Abstract
Nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) is a distinct subtype of Hodgkin lymphoma. We report our results of relapsed/refractory NLPHL patients who received high-dose chemotherapy and autogenic stem cell transplantation (HDC auto-SCT). Seventeen NLPHL patients received HDC auto-SCT (1996–2014): male 14 and female 3, with median age at diagnosis of 22 years, at HDC auto-SCT 28 years (15–58 years). At the time of relapse/progression, 13 (76 %) had NLPHL and 4 (24 %) had transformed diffuse large B cell lymphoma. The reason for HDC auto-SCT was refractory NLPHL in 12 patients and relapsed in 5 patients. Salvage chemotherapy was etoposide, methylprednisolone, cisplatinum, and Ara-C (ESHAP); eight patients also received rituximab with ESHAP. HDC was carmustine, etoposide, cytarabine, and melphalan (BEAM). Post-auto-SCT, complete remission was achieved in 14 (82 %), partial remission in 1 (6 %), and progressive disease in 2 (12 %) patients. The median follow-up is 63 months from auto-SCT (6–124 months). Of the nine patients who received only ESHAP, four had post-auto-SCT events versus no event in all eight patients who received rituximab + ESHAP. Kaplan–Meier estimates of 5-year event-free survival for the whole group is 76 %: rituximab + salvage (100 %) versus salvage alone (56 %), P = 0.041. Overall survival is 94 %: 100 versus 89 %, respectively, P = not significant (NS). Even in refractory NLPHL patients, long-term disease-free survival is possible after HDC auto-SCT. Post-auto-SCT relapse or progression can still be managed with chemo/chemo + immunotherapy/radiation. These encouraging results of rituximab in salvage setting should be explored further in a clinical trial setting for this patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) is a relatively uncommon disease that accounts for about 5–6 % of all Hodgkin lymphoma (HL) cases. NLPHL is a distinct subtype of HL with unique clinicopathological, morphologic, and immunohistochemical features. The most characteristic histological feature of NLPHL is the presence of atypical “lymphocyte-predominant cells” (LP cells) or “pop-corn” cells in a background of non-neoplastic and reactive nodular small mature B lymphocytes. These LP cells are CD20-positive, CD15-negative, and CD30-negative [1–3]. Both Revised European-American Lymphoma (REAL) classification and subsequently the World Health Organization (WHO) classification recognized NLPHL as a distinguished type of HL different from classical HL [1]. According to the WHO 2008 definition, at least a partial nodular pattern is required for the diagnosis of NLPHL. Patients are predominantly male and mostly in the 30–50 years age group [4, 5]. Although long-term survival is better than in classical HL, frequent relapses are common and progression/transformation to aggressive non-Hodgkin lymphoma (NHL) is a concern [2, 3, 6–8]. Due to the presence of CD20-positive cells, rituximab has been tried for these patients at different disease statuses with variable success [9, 10]. Due to the relatively small number of cases, most of the information is gained from a small series or subset analysis of larger HL reports. Furthermore, the role of high-dose chemotherapy and autogenic stem cell transplantation (HDC auto-SCT) in relapsed and refractory NLPHL is not well reported due to paucity of data. We present our results of patients with NLPHL who underwent HDC auto-SCT with and without rituximab-containing salvage chemotherapy.
Patients and methods
The Institutional Research Advisory Council approved this retrospective study. The details of methodology were fully explained in our previous report [11]. All HL patients with relapsed or primary refractory NLPHL who underwent HDC auto-SCT from 1996 to 2014 were reviewed. All patients were staged according to Ann Arbor/Cotswolds modification staging system. International Working Group response criteria were used for response [12]. Refractory disease is defined as partial response (PR), no response (NR), stable disease (SD), progressive disease (PD), or relapsing within 3 months of finishing the planned treatment [11]. Refractory relapse is defined as any relapsed disease that did not respond to salvage chemotherapy and required second line of salvage therapy. Kaplan–Meier method was used for overall survival (OS) and event-free survival (EFS) analysis from day 0 of auto-SCT. Event is defined as post–HDC auto-SCT presence of disease, relapse, progression, or death from any cause. All cases were discussed and reviewed in the combined lymphoma conference (medical oncologist, radiation oncologist, radiologist, and hematopathologist) and approved in the transplant meeting for HDC auto-SCT.
Results
From 1996 to December 2014, 306 patients with relapsed/refractory HL received HDC auto-SCT. Nineteen of those patients had primary diagnosis of NLPHL (representing 6.2 % of total HL patients who underwent HDC auto-SCT). Two patients are excluded from the analysis as they were transplanted only a few weeks ago. All analysis is for 17 patients: male 14 (82 %) and female 3 (18 %). Median age at diagnosis was 22 years (10–54 years) and at HDC auto-SCT 28 years (15–58 years). At initial presentation, patients were at stages I/II/III/IV, 2:6:5:4 respectively. First-line chemotherapy was administered in 16 patients (94 %), while only 1 patient (6 %) had radiotherapy (XRT) alone. Primary treatment was adriamycin, bleomycin, vinblastin, and dacarbazine (ABVD) for 14 (82 %) patients. One patient received mechlorethamine , vincristine, procarbazine, and prednisone (MOPP), and one patient had rituximab + cyclophosphamide, adriamycin, vincristine, and prednisone (CHOP) and so received XRT alone. Three patients also had XRT after chemotherapy. Thirteen patients (76 %) had tissue confirmation at relapse/progression prior to salvage chemotherapy: 8/12 with refractory and 5/5 with relapsed disease had biopsy.
Six patients received >1 line of salvage chemotherapy. Three patients with first relapse were not responding to first-line salvage and required second-line salvage chemotherapy. Two patients had disease refractory to both primary chemotherapy and first-line salvage and received second-line salvage before HDC auto-SCT. One patient with frequent relapses received HDC auto-SCT for third relapse. The reason for HDC auto-SCT was primary refractory disease in ten, first relapse in six (two of them had refractory relapse), and third relapse in one patient. Overall, 12 patients had HDC auto-SCT for refractory and 5 for relapsed disease.
At the time of relapse/progression, prior to starting salvage chemotherapy and HDC auto-SCT, 13 (76 %) had NLPHL and 4 (24 %) had transformed diffuse large B cell lymphoma (DLBCL; 2 had DLBCL and 2 had T cell histiocytic rich B cell lymphoma type). The reason for HDC auto-SCT was refractory NLPHL in 12 patients: PD (4), PR (4), relapse within 3 months after initial chemotherapy (2), and refractory relapse in 2 patients. Five patients had relapsed disease. Prior to salvage chemotherapy, stages I/II/III/IV were 2:4:6:5 respectively. Detailed patient’s characteristics are shown in Table 1. With respect to the number of salvage regimens used before auto-SCT, 11 patients had only one line of salvage chemotherapy (primary chemotherapy therapy followed by salvage), 5 with two salvage chemotherapy (one primary and two lines of salvage), and only 1 with three lines of salvage prior to HDC auto-SCT. Etoposide, solumedrol, cisplatinum, and Ara-C (ESHAP) as salvage chemotherapy was used in 16/17 patients: as first-line salvage in 11 (65 %), second-line in 0, and third-line in 5 (29 %); median number of ESHAP cycles given were 3 (1 patient 14 years of age had pediatrics version/doses of ESHAP). Eight patients also received rituximab with ESHAP (one dose in one, three doses in five, and four doses in two patients as rituximab + ESHAP).
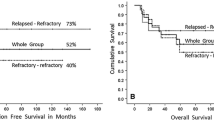
Response to salvage chemotherapy was PR in seven patients (41 %) and CR in ten patients (59 %). We observed 75 % CR (6/8 patients) after salvage chemotherapy/prior to HDC in patients who received rituximab + ESHAP as compared to 44 % (4/9 patients) with non-rituximab-based salvage chemotherapy. All patients had carmustine, etoposide, cytarabine, and melphalan (BEAM) as conditioning regimen [11, 13]. Neutrophil (10 days, range 8–20 days) and platelet engraftment (16 days, range 10–28 days) were the same as reported before for 168 patients [13]. There was no treatment-related mortality, any myelodysplasia/leukemia, or second cancer in this cohort so far. Post-auto-SCT overall response was observed in 15 patients (88 %), CR or CR-unconfirmed in 14 (82 %), PR in 1 (6 %), and progressive disease in 2 (13 %). Six (38 %) patients had XRT post–HDC auto-SCT, for consolidation post-CR in four patients, and one each for eradication of residual disease and for palliation in a patient with rapid PD. Of the nine patients who received ESHAP without rituximab, four of them had post–HDC auto-SCT disease specific events (persistent disease in one, relapsed in one, and PD in two patients) as compared to no event in eight patients who received rituximab + ESHAP. Currently, 16 (94 %) patients are alive, 15 with no evidence of disease, and only 1 with disease (on observation for slowly progressive disease for 8 years). One patient with transformed DLBCL had rapidly progressive disease post–HDC auto-SCT and received only palliative XRT and died shortly. One patient with persistent disease received XRT and went into CR. One patient had slowly progressive disease for the last 8 years and is on observation only. One patient who had HDC in 2005 relapsed three times after HDC: at first relapse received gemcitabine + rituximab and went into CR; at second relapse received rituximab × 8 doses and XRT and went into CR; at third post–HDC relapse, he received rituximab + COPP, went into CR and currently on maintenance rituximab. Median follow-up is 63 months from auto-SCT (9 to 124 months), rituximab + salvage with 65 months follow-up (9 to 121 months, except for one patient, all with >60 months of follow-up), and salvage alone with 73 months (36 to 124 months). In our institution, routine use of fluorodeoxyglucose–positron emission tomography (FDG-PET) after salvage chemotherapy started in 2005. Ten out of 17 patients also had FDG-PET after salvage chemotherapy; 2 patients had positive FDG-PET after salvage and 1 of them had PD. Eight patients had negative FDG-PET, and no disease-related event was observed in these patients. Kaplan–Meier estimates of 5-year event-free survival for the whole group is 76 % (rituximab + salvage vs salvage alone is 100 vs 56 %, P = 0.041) and overall survival is 94 % (rituximab + salvage vs salvage alone is 100 vs 89 % respectively, P = not significant (NS)).
Four patients had transformed DLBCL. Two of them had relapsed disease, one had rituximab-based salvage, and both are alive. Two patients had refractory disease; one patient had rituximab-based salvage and is alive, and the other patient rapidly progressed and died. Overall, three out of four transformed patients are alive.
We also compared the outcome of NLPHL with our 272 relapsed/refractory HL and 143 aggressive non-Hodgkin lymphoma who had HDC auto-SCT during the same time period. Five-year disease-specific EFS was 76 versus 56 versus 61 % (P = 0.52), and disease-specific OS was 94 vs 67 vs 63 % (P = 0.07), respectively (Table 2 and Fig. 1). Despite the small sample size, we can discern a clear trend favoring superior OS of NLPHL patients. The outcomes of patients with NLPHL with a disease event after HDC auto-SCT (persistent disease, relapse, progression) were significantly different as compared to relapsed/refractory HL and aggressive non-Hodgkin lymphoma. One out of 4 (25 %) NLPHL patients died after an event as compared to the fact that 87/117 (75 %) HL and 44/52 (85 %) with aggressive non-Hodgkin lymphoma patients died after having post–HDC auto-SCT persistent disease, relapse, or progression (P = 0.002). However, these numbers are too small to make a concrete interpretation.
Discussion
HDC auto-SCT is a well-established treatment modality for relapsed and refractory HL. The role of HDC auto-SCT in relapsed and refractory NLPHL is not well reported due to the small number of patients, making it difficult to conduct a prospective trial or to even perform a retrospective analysis of a large cohort of NLPHL patients who underwent HDC auto-SCT. Limited studies have reported a selected number of NLPHL cases alone or as a part of classical HL patients who underwent HDC auto-SCT [14–18]. We are reporting our cohort of 17 patients, representing 5.55 % of all HLs transplanted in our institution. Unlike other reports, where many patients were heavily pretreated, most of our patients, as shown in Table 1, are minimally pretreated and two thirds after primary chemotherapy failure received salvage chemotherapy and HDC. Furthermore, only 35 % of the patients had two or more lines of salvage chemotherapy prior to HDC auto-SCT. Despite these high risk characteristics, long-term overall survival is extremely encouraging and only one patient had died of disease. Rituximab was also used in eight patients (from 2004 to 2014) with salvage, and we observed 75 % CR after rituximab + salvage chemotherapy as compared to 44 % with non-rituximab-based salvage chemotherapy (P = NS due to small sample size).
Jackson et al. [14] reported eight patients who received HDC auto-SCT and only three (37.5 %) remained in CR after a median follow-up of 39.2 months (range 28.6–138.5). Of these three patients who are in CR, two patients received rituximab-based salvage and their CR duration is 28 and 50 months. They also reported encouraging result of rituximab as second to sixth line without HDC auto-SCT in ten patients of whom six are in CR. Karuturi et al. [15] reported a large series of 26 patients and reported 5-year OS and EFS of 76 and 69 %, respectively. Eleven patients received rituximab in salvage setting and nine in conditioning regimen before HDC. Separate analysis of rituximab + salvage/conditioning is not provided. Basioli et al. [16] reported 16 patients; 9 of them had HDC auto-SCT after histological transformation. Patients with histological transformation treated with and without HDC auto-SCT had no significant difference in outcome (most likely due to small sample size). They also reported 21 patients who received rituximab, 1 during first line, 13 during second, and 7 during third or later treatment. Separate analysis of rituximab in any setting is not provided. Patients with histological transformation at relapse had significantly inferior outcome as compared to patients who had NLPHL diagnosis at relapse (P = 0.006). Bierman et al. [17] reported 19 patients who underwent HDC auto-SCT for relapsed and refractory NHPHL from 1987 to 2002. He also compared their outcome with 229 nodular sclerosis HLs during the same time period. Five-year progression-free survival for NLPHL (40 %) was similar to that for nodular sclerosis HL patients (39 %). Five-year OS was also similar, 56 versus 53 %, respectively. Rituximab was not used in this study. Xing et al. reported seven NLPHL patient cases that underwent HDC auto-SCT, but details are not provided [18].
These reports, along with our data, highlight that for patients with NLPHL, even with refractory disease or after multiple relapses, long-term disease-free survival is possible after HDC auto-SCT. Although only a limited number of patients received rituximab, results are encouraging and rituximab has shown promising results. These results should be interpreted with caution due to selection bias and very small sample size of most reports. Integration of rituximab in salvage setting should be explored further in this unique set of patient population. Post–HDC auto-SCT relapse or progression can still be managed with chemotherapy/chemotherapy + immunotherapy.
References
Harris NL, Jaffe ES, Stein H et al (1994) A revised European-American classification of lymphoid neoplasms: a proposal from the international lymphoma study group. Blood 84:1361–1392
Illes A, Simon Z, Toth E et al (2008) Nodular lymphocyte predominant Hodgkin lymphoma (NLPHL)-clinicopathological features based on the data of two Hungarian lymphoma centres. Pathol Oncol Res 14:411–421
Fan Z, Natkunam Y, Bair E et al (2003) Characterization of variant patterns of nodular lymphocyte predominant Hodgkin lymphoma with immunohistologic and clinical correlation. Am J Surg Pathol 27:1346–1356
Nogova L, Reineke T, Brillant C et al (2008) Lymphocyte-predominant and classical Hodgkin's lymphoma: a comprehensive analysis from the German Hodgkin study group. J Clin Oncol 26:434–439
Nogova L, Reineke T, Eich HT et al (2005) Extended field radiotherapy, combined modality treatment or involved field radiotherapy for patients with stage IA lymphocyte-predominant Hodgkin's lymphoma: a retrospective analysis from the German Hodgkin study group (GHSG). Ann Oncol 16:1683–1687
Feugier P, Labouyrie E, Djeridane M et al (2004) Comparison of initial characteristics and long-term outcome of patients with lymphocyte-predominant Hodgkin lymphoma and classical Hodgkin lymphoma at clinical stages IA and IIA prospectively treated by brief anthracycline-based chemotherapies plus extended high-dose irradiation. Blood 104:2675–2681
Hartmann S, Eray M, Doring C et al (2014) Diffuse large B cell lymphoma derived from nodular lymphocyte predominant Hodgkin lymphoma presents with variable histopathology. BMC Cancer 14:332
Huang JZ, Weisenburger DD, Vose JM et al (2003) Diffuse large B-cell lymphoma arising in nodular lymphocyte predominant Hodgkin lymphoma. A report of 21 cases from the Nebraska lymphoma study group. Leuk Lymphoma 44:1903–1910
Advani RH, Horning SJ, Hoppe RT et al (2014) Mature results of a phase II study of rituximab therapy for nodular lymphocyte-predominant Hodgkin lymphoma. J Clin Oncol 32:912–918
Eichenauer DA, Fuchs M, Pluetschow A et al (2011) Phase 2 study of rituximab in newly diagnosed stage IA nodular lymphocyte-predominant Hodgkin lymphoma: a report from the German Hodgkin study group. Blood 118:4363–4365
Akhtar S, Al-Sugair AS, Abouzied M et al (2013) Pre-transplant FDG-PET-based survival model in relapsed and refractory Hodgkin's lymphoma: outcome after high-dose chemotherapy and auto-SCT. Bone Marrow Transplant 48:1530–1536
Cheson BD, Pfistner B, Juweid ME et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25:579–586
Akhtar S, Weshi AE, Rahal M et al (2008) Factors affecting autologous peripheral blood stem cell collection in patients with relapsed or refractory diffuse large cell lymphoma and Hodgkin lymphoma: a single institution result of 168 patients. Leuk Lymphoma 49:769–778
Jackson C, Sirohi B, Cunningham D et al (2010) Lymphocyte-predominant Hodgkin lymphoma—clinical features and treatment outcomes from a 30-year experience. Ann Oncol 21:2061–2068
Karuturi M, Hosing C, Fanale M et al (2013) High-dose chemotherapy and autologous stem cell transplantation for nodular lymphocyte-predominant Hodgkin lymphoma. Biol Blood Marrow Transplant 19:991–994
Biasoli I, Stamatoullas A, Meignin V et al (2010) Nodular, lymphocyte-predominant Hodgkin lymphoma: a long-term study and analysis of transformation to diffuse large B-cell lymphoma in a cohort of 164 patients from the adult lymphoma study group. Cancer 116:631–639
Bierman P, Naushad H, Loberiza F et al (2006) High-dose chemotherapy followed by autologous hematopoietic stem cell transplantation (AHSCT) for lymphocyte predominant Hodgkin’s disease. Blood 108:1a–1062a
Xing KH, Connors JM, Lai A et al (2014) Advanced-stage nodular lymphocyte predominant Hodgkin lymphoma compared with classical Hodgkin lymphoma: a matched pair outcome analysis. Blood 123:3567–3573
Acknowledgments
We appreciate Ms. Ruqaya Belkhedim, Mr. Fayez Abu Zeid, and Ms. Reena Ulahannan from BMT clinic, Mr. Edgardo Colcol, Riad Youniss, and Dr. Abida Rehman from Oncology Research Unit, and Ms. Iman Youssef, clinical nurse coordinator, and Haris Syed for reviewing the manuscript for their valuable contribution.
Author contribution
All authors had full access to the manuscript and approved the final version.
Contributions: S.A. was the principal investigator, designed research, collected data, analyzed and interpreted data, and wrote the manuscript. T. Elhassan performed statistical analysis, wrote statistical portion of the manuscript, and provided final approval of the manuscript. S.M.R, M.N.Z, and W.E helped in interpretation of data, drafting the article, and provided final approval of the manuscript. I.M. designed research, collected data, interpreted data, helped in manuscript writing, and provided final approval of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
No conflict of interest.
Funding
No research grant or support for this project
Approved by Institutional Research Advisory Counsel
All authors had full access to all data.
Human and Animal Rights and Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Rights and permissions
About this article
Cite this article
Akhtar, S., Elhassan, T.A.M., Edesa, W. et al. High-dose chemotherapy and autologous stem cell transplantation for relapsed or refractory nodular lymphocyte predominant Hodgkin lymphoma. Ann Hematol 95, 49–54 (2016). https://doi.org/10.1007/s00277-015-2527-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-015-2527-4