Abstract
The optimal first-line treatment for advanced low-grade non-Hodgkin lymphomas (LG-NHL) is still highly debated. Recently, the StiL and the BRIGHT trials showed that the combination of rituximab and bendamustine (R-B) is non-inferior to rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) with a better toxicity profile. Utilizing a retrospective analysis, we compared the efficacy and safety of both regimens in clinical practice. From November 1995 to January 2014, 263 LG-NHL patients treated with either R-B or R-CHOP were retrospectively assessed in seven European cancer centers. Ninety patients were treated with R-B and 173 with R-CHOP. Overall response rate was 94 and 92 % for the R-B and the R-CHOP group, respectively. The percentage of complete response was similar for both groups (63 vs. 66 % with R-B and R-CHOP, respectively; p = 0.8). R-B was better tolerated and less toxic than R-CHOP. The median follow-up was 6.8 and 5.9 years for the R-CHOP and the R-B group, respectively. Overall, no difference in progression-free survival (PFS) (108 vs. 110 months; p = 0.1) was observed in the R-B group compared to the R-CHOP cohort. Nevertheless, R-B significantly prolonged PFS in FL patients (152 and 132 months in the R-B and R-CHOP group, respectively; p = 0.05). However, this result was not verified in multivariate analysis probably due to the limits of the present study. We confirm that the R-B regimen administered in patients with LG-NHL is an effective and less toxic therapeutic option than R-CHOP in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low-grade non-Hodgkin lymphomas (LG-NHL), consisting mainly of follicular lymphoma (FL) (grade 1–2), lymphoplasmacytic lymphoma (LPL), small lymphocytic lymphoma (SLL), marginal zone lymphoma (MZL), and Waldenstrom’s macroglobulinemia (WM), represent about 40 % of all NHLs [1]. Despite the recent advances in anti-lymphoma treatment, a standard first-line treatment has not yet been well established [2]. In the last decades, the most commonly used regimens have included rituximab, a monoclonal antibody anti-CD20, combined with either cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) [3] or cyclophosphamide, vincristine, and prednisone (R-CVP) [4]. Recently, in the FOLL05 study, a multicenter randomized trial comparing R-CVP, R-CHOP and rituximab, fludarabine, and mitoxantrone (R-FM) in chemo-naïve patients with advanced FL, demonstrated that R-CHOP has the best efficacy and risk-benefit ratio [5]. However, despite high overall response rates (ORR), nearly all LG-NHL patients relapse and some succumb to their disease. Given the lack of long-term disease control with the current standard of care, new therapeutic options are needed. Between 1971 and 1992, bendamustine, a unique mechlorethamine alkylating agent containing a benzimidazole heterocyclic ring, developed in the 1960s in the German Democratic Republic, was successfully used for treating B cell malignancies [6–8]. In 2008, Leoni et al. provided evidence that bendamustine is able to kill neoplastic B cells [9]. Subsequently, the STiL-1 trial demonstrates the superiority of bendamustine plus rituximab (R-B) with respect to R-CHOP as first-line treatment for LG-NHL and mantle cell lymphomas (MCL) [10]. Although R-B was associated with improvement in progression-free survival (PFS), this did not translate into an overall survival (OS) advantage [10]. Moreover, R-B was associated with less toxicity and an improved quality of life when compared to other regimens [10–12]. Based on these data, R-B is currently the most used regimen in North America and Europe as first-line therapy in indolent NHL [13]. However, up to now, it is not known whether these positive results of R-B in comparison to R-CHOP obtained in highly selected patients in prospective trials can be reproduced in everyday clinical routine. Therefore, in order to compare efficacy and toxicity of these two different regimens in a real-life setting, we retrospectively assessed all patients affected by LG-NHL treated by either R-B or R-CHOP in first line in seven European cancer centers.
Methods
Patients
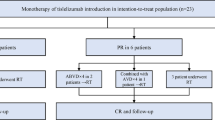
From November 1995 to January 2014, 271 patients affected by LG-NHL (FL grade 1–2, LPL, SLL, MZL, WM) were retrospectively assessed in five Italian and two Austrian cancer centers. Histologic diagnosis was performed according to the international guidelines by an expert pathologist of each participating cancer center [14, 15]. All patients treated in the participating centers who met the following criteria were included in the present analysis: newly diagnosed LG-NHL, first-line treatment of either R-B or R-CHOP, age ≥18 years, performance status ≤2, and a clear treatment indication such as systemic symptoms, large tumor mass (characterized by lymphomas with a diameter >3 cm in three or more regions or by a lymphoma with a diameter >7 cm in one region), presence of lymphoma-related complications, progressive disease defined as a more than 50 % increase of tumor mass within 6 months, and/or a hyperviscosity syndrome. Patients with a history of a severe cardiac disease, previous malignancy, inadequate hepatic, renal, or cardiac function, active infection of HIV, and/or hepatitis B or C were excluded.
This analysis was approved by the local Ethical Committee (Prot. 0042654-BZ). Due to the retrospective and anonymous data collection, informed consent was not necessary.
Treatment plan
All patients underwent immunochemotherapy consisting of rituximab in association with either bendamustine or CHOP based on the physician’s choice. Each center collected patient data from the date when the first patients were treated with R-B (for example the Medical University of Innsbruck in 1995). Since rituximab and bendamustine at that time were not yet part of clinical routine, only limited patient data was available for the early years of data assessment. The standard rituximab dose was the same for both groups, namely 375 mg/m2 on day 1 of each cycle. Bendamustine was administered at the same dose as in the prospective trials (90 mg/m2) on days 1 and 2 every 4 weeks for up to 6 cycles [10, 11]. CHOP was administered at standard doses every 3 weeks for a maximum of 6 cycles [10, 11]. No maintenance or consolidation treatment was given. All patients received standard antiemetic prophylaxis but no prophylactic antibiotic or antiviral treatment. The use of granulocyte-colony stimulating factors (G-CSF) and allopurinol was allowed at investigators’ discretion.
All patients were evaluated for response to therapy according to international criteria [13, 14] and toxicity according to the National Cancer Institute’s Common Toxicity Criteria (NCI-CTC). Treatment response was assessed about 1 month after treatment completion by a full physical examination, blood testing, bone marrow aspirate, and biopsy in case of bone marrow involvement at diagnosis, as well as imaging studies with computed tomography (CT). Follow-up visits were performed every 3–6 months for 5 years and annually thereafter in all participating centers.
Statistical analyses
Chi-square test was performed to assess the significance of differences between categorical variables. OS and PFS were plotted as curves using the Kaplan–Meier method and were defined as time from diagnosis until death from any cause and as time from diagnosis until disease progression or death from any cause, respectively [16, 17]. Log-rank test was employed to assess the impact on survival of categorical variables, and the Cox proportional hazards model was used to evaluate whether the type of treatment (R-B vs. R-CHOP) influenced OS and/or PFS independently of clinical prognosticators. Statistical analyses were performed with MedCalc (version 11.0; MedCalc Software, Acacialaan, Ostend, Belgium) software and the GraphPad Prism (version 5.0; GraphPad Software, Inc., San Diego, CA, USA) package. The limit of significance for all analyses was defined as p < 0.05.
Results
Clinical characteristics at time of diagnosis
Clinical features according to the two different treatment groups are summarized in Table 1. In the R-B group, the median age at time of diagnosis was 65 years (range 42–87 years) compared to 57 years (range 30–80 years) in the R-CHOP cohort (p < 0.001). A male predominance was observed in the R-B group (63 vs. 48 %; p = 0.02). Most patients in both groups had stage III–IV disease (87 and 75 % in the R-B and R-CHOP groups, respectively). A significantly higher rate of B-symptoms and bone marrow involvement was recorded in the R-B group in contrast to the R-CHOP one (32 vs. 24 %; p = 0.05 and 48 vs. 36 %; p = 0.05, in R-B and R-CHOP group, respectively). The different lymphoma entities were similarly distributed between both groups (Table 1). In detail, most patients were affected by follicular lymphoma (60 % in R-B group and 80 % in the R-CHOP group), followed by marginal zone lymphoma (22 % in R-B group and 13 % in the R-CHOP group). The Follicular Lymphoma International Prognostic Index (FLIPI) [18] was calculated for patients with FL, and about one-third of the patients in both groups were in the poor-risk category.
Treatment and response
Ninety patients were treated with R-B and 173 with R-CHOP. Overall, 471 cycles of R-B (median 5 cycles; range 2–6) and 955 of R-CHOP (median 6 cycles; range 2–8) were delivered. Dose reduction of immunochemotherapy was necessary in 3 and 12 % of patients who underwent R-B and R-CHOP, respectively (p = 0.02). ORR was 94 % for the R-B treatment group and 92 % for the standard treatment group. The percentage of complete remission (CR) was similar for both groups, namely 63 % in those patients who underwent R-B versus 66 % in the others (p = 0.8). Also, the rate of partial remissions (PR) (25 vs. 20 %; p = 0.6) and stable disease (SD) (5 vs. 6 %; p = 0.6) was similar in the two groups. Progressive disease (PD) developed in 5 and 7 % of patients who underwent R-B and R-CHOP, respectively.
Hematologic toxicity was clearly less frequent in the R-B group: grade 1/2 events were observed in 14 versus 55 % (p < 0.001) while grade 3/4 toxicity occurred in 15 versus 71 % (p < 0.001). In particular, grade 3–4 neutropenia and thrombocytopenia were significantly less frequent in the R-B group (11 vs. 54 %; p < 0.0001 and 2 vs. 10 %; p < 0.0001; Table 2). Non-hematological toxicity varied significantly as well (Table 3). Alopecia, a frequent event in R-CHOP-treated patients (100 %), did not occur in the R-B group. Peripheral neuropathy (1 vs. 32 %; p < 0.001) and drug-associated erythematous skin reaction (urticaria, rash) (2 vs. 12 %; p = 0.005) were significantly less common in the R-B group as well. In addition, the incidence of infections was significantly higher in the R-CHOP arm (57 vs. 3 % overall; p < 0.001).
We recorded five (5 %) secondary malignancies in the R-B group compared with 16 (9 %) in the R-CHOP group, namely one case of myelodysplastic syndrome (MDS) and four solid tumors (one breast cancer, one pancreatic cancer, one colon cancer, and one basalioma) in the former compared to six cases of hematological malignancy (four acute myeloid leukemia, one MDS, and one diffuse large B cell lymphoma) and ten solid tumors (three lung cancer, three melanoma, two colon cancer, and two prostatic cancers) in the latter.
Follow-up
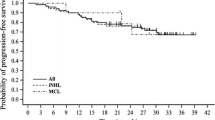
The median follow-up was 6.8 years (range 8–185 months) and 5.9 years (range 9–185 months) for the R-CHOP and R-B group, respectively. On the whole, there was no difference in PFS between the R-CHOP and the R-B group (108 vs. 110 months; p = 0.1; Fig. 1a). However, a clear PFS advantage of R-B was observed in the subgroup of patients affected by FL (median PFS of 152 and 132 months in the R-B and R-CHOP group, respectively; p = 0.05; Fig. 1b). As expected, OS did not differ between the two groups (median OS not reached vs. 168 months in R-B and R-CHOP group, respectively; p = 0.7) (Fig. 2). In the univariate analysis, survival was significantly influenced by age, B-symptoms, bone marrow (BM)-involvement, extranodal site involvement, and LDH levels. As expected, confidence intervals in multivariate analysis were very variable due to the retrospective nature of the analysis and the relatively low number of patients. However, in FL patients, the most important PFS prognosticators were bone marrow involvement (p = 0.024; hazard ratio (HR) 1.775; confidence interval (CI) 0.077–2.923), >2 extranodal sites (p = 0.019; HR 2.021; CI 1.121–3.644), and elevated LDH (p < 0.001; HR 3.241; CI 1.939–5.418), while elevated LDH was the most important OS prognosticator (p < 0.001; HR 5.427; CI 2.280–12.920). PFS of patients affected by other lymphoma subtypes was independently influenced by elevated LDH (p = 0.014; HR 4.589; CI 1.360–15.490) and OS by elevated LDH (p = 0.012; HR 27.858; CI 2.086–372.086) and intermediate risk FLIPI (p = 0.040; HR 24.091; CI 1.160–500.495) (Tables 4 and 5).
Discussion
The current standard treatment for advanced LG-NHL consists of immunochemotherapy, but the choice of first-line treatment is highly controversial. Initial treatment consists of R-CHOP or R-B [6]. In two prospective trials, R-B proved to be less toxic and non-inferior to R-CHOP [10, 11]. However, clinical data regarding efficacy and toxicity are lacking. This study provides evidence that the R-B regimen has similar efficacy and less toxicity in LG-NHL than R-CHOP.
The strengths of this analysis were the relatively long-term follow-up (nearly twice that of the previous reports [10, 11]) and the well-controlled treatment in a multicenter setting despite the fact that patients were treated outside a clinical trial, avoiding the known overestimation effects in pivotal trials [19]. The main limitation of this study was the retrospective data assessment, the long accrual period with the consequent absence of uniform criteria for response evaluation. An additional limitation was that a central pathology review was not performed. However, all participating centers demonstrate a lengthy experience in lymphoma diagnosis and management, along with the active involvement of expert hemopathologists.
In the present analysis, we included only patients affected by LG-NHL, which is in contrast to the two prospective trials [10, 11] that also allowed the randomization of patients affected by MCL (almost 20 % of each arm). Due to the often aggressive clinical course and high relapse rate leading to a dismal outcome, we did not consider this entity as an LG-NHL. Another difference in comparison to the above mentioned studies was that the clinical factors assessed at time of diagnosis were not well balanced between the two treatment groups since patients who underwent R-B had a higher percentage of negative prognostic parameters (higher median age and bone marrow infiltration, poor FLIPI score, and increased concentrations of beta2 microglobulin) than others. This was expected, because in clinical routine R-B was initially reserved for elderly and unfit patients who were ineligible for an R-CHOP treatment.
Despite the higher number of patients with an unfavorable risk profile at time of diagnosis, the ORR (94 % in R-B vs. 92 % in R-CHOP; p = 0.7) and especially the percentage of CR (63 vs. 66 %; p = 0.8) were similar in both groups, suggesting that R-B was at least as efficient in response induction as R-CHOP. Due to the nature of the present analysis, we expected a lower ORR than in prospective trials, which was neither the case for R-CHOP [5, 11] nor for R-B [10, 11]. The much higher CR rate after R-B in the StiL and BRIGHT trial [10, 11] in comparison to the present analysis can be easily explained by the better risk profile at time of diagnosis. Moreover, due to the retrospective nature of the present analysis imaging and histologic samples of disease, restaging after the end of treatment were not centrally reviewed which might at least explain in part the discordance to the prospective trials.
Similar to what was observed in the two prospective studies [10, 11], R-B was clearly less toxic than R-CHOP. In particular, grade 3–4 neutropenia was significantly less frequent in the R-B treatment group (11 vs. 54 %; p < 0.001), translating into a significantly reduced occurrence of infections in comparison to R-CHOP (57 vs. 3 %; p < 0.0001). Likewise, in the StiL and in the BRIGHT trial [10, 11], grade 3–4 neutropenia was reported 29 % with R-B versus 69 % with R-CHOP and 39 vs. 87 %, respectively. Also, non-hematologic toxicities, such as neuropathy, skin reactions, alopecia, and nausea, were more frequently observed in R-CHOP patients as well. The relatively high percentage of skin reactions in the R-CHOP group could be explained by the routine use of allopurinol in patients with large tumor masses, while the same drug is not recommended with bendamustine [20, 21; therefore, it might have been omitted by some physicians. However, due to the retrospective nature of this analysis, data regarding allopurinol administration was not assessed. These findings are in line with the StiL trial [10], but they differ from the BRIGHT study [11] where R-B patients had a higher incidence of nausea, vomiting, and skin reactions. The overall more favorable R-B toxicity profile translated into a higher feasibility since a dose reduction of immunochemotherapy was necessary in 12 % of R-CHOP patients compared to only 3 % in the RB group (p = 0.02). Similar results were obtained in the StiL trial (11.2 vs. 4 %) [10], but were less pronounced in the American one (6 vs. 4 %). The number of secondary primary malignancies was similar in both groups (9 % with R-CHOP vs. 5 % with R-B; p = 0.3) which is in line with the StiL trial [10], while the follow-up of the BRIGHT study was too short to draw any conclusions.
Unlike the StiL trial [10], overall, there was no difference in PFS between the two treatment groups (p = 0.1). Although R-B led to a significantly longer PFS in FL patients (p = 0.05) in univariate analysis, this could not be confirmed by cox-regression probably due to the limits of the present cohort. In contrast to Rummel et al. [10] in the present analysis, the R-CHOP survival data are similar to those noted by Czuczman et al. [3] and Hiddemann et al. [22]. As expected, due to the often indolent clinical course of LG-NHL and efficient salvage treatments, OS was similar in both groups.
In conclusion, R-B has an at least similar efficacy to the standard R-CHOP regimen in indolent lymphomas but with a much better toxicity profile. Therefore, R-B combination can be considered as a feasible alternative regimen in LG-NHL patients.
Headings
-
Bendamustine plus rituximab (R-B) has demonstrated to be at least as efficient as R-CHOP in indolent B cell non-Hodgkin lymphoma.
-
R-B has shown a clearly better toxicity profile than R-CHOP with significantly grade 3–4 hematologic and non-hematologic toxicities.
-
R-B treatment led to an improvement of progression-free survival in follicular lymphoma; however, randomized clinical trials are warranted in order to confirm this superiority.
References
Harris NL, Jaffe ES, Diebold J et al (1999) The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November, 1997. Ann Oncol 10:1419–1432
Press OW (2013) Selection of first-line therapy for advanced follicular lymphoma. J Clin Oncol 31:1496–1498
Czuczman MS, Grillo-López AJ, White CA et al (1999) Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol 17:268–276
Marcus R, Imrie K, Belch A et al (2005) CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood 105:1417–1423
Federico M, Luminari S, Dondi A et al (2013) R-CVP versus R-CHOP versus R-FM for the initial treatment of patients with advanced-stage follicular lymphoma: results of the FOLL05 trial conducted by the Fondazione Italiana Linfomi. J Clin Oncol 31:1506–1513
Kath R, Blumenstengel K, Fricke HJ et al (2001) Bendamustine monotherapy in advanced and refractory chronic lymphocytic leukemia. J Cancer Res Clin Oncol 127:48–54
Ruffert K, Jahn H, Syrbe G et al (1989) Bendamustine as an alternative approach to treat malignant non-Hodgkin’s lymphoma. Z Klin Med 8:671–674
Herold M, Keinert K, Anger G (1992) Risk-adapted combined radiotherapy and chemotherapy for Hodgkin’s disease. Onkologie 15:501–505
Leoni LM, Bailey B, Reifert J et al (2008) Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res 14:309–317
Rummel MJ, Niederle N, Maschmeyer G et al (2013) Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 381:1203–1210
Flinn IW, van der Jagt R, Kahl BS et al (2014) Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood 123:2944–2952
Burke JM, Van der Jagt RH, Kahl BS, et al. (2012) Differences in quality of life between bendamustine plus rituximab compared with standard first- line treatments in patients with previously untreated advanced indolent non-Hodgkin’s lymphoma or mantle cell lymphoma. Blood a155
Morschhauser F, Seymour JF, Feugier P et al (2011) Impact of induction chemotherapy regimen on response, safety and outcome in the PRIMA study. Ann Oncol 22(suppl 4):89
Harris NL, Jaffe ES, Stein H et al (1994) A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 84:1361–1392
Swerdlow SH, Campo E, Harris NL, Swerdlow SH, Campo E, Harris NL (2008) WHO classification of tumours of haematopoietic and lymphoid tissues, 4th edn. IARC Press, Lyon
Cheson BD, Horning SJ, Coiffier B et al (1999) Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol 17:1244
Cheson BD, Pfistner B, Juweid M et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25:579–586
Solal-Celigny P, Roy P, Colombat P et al (2004) Follicular Lymphoma International Prognostic Index. Blood 104:1258–1265
Singal AG, Higgins PD, Waljee AK (2014) A primer on effectiveness and efficacy trials. Clin Transl Gastroenterol 5:e45
Alamdari HS, Pinter-Brown L, Cassarino DS, Chiu MW (2010) Severe cutaneous interface drug eruption associated with bendamustine. Dermatol Online J 16:1
Fallon MJ, Heck JN (2015) Fatal Stevens-Johnson syndrome/toxic epidermal necrolysis induced by allopurinol-rituximab-bendamustinetherapy. J Oncol Pharm Pract 21:388–392
Hiddemann W, Kneba M, Dreyling M et al (2005) Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 106:3725–3732
Acknowledgments
This article did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This analysis was approved by the local Ethical Committee (Prot. 0042654-BZ). Due to the retrospective and anonymous data collection, informed consent was not necessary.
Conflict of interest
The authors have no conflicts of interest. PM, NS, IW, FZ, RZ, AV, EM, PG, VP, and SC have no financial disclosure. SF and MM have received honoraria from Mundipharma; WW has received honoraria and research funding from Mundipharma and Roche.
Rights and permissions
About this article
Cite this article
Mondello, P., Steiner, N., Willenbacher, W. et al. Bendamustine plus rituximab versus R-CHOP as first-line treatment for patients with indolent non-Hodgkin’s lymphoma: evidence from a multicenter, retrospective study. Ann Hematol 95, 1107–1114 (2016). https://doi.org/10.1007/s00277-016-2668-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-016-2668-0