Abstract
Myelodysplastic syndromes (MDSs) are heterogeneous hematopoietic disease characterized by ineffective haematopoiesis that frequently transforms into acute leukaemia. Alterations in many individual biologic pathways have been reported in MDS pathophysiology. Disease progression along the MDS, acute myeloid leukemia (AML) continuum is believed to be a consequence of stepwise accumulation of DNA mutations which infers a defect in DNA repair. The present study investigated the association between DNA repair genes (XRCC1, XRCC3, OGG1, XPD and RAD51) and the risk of developing MDS. The study was carried out in 92 primary MDS patients. The genotyping study was carried out by PCR-RFLP technique. We have studied seven single-nucleotide polymorphisms (SNPs) of five DNA repair genes (XRCC1 (Arg194Trp, Arg280His, Arg399Gln), XRCC3, XPD, RAD51 and OGG1). Significantly, a high frequency of DNA repair gene XRCC1 (Arg280His) (p = 0.05) and XPD (Lys751Gln) (p = 0.01) polymorphism was observed in MDS patients compared to controls. The distribution of polymorphisms in MDS subgroups showed a significant association of XRCC1 with RAEB I compared to other subgroup. Though a high frequency of XRCC1 gene polymorphism was observed in farmers and tobacco chewers, it was not statistically significant. Our study suggests that XRCC1 (Arg280His) and XPD polymorphisms are associated with risk of MDS and XRCC1 polymorphism strongly associated with advanced MDS subgroup. Hence, these polymorphisms can be used as a prognostic marker in MDS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myelodysplastic syndromes (MDSs) are heterogeneous hematopoietic disease characterized by ineffective haematopoiesis that frequently transforms into acute leukaemia. MDS is primarily a disease of the elderly, whereas some cases can occur following large genotoxic insults such as chemotherapy drug treatment [1]. Alterations in many individual biologic pathways have been reported in MDS pathophysiology. However, despite a hypercellular bone marrow, the generally accepted primary hypothesis involves an initial deleterious genetic event within a hematopoietic stem cell, subsequent development of excessive cytokines/inflammatory response leading to a pro-apoptotic/ proliferative state and resultant peripheral cytopaenias [2].
DNA damage repair pathways are important for removing different types of DNA damage. The base excision repair (BER), nucleotide excision repair (NER) and double-strand break repair (DSB repair) are the most important DNA repair pathways. Mutations are early events in carcinogenesis, and impaired DNA repair might be a risk factor for many cancers [3]. Common genetic polymorphisms in DNA repair genes might affect protein function and, thus, the capacity of repair DNA damage, which in turn could lead to genetic instability and leukemogenesis. Polymorphisms in DNA repair genes are thought to be a risk factor for cancer as a result of increased rate of mutations. Among them, polymorphisms of X-ray repair cross complementing group 1 (XRCC1), X-ray repair cross complementing group 3 (XRCC3) and xeroderma pigmentosum Complementation group D (XPD) have been studied extensively. Several single-nucleotide polymorphisms (SNPs) in XRCC1, XRCC3 and XPD genes have been identified. Among them, XRCC1 Arg399Gln, Arg280His, Arg194Trp, XRCC3 Thr241Met and XPD Lys751Gln polymorphisms are the most studied in cancers, including leukaemia [4]. The DNA repair enzyme human oxoguanine glycosylase 1 (hOGG1) is a DNA glycosylase/AP lyase that has been indicated to play an important role in preventing carcinogenesis by repairing oxidative damage to DNA. Specifically, glycosylase/AP lyase could efficiently catalyze the excision and removal of 8-OH-dG adducts. HOGG1 may play a vital role in maintaining genome integrity and preventing the development of cancer [5].
Human RAD51, known to function in DNA repair, interacts with a number of proteins implicated in breast cancer, including BRCA1 and BRCA2. In vitro, RAD51 protein promotes DNA homologous pairing and strand exchange, in association with other proteins of the gene conversion complex [6]. Two SNP polymorphisms have been described in the 5′-untranslated region (5′-UTR) of RAD51 gene, a G to C substitution at position +135 bp and a G to T substitution at position +172 bp from the start of the cDNA sequence. Several studies have linked the RAD51 135C allele with altered susceptibility to both breast cancer and ovarian cancer [7, 8].
Disease progression along the MDS-acute myeloid leukaemia (AML) continuum is believed to be a consequence of stepwise accumulation of DNA mutations which infers a defect in DNA repair [9]. Genetic background is thought to influence the risk of developing MDS; several case–control studies have investigated the relationships between specific genetic polymorphisms and the risk of MDS [10]. The present study investigated the seven SNPs of DNA repair genes (XRCC1, XRCC3, OGG1, XPD and RAD51) in MDS, as these SNPs associated with various cancers.
Materials and methods
Patients
The study was carried out in 92 primary MDS patients including 52 males and 40 females. The MDS patients were diagnosed according to WHO classification [11]. Sixty age- and sex-matched controls were also recruited. The study was carried out with consent of patients and controls. The patients’ demographic details such as occupational exposure and habits (tobacco chewing, smoking, alcohol, etc.) were recorded in our proforma. The study protocols were approved by the Institutional Ethics Committee.
DNA isolation
Genomic DNA was extracted from the peripheral blood of the patients’ sample collected in EDTA Vacutainers. The DNA was extracted using QIAamp DNA Blood Mini Kit (Qiagen), based on column extraction method.
Genotyping
Seven SNPs of five DNA repair genes (XRCC3, XRCC1, OGG1, XPD, RAD51) were selected for the study. The genotypes were determined by the PCR-RFLP method, and primers, annealing temperature, length of amplified fragments, restriction pattern and restriction enzymes used are listed in Supplementary Table. The PCR was done using 100 ng of genomic DNA, 0.2 μmol/L of each primer, 1× PCR buffer, 0.2 μmol/L of each deoxynucleotide triphosphate, 2.0 mmol/L MgCl2 and 0.625 units of Taq in a 25-μL reaction volume. PCR program was a 5-min denaturation step at 95 °C followed by 35 cycles of 94 °C for 50 s, 55 to 63 °C for 30 to 50 s, 72 °C for 30 to 50 s (according to the length of fragment amplified), respectively, and a final extension step at 72 °C for 10 min. The amplified PCR products were digested at 37 °C for 5 min using restriction enzymes (Fermentas, Inc.). The digested products were resolved on 3 % agarose gels and analyzed under UV light (Supplementary Methods, Supplementary Fig. 1).
All data were analyzed by GraphPad InStat software, version 3 (GraphPad, San Diego, CA, USA). Fischer’s exact test (two-sided) was used to compare the distribution of qualitative variables between cases and controls. A value less than 0.05 was considered as statistically significant.
Results
The male/female ratio of 92 MDS was 1.3:1 and the age group ranging from 15 to 92 years with a median age of 47 years. The subgroups of MDS were RA (43 %), RAEB I (8.6 %), RAEB II (9.7 %), RARS (4.3 %), RCMD (32.6 %) and MDS-U (1.08 %) (Fig. 1). Clinical characteristics of MDS patients are presented in Table 1.
Our study investigated the frequency of seven polymorphisms in 92 MDS patients and 60 controls. The distributions of genotypes and alleles of XRCC1 (Arg194Trp, Arg280His, Arg399Gln), XRCC3, XPD, RAD51 and OGG1 genes in MDS patients are summarized in Table 2. Among seven polymorphisms, XPD (p = 0.011) and XRCC1 (Arg280His) (p = 0.05) were found to be significantly associated with MDS. The frequency of homozygous variant His/His of XRCC1 Arg280His genotype was higher in patients (64 %) compared to controls (20 %). It was observed that the frequency of heterozygous variant Arg/His and Arg/Arg genotype was high in controls (22 and 58 %) compared to patients (15 and 21 %). XPD Lys751Gln showed a significantly higher frequency of homozygous variant Gln/Gln in patients (15 %) compared to controls (3 %). The frequency of wild-type variant Lys/Lys was high in controls (57 %) compared to patients (48 %). In case of XRCC1 (Arg194Trp, Arg399Gln), XRCC3, RAD51 and OGG1 DNA repair genes, no significant difference was observed between MDS patients and controls.
The distribution of variant allele and genotype frequency of MDS subtypes revealed that the XRCC1 Arg280His homozygous variant (His/His) allele was higher in MDS RAEB I and II compared to other subgroups (Table 3). The frequency of this variant allele when compared between MDS patients with and without blast and the frequency of XRCC1 homozygous variant (his/his) were high in patients with blast (76.47 %) compared to patients without blast (38.02 %).
In this study, the data on occupational exposure revealed a high frequency (63.2 %) of homozygous variant of XRCC1 (Arg280His) in farmers exposed to pesticides compared to other occupations (exposed to polyvinyl chloride, toluene and zinc oxide). However, the genotype was not found to be significantly associated (p > 0.05) with occupational exposure (Table 4). The lifestyle of MDS patients such as smoking, alcohol and tobacco consumption was studied. In our study group, 39.76 % MDS patients had a history of smoking (4.3 %); tobacco chewing (21.5 %); being alcoholics (5.37 %); smoking and drinking alcohol (1.07 %); smoking and using tobacco (4.30 %); drinking alcohol and using tobacco (2.15 %); and smoking, drinking alcohol and using tobacco (1.07 %) (Fig. 2). DNA repair gene polymorphisms showed a high frequency (67.8 %) of homozygous variant His/His of XRCC1 Arg280His polymorphisms in tobacco chewers compared to controls. However, these results were statistically not significant (p > 0.05) (Table 5).
Discussion
DNA damage occurs due to several factors such as exposure to environmental factors (chemicals, toxins, ultraviolet light and ionizing radiation) or cellular metabolisms (replications) [12]. The DNA damage is controlled by several DNA repair genes (XRCC1, XRCC3, XPD, OGG1, RAD51, etc.). We have studied seven polymorphisms in five DNA repair genes (OGG1, XRCC1 (Arg194Trp, Arg280His, Arg399Gln), XRCC3, XPD and RAD51) in primary MDS patients. Our study showed a high frequency (15 %) of Gln/Gln genotype of XPD gene in MDS compared to controls. We also observed statistically significant (p = 0.05) association of His/His genotype in XRCC1 gene between MDS patients and controls. The results suggest that XRCC1 (Arg280His) and XPD (Lys751Gln) polymorphisms exist in Indian patients with MDS, and these polymorphisms may be playing a role in progression of the disease. However, the frequency of 751Lys (G) allele was found to be high (0.664) as compared to 751Gln (C) allele (0.336) in MDS patients. The 280Arg (G) allele frequency was 0.282 as compared to 280His (A) allele (0.717). Several studies have shown the association of genetic polymorphisms of DNA repair genes with haematological malignancies such as AML [13], ALL [14] and CML [15]. Recent studies demonstrated the potential impact of genetic polymorphisms on the risk to develop MDS. The study carried out by Aktuglu et al. [16] showed the association of XRCC3, XPD and OGG1 genotypes with an increased MDS risk in Turkish population. Similarly, Li et al. [17] showed a significant difference of RAD51 between MDS patients and controls. Belickova et al. [18] reported a significant association of LIG1, RAD52, MSH3 and GPX3 genotypes with MDS, thus indicating that these genes might have important role on MDS. However, a study carried out by Fabiani et al. [10] showed that the difference of frequencies of DNA repair gene polymorphisms (RAD51, XRCC3 and XPD) was not significant between MDS patients and controls. Kreuziger et al. [19] did not find any correlation of RAD51 and XRCC3 gene polymorphisms with MDS. A study carried out by Sekar et al. [20] suggests that Gln/Gln genotype of XPD poses a great risk for cancer. The protective effect of variant allele 399Gln of XRCC1 also has been reported in the development of t-AML [21]. A study from British population demonstrated an increased risk of AML in individuals with XPD 751Gln genotype [22]. Similar results were observed in paediatric AML cases in a study carried out in USA [23]. Our extensive literature survey indicates that the XRCC3, XPD and RAD51 gene polymorphisms are frequently associated with MDS. However, our results are slightly different from the existing literature on polymorphisms of DNA repair genes. In our cohort, the XRCC1 polymorphism was observed in significant number of patients (Table 2). Hence, XRCC1 polymorphism might be associated with risk of MDS in Indian population. However, the prognostic significance of the XRCC1 polymorphism needs to be established. Our interesting observation is that the XRCC1 polymorphism was significantly associated with RAEB I and II subgroup MDS (Table 4). The frequency was found to be high in patients with excess blasts as compared to patients without excess blasts. However, a large sample size of RAEB I and II needs to be studied to show the involvement of these polymorphisms in advanced MDS group.
Few studies have shown that the genetic variant of genes may alter endonuclease and DNA binding activity and reduce ability to communicate with BER proteins [24, 25]. XRCC1 is an important component of BER pathway. After excision of a damaged base, it stimulates endonuclease action and acts as a scaffold in the subsequent restoration of the site by complexing with DNA ligase III via a BRCT domain in its COOH terminus and with DNA polymerase h via the NH2-terminal domain [26]. XPD plays an important role in recognizing damaged DNA in nucleotide excision repair (NER) pathway by ATP-dependent helicase. Functional disorder in helicase that is responsible for the NER function usually results from chemotherapy that leads to DNA defects and replacement of Lys amino acid with glutamine amino acid in XPD gene codon 751 [22]. The defects in these genes affect the DNA repair process leading to genomic instability and tumorigenesis.
The investigation of gene-environment interaction is important in studying the impact of repair genes on cancers, since the effects of genetic polymorphisms may be apparent only in presence of carcinogenic agents such as pesticides, etc. Several etiological factors have been reported in MDS. Importantly, exposure to tobacco, smoking and alcohol found to have association with MDS. In our study group, majority of the MDS patients had a history of pesticide exposure and tobacco chewing habit (Tables 4 and 5). The MDSs were distributed according to gene polymorphisms, and we have observed a high frequency (62 %) of XRCC1 gene polymorphisms in MDS patients who had a history of exposure to pesticides and a habit of tobacco chewing. However, the results were not statistically significant. There are several studies that have shown the association of smoking, alcohol and tobacco usage with MDS [27, 28]. Ma et al. [29] suggested that the smoking exposure in low-risk MDS is associated with increased mortality.
In conclusion, XRCC1 (Arg280His) and XPD genotypes are associated with an increased MDS risk, suggesting the involvement of these genes in the pathogenesis of disease. XRCC1 gene polymorphism is significantly present in Indian patients with MDS and associated may be with RAEB I and II subgroup, and this needs to be confirmed with large sample size.
References
Ma W, Kantarjian H, Zhang K, Zhang X, Wang X, Chen C, Donahue AC, Zhang Z, Yeh CH, O’Brien S, Garcia-Manero G, Caporaso N, Landgren O, Albitar M (2010) Significant association between polymorphism of the erythropoietin gene promoter and myelodysplastic syndrome. BMC Med Genet 11:163
Rund D, Ben Yehuda D (2004) Therapy-related leukemia and myelodysplasia: evolving concepts of pathogenesis and treatment. Hematology 9(3):179–187
Ronen A, Glickman BW (2001) Human DNA repair genes. Environ Mol Mutagen 37(3):241–283
Bsnescu C, Trifa AP, Demian S, Lazar EB, Dima D, Duicu C, Dobreanu M (2014) Polymorphism of XRCC1, XRCC3, and XPD genes and risk of chronic myeloid leukemia. Biomed Res Int 2014:213790. doi:10.1155/2014/213790
Yamane A, Kohno T, Ito K, Sunaga N, Aoki K et al (2004) Differential ability of polymorphic OGG1 proteins to suppress mutagenesis induced by 8-hydroxyguanine in human cell in vivo. Carcinogenesis 25:1689–1694
Benson FE, Baumann P, West SC (1998) Synergistic actions of Rad51 and Rad52 in recombination and DNA repair. Nature 391(6665):401–404
Jara L, Acevedo ML et al (2007) RAD51 135G>C polymorphism and risk of familial breast cancer in a South American population. Cancer Genet Cytogenet 178(1):65–69
Jakubowska A, Gronwald J et al (2007) The RAD51 135 G>C polymorphism modifies breast cancer and ovarian cancer risk in Polish BRCA1 mutation carriers. Cancer Epidemiol Biomarkers Prev 16(2):270–275
Rowley JD (1999) The role of chromosome translocations in leukemogenesis. Semin Hematol 36:59–72
Fabiani E, D’Alò F, Scardocci A, Greco M, Di Ruscio A, Criscuolo M, Fianchi L, Pagano L, Hohaus S, Leone G, Voso MT (2009) Polymorphisms of detoxification and DNA repair enzymes in myelodyplastic syndromes. Leuk Res 33:1068–1071
Vardiman JW, Thiele J, Arber DA et al (2009) The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 114:937–951
Spry M, Pierce H (2007) DNA repair pathways and hereditary cancer susceptibility syndromes. Front Biosci 12:4191–4207
El-Din MS, Raslan H, Abdel-Hamid S, Makhlouf M (2012) Detection of XRCC1 gene polymorphisms in Egyptian patients with acute myeloid leukemia. Comp Clin Pathol 21(5):505–513
Joseph T, Kusumakumary P, Chacko P, Abraham A, Pillai MR (2005) DNA repair gene XRCC1 polymorphisms in childhood acute lymphoblastic leukemia. Cancer Lett 217(1):17–24
Annamaneni S, Gorre M, Kagita S, Addepalli K, Digumarti RR, Satti V, Battini MR (2013) Association of XRCC1 gene polymorphisms with chronic myeloid leukemia in the population of Andhra Pradesh, India. Hematology 18(3):163–168
Aktuglu MB, Ayer M, Bireller ES, Rencuzogullari C, Acik H, Karaali Z, Alioglu T, Yigit N, Velet M, Atalay E, Ure OS, Cakmakoglu B (2014) Investigation of DNA repair gene variants on myelodysplastic syndromes in a Turkish population. Med Oncol 31:174
L L, Yang L, Zhang Y, Xu Z, Qin T, Hao Y, Xiao Z (2011) Detoxification and DNA repair genes polymorphisms and susceptibility of primary myelodysplastic syndromes in Chinese population. Leuk Res 35:762–765
Belickova M, Merkerova MD, Stara E, Vesela J, Sponerova D, Mikulenkova D, Brdicka R, Neuwirtova R, Jonasova A, Cermak J (2013) DNA repair gene variants are associated with an increased risk of myelodysplastic syndromes in a Czech population. J Hematol Oncol 6:1–6
Baumann Kreuziger LM, Steensma DP (2008) RAD51 and XRCC3 polymorphism frequency and risk of myelodysplastic syndromes. Am J Hematol 83(10):822–823
Seker H, Butkiewicz D, Bowman ED, Rusin M, Hedayati M, Grossman L, Haris CC (2001) Functional significance of XPD polymorphic variants: attenuated apoptosis in human lymphoblastoid cells with the XPD 312 Asp/Asp genotype. Cancer Res 61:7430–7434
Seedhouse C, Bainton R, Lewis M, Harding A, Russell N, Das-Gupta E (2002) The genotype distribution of the XRCC1 gene indicates a role for base excision repair in the development of therapy-related acute myeloblastic leukemia. Blood 100:3761–3766
Allan JM, Smith AG, Wheatley K, Hills RK, Travis LB, Hill DA, Travis LB, Hill DA, Swirsky DM, Morgan GJ, Wild CP (2004) Genetic variation in XPD predicts treatment outcome and risk of acute myeloid leukemia following chemotherapy. Blood 104:3872–3877
Mehta PA, Alonzo TA, Gerbing RB, Elliott JS, Wilke TA, Kennedy RJ, Ross JA, Perentesis JP, Lange BJ, Davies SM (2006) XPD Lys751Gln polymorphism in the etiology and outcome of childhood acute myeloid leukemia: a Children’s Oncology Group report. Blood 107:39–45
Griffin CS, Simpson PJ, Wilson CR, Thacker J (2000) Mammalian recombination-repair genes XRCC2 and XRCC3 promote correct chromosome segregation. Nat Cell Biol 2:757–761
Cui X, Brenneman M, Meyne J, Oshimura M, Goodwin EH, Chen DJ (1999) The XRCC2 and XRCC3 repair genes are required for chromosome stability in mammalian cells. Mutat Res 434:75–88
Vidal A-E, Boiteux S, Hickson I-D, Radicella J-P (2001) XRCC1 coordinates the initial and late stages of DNA abasic site repair through protein-protein interactions. EMBO J 20:6530–6539
Bjork J, Albin M, Mauritzson N, Stromberg U, Johannson B, Hagmar L (2000) Smoking and myelodysplastic syndromes. Epidemiology 11:285–291
Du Y, Fryzek J, Sekeres MA, Taioli E (2010) Smoking and alcohol intake as risk factors for myelodysplastic syndromes (MDS). Leuk Res 34(1):1–5
Ma X, Wang R, Galili N, Mayne ST, Wang SA, Yu H, Raza A (2011) Cigarette smoking shortens the survival of patients with low-risk myelodysplastic syndromes. Cancer Causes Control 22(4):623–629
Acknowledgments
Author (DJ) thanks the Indian Council of Medical Research (ICMR) for the award of Postdoctoral fellowship. The part of the study was carried out from an ICMR core grant to the institute. Thanks are also due to the haematologists of KEM Hospital and technical staff of the Cytogenetics Department.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Methods 1
(DOCX 11 kb)
Supplementary Table 1
(DOCX 16 kb)
Supplementary Fig. 1
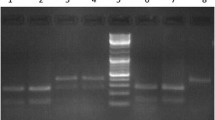
PCR-RFLP analysis on 3 % polyacrylamide gel containing ethidium bromide. Arg280His (B) Arg399Gln (C) Arg194Trp polymorphism of XRCC1 gene, (D) Ser326Cys polymorphism of OGG1 (E) Thr241Met polymorphism of XRCC3 (F) 135 G > C polymorphism of RAD51 (G) Lys751Gln polymorphism (JPEG 131 kb).
Rights and permissions
About this article
Cite this article
Joshi, D., Korgaonkar, S., Shanmukhaiah, C. et al. Association of XPD (Lys751Gln) and XRCC1 (Arg280His) gene polymorphisms in myelodysplastic syndrome. Ann Hematol 95, 79–85 (2016). https://doi.org/10.1007/s00277-015-2528-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-015-2528-3