally N, Nemets A, Rouvio O, Ronson A, Herzog-Tzarfati K, Akria L, Braester A, Hellmann I, Yeganeh S, Nagler A, Leiba R, Mittelman M, Ofran Y (2013) Predicting infections in high-risk patients with myelodysplastic syndrome/acute myeloid leukemia treated with azacitidine: a retrospective multicenter study. Am J Hematol 88:130–134
Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, Ohyashiki K, Toyama K, Aul C, Mufti G, Bennett J (1997) International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 89:2079–2088
Cordoba I, Gonzalez-Porras JR, Such E, Nomdedeu B, Luno E, de Paz R, Carbonell F, Vallespi T, Ardanaz M, Ramos F, Marco V, Bonanad S, Sanchez-Barba M, Costa D, Bernal T, Sanz GF, Canizo MC (2012) The degree of neutropenia has a prognostic impact in low risk myelodysplastic syndrome. Leuk Res 36:287–292
Gonzalez-Porras JR, Cordoba I, Such E, Nomdedeu B, Vallespi T, Carbonell F, Luno E, Ardanaz M, Ramos F, Pedro C, Gomez V, de Paz R, Sanchez-Barba M, Sanz GF, Del Canizo AC, Spanish Myelodysplastic Syndrome R (2011) Prognostic impact of severe thrombocytopenia in low-risk myelodysplastic syndrome. Cancer 117:5529–5537
Sorror ML, Giralt S, Sandmaier BM, De Lima M, Shahjahan M, Maloney DG, Deeg HJ, Appelbaum FR, Storer B, Storb R (2007) Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood 110:4606–4613
Maertens J, Marchetti O, Herbrecht R, Cornely OA, Fluckiger U, Frere P, Gachot B, Heinz WJ, Lass-Florl C, Ribaud P, Thiebaut A, Cordonnier C, Third European Conference on Infections in L (2011) European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3--2009 update. Bone Marrow Transplant 46:709–718
Schuck A, Goette MC, Neukirchen J, Kuendgen A, Gattermann N, Schroeder T, Kobbe G, Germing U, Haas R (2017) A retrospective study evaluating the impact of infectious complications during azacitidine treatment. Ann Hematol 96(7):1097–1104
Giles FJ, Borthakur G, Ravandi F, Faderl S, Verstovsek S, Thomas D, Wierda W, Ferrajoli A, Kornblau S, Pierce S, Albitar M, Cortes J, Kantarjian H (2007) The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol 136:624–627
Malfuson JV, Etienne A, Turlure P, de Revel T, Thomas X, Contentin N, Terre C, Rigaudeau S, Bordessoule D, Vey N, Gardin C, Dombret H, Acute Leukemia French A (2008) Risk factors and decision criteria for intensive chemotherapy in older patients with acute myeloid leukemia. Haematologica 93:1806–1813
Schellongowski P, Staudinger T, Kundi M, Laczika K, Locker GJ, Bojic A, Robak O, Fuhrmann V, Jager U, Valent P, Sperr WR (2011) Prognostic factors for intensive care unit admission, intensive care outcome, and post-intensive care survival in patients with de novo acute myeloid leukemia: a single center experience. Haematologica 96:231–237
Molina R, Bernal T, Borges M, Zaragoza R, Bonastre J, Granada RM, Rodriguez-Borregan JC, Nunez K, Seijas I, Ayestaran I, Albaiceta GM, Investigators Es (2012) Ventilatory support in critically ill hematology patients with respiratory failure. Crit Care 16:R133
Itzykson R, Thepot S, Quesnel B, Dreyfus F, Beyne-Rauzy O, Turlure P, Vey N, Recher C, Dartigeas C, Legros L, Delaunay J, Salanoubat C, Visanica S, Stamatoullas A, Isnard F, Marfaing-Koka A, de Botton S, Chelghoum Y, Taksin AL, Plantier I, Ame S, Boehrer S, Gardin C, Beach CL, Ades L, Fenaux P, Groupe Francophone des M (2011) Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood 117:403–411
Falantes JF, Calderon C, Marquez-Malaver FJ, Aguilar-Guisado M, Martin-Pena A, Martino ML, Montero I, Gonzalez J, Parody R, Perez-Simon JA, Espigado I (2014) Patterns of infection in patients with myelodysplastic syndromes and acute myeloid leukemia receiving azacitidine as salvage therapy. Implications for primary antifungal prophylaxis. Clin Lymphoma Myeloma Leuk 14:80–86
Bow EJ (2009) Neutropenic fever syndromes in patients undergoing cytotoxic therapy for acute leukemia and myelodysplastic syndromes. Semin Hematol 46:259–268
Sekeres MA, Stone RM, Zahrieh D, Neuberg D, Morrison V, De Angelo DJ, Galinsky I, Lee SJ (2004) Decision-making and quality of life in older adults with acute myeloid leukemia or advanced myelodysplastic syndrome. Leukemia 18:809–816
Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106:2258–2266
Santini V, Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Silverman LR, List A, Gore SD, Seymour JF, Backstrom J, Beach CL (2010) Management and supportive care measures for adverse events in patients with myelodysplastic syndromes treated with azacitidine*. Eur J Haematol 85:130–138
Diamantopoulos P, Zervakis K, Papadopoulou V, Iliakis T, Kalala F, Giannakopoulou N, Rougala N, Galanopoulos A, Bakarakos P, Variami E, Dimitrakopoulou A, Viniou NA (2015) 5-Azacytidine in the treatment of intermediate-2 and high-risk myelodysplastic syndromes and acute myeloid leukemia. A five-year experience with 44 consecutive patients. Anticancer Res 35:5141–5147
Bernal T, Martinez-Camblor P, Sanchez-Garcia J, de Paz R, Luno E, Nomdedeu B, Ardanaz MT, Pedro C, Amigo ML, Xicoy B, del Canizo C, Tormo M, Bargay J, Valcarcel D, Brunet S, Benlloch L, Sanz G, Spanish Group on Myelodysplastic S, Foundation P, Spanish Society of H (2015) Effectiveness of azacitidine in unselected high-risk myelodysplastic syndromes: results from the Spanish registry. Leukemia 29:1875–1881
Acknowledgements
Instituto Universitario de Oncología del Principado de Asturias is supported by Fundación Bancaria Caja de Ahorros de Asturias.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Lorenzana, N., Avila, L.F., Alonso, S. et al. The impact of antimicrobial prophylaxis in morbidity and infections during azacitidine treatment. Ann Hematol 96, 1833–1840 (2017). https://doi.org/10.1007/s00277-017-3091-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-017-3091-x