Abstract
We retrospectively assessed the clinical characteristics of patients with paroxysmal nocturnal hemoglobinuria (PNH) according to severity of cytopenia. A total of 282 patients with hematological parameters assessed at the time of diagnosis of PNH were included. There were 24 patients with PNH/severe aplastic anemia (SAA) (at least two of the three criteria; hemoglobin ≤8 g/dL; absolute neutrophil count (ANC) <0.5 × 109/L; platelet count <20 × 109/L), 96 patients with PNH/aplastic anemia (AA) (at least two of the three criteria; hemoglobin ≤10 g/dL; ANC 0.5–1.5 × 109/L; platelet count 20–100 × 109/L), and 162 classic PNH patients. Compared with the classic PNH subgroup, the PNH/SAA subgroup had a significantly lower median granulocyte PNH clone size (26.7 vs. 51.0 %, P = 0.021) and lower incidence of lactate dehydrogenase ≥1.5 times the upper limit of normal (52.9 vs. 80.0 %, P = 0.049). The incidence of thromboembolism was similar in both subgroups. Overall survival was significantly lower in the PNH/SAA subgroup than in the classic PNH subgroup (P = 0.033). Our findings suggest that identification of patients with PNH/SAA at the time of diagnosis is important because of different clinical manifestations and poorer outcome compared with patients with classic PNH (clinicaltrials.gov identifier: #NCT01224483).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, progressive, and life-threatening disease driven by chronic hemolysis leading to thrombosis, renal impairment, pain, severe fatigue, poor quality of life, and death [1, 2]. Thrombosis has been considered as a significant risk factor for mortality and the leading cause of death in PNH patients [1–5]. Because immune-mediated bone marrow (BM) failure is essential for the evolution of PNH, patients frequently have an element of BM failure such as aplastic anemia (AA) either before or after the diagnosis of PNH [6, 7]. About half of newly diagnosed PNH patients had a preceding history of AA prior to diagnosis of PNH [5, 8]. Therefore, PNH is usually classified into three subgroups: classic PNH (hemolysis with no BM disorder), PNH in the setting of another specified BM disorder (with or without evidence of hemolysis), and subclinical PNH (usually associated with no hemolysis) [8, 9]. Although PNH clones may be detected in patients with myelodysplastic syndrome (MDS), AA is the most frequently associated BM failure disorder in PNH [1, 3, 6]. Approximately half of all patients with AA have detectable populations of PNH clones although in general, the clone size is smaller in patients with AA than in patients with classic PNH [10–12]. It has been assumed that patients with severe aplastic anemia (SAA) do not exhibit hemolysis and are not at risk for thromboembolism (TE) due to a lack of significant erythropoiesis to generate hemolysis. However, this assumption has not been widely assessed in studies evaluating the clinical characteristics and risk of TE in PNH patients with aplasia/cytopenias (PNH-cytopenia) compared with classic PNH.
Although eculizumab, a monoclonal antibody to complement protein 5 (C5), can be effective in controlling intravascular hemolysis in both classic PNH and PNH-cytopenia, it does not improve underlying cytopenias related to BM failure [13, 14]. This point is important when considering the high risk of serious infection and/or bleeding in patients with SAA. Therefore, patients with PNH-cytopenia should be further subdivided according to the severity of cytopenia into those PNH patients with concomitant SAA (PNH/SAA) or non-SAA (PNH/AA). Patients with PNH/SAA may require additional treatment such as immunosuppressive therapy (IST) or allogeneic hematopoietic stem cell transplantation (HSCT) [15, 16].
The aim of this study was to identify the clinical characteristics and outcomes of patients with PNH-cytopenia stratified according to the severity of cytopenia. In addition, we also evaluated the natural course and pattern of clonal evolution in PNH patients with a history of AA prior to diagnosis of PNH.
Patients and methods
Patients and study design
The AA Working Party of the Korean Society of Hematology has established a web-based nationwide registry of PNH patients. Between July 2009 and November 2010, the medical records of 301 patients from nine institutions were collected in this registry. PNH was confirmed at each individual site using a flow cytometric method based on the analysis of expression of CD55 and CD59. About 50,000 cells were analyzed in each sample. Minimum sensitivity varied over time and between institutions, depending upon the technology employed at each site. However, in the most recent assessments, the minimum clone size detection at most of the sites was 0.1 % for white blood cells and 3 % for red blood cells (RBCs). The percentage of glycosylphosphatidylinositol-anchored proteins (GPI-AP)-deficient cells was determined at the closest time point to diagnosis (n = 236, 78.4 %). In patients diagnosed before the establishment of flow cytometry, a positive Ham or sucrose lysis test was used. The PNH granulocyte and RBC clone sizes were based on available data from chart reviews and were not limited to minimum clone size or flow methodology.
A total of 282 patients (92.2 %) with hematological parameters, including hemoglobin (Hb), absolute neutrophil count (ANC), and platelet count, measured at the time of diagnosis of PNH were included in this analysis. Among them, 214 patients (75.9 %) had a result of GPI-AP analysis (granulocyte) at the time of diagnosis. Clinically significant elevated hemolysis was defined as a lactate dehydrogenase (LDH) levels of 1.5 times or more the upper limit of normal (ULN) based on previously published, multinational, registration clinical trials for the treatment of PNH [5, 17]. We chose LDH at diagnosis of PNH as a consistent point of reference for all analyses. The cumulative incidence of TE was collected for the time periods prior to and post diagnosis of PNH. Clinical symptoms related with PNH, including abdominal pain, chest pain, dyspnea, and hemoglobinuria, were evaluated based on the physician’s reporting after diagnosis of PNH.
Among 282 patients with PNH with hematological parameters at the time of diagnosis of PNH, 93 patients (33.0 %) had been diagnosed with AA before the diagnosis of PNH (Table 1). We further analyzed the pattern of clonal evolution from AA to PNH subtypes according to the IST in 93 PNH patients with preceding AA. This study was conducted in accordance with the Declaration of Helsinki and was reviewed and approved by the Institutional Review Boards of each participating hospital.
Definition of subgroups of PNH patients
Patients were classified into three subgroups according to the presence and severity of cytopenia at the time of diagnosis of PNH: PNH with concomitant SAA (PNH/SAA, n = 24), non-SAA (PNH/AA, n = 96), and classic PNH (n = 162). We defined patients with PNH/SAA for this study with evidence of at least two of the following three hematological parameters at diagnosis: Hb ≤8 g/dL, ANC <0.5 × 109/L, and platelet count <20 × 109/L. Patients who did not fulfill the criteria of PNH/SAA and met at least two of the following three peripheral blood cytopenias were classified to a PNH/AA: Hb ≤10 g/dL, ANC 0.5–1.5 × 109/L, and platelet count 20–100 × 109/L. Classic PNH included the patients with clinical evidence of intravascular hemolysis but no evidence of other BM failure.
Statistical analyses
Statistical analysis was performed by using analysis of variance followed by a post hoc Tukey honest significant difference (HSD) test applied for multiple paired comparisons. The cumulative incidences of the first TE event were estimated using Kaplan-Meier methods and tested for the associated BM failure effect by an unstratified log-rank statistic. Overall survival rate was analyzed using the Kaplan-Meier methods, and survival curves were compared using log-rank test. A P value of <0.05 was defined as statistically significant. All statistical calculations were performed with PASW software version 20.0 (SPSS, Inc., Chicago, IL 60606, USA).
Results
Patient characteristics
Among the 282 patients, there were 24 patients with PNH/SAA (8.5 %), 96 patients with PNH/AA (34.0 %), and 162 classic PNH patients (57.4 %). Baseline demographic and clinical characteristics are summarized in Table 1. There were no statistically significant differences between the subgroups in the median age at diagnosis. The median baseline hematological parameters of Hb, ANC, and PLT were significantly lower in patients with PNH/SAA than in those with PNH/AA or classic PNH. However, there was no difference between the subgroups in the incidence of common symptoms related to PNH such as abdominal pain, chest pain, dyspnea, and hemoglobinuria. With regard to treatment for PNH, significantly more patients in the PNH/SAA or PNH/AA subgroups than in the classic PNH subgroup required RBC transfusions although there were no significant differences between the three PNH subgroups in the percentage of patients who underwent allogeneic HSCT (Table 1). Results from BM analyses performed at the time of diagnosis were available for 251 patients (89.0 %). Based on these BM findings, a total of 18/251 patients (7.2 %) were diagnosed with MDS although none of the patients was considered to have myeloproliferative neoplasms or leukemia. None of the patients had received treatment with eculizumab.
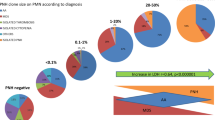
Median granulocyte PNH clone sizes were 26.7 % in PNH/SAA, 39.0 % in PNH/AA, and 50.8 % in classic PNH patients. Post hoc analyses showed that the median clone size was significantly lower in the PNH/SAA subgroup compared with the classic PNH subgroup (P = 0.021, Fig. 1a). The percentage of the patients with a high granulocyte PNH clone size (>50 %) was reported in 4 patients with PNH/SAA (22.2 %), 33 patients with PNH/AA (42.9 %), and 60 patients with classic PNH (50.4 %) with the differences between subgroups being statistically significant for the PNH/SAA versus the classic PNH subgroups (P = 0.039) but not between the PNH/SAA versus the PNH/AA subgroups (P = 0.165) or the PNH/AA versus the classic PNH subgroups (P = 0.578). At diagnosis, LDH levels were significantly lower in the PNH/SAA subgroup compared with the classic PNH subgroup (1.8-fold vs. 4.5-fold greater than ULN; P = 0.008) but were not statistically significant in any of the other subgroup comparisons. The percentages of patients with elevated levels of hemolysis (LDH levels ≥1.5 times ULN) at diagnosis was also significantly lower in the PNH/SAA subgroup than in the classic PNH subgroup (P = 0.049, Fig. 1b). Compared with PNH patients without hemolysis, the patients with hemolysis were not likely to have received treatment with corticosteroid (P = 0.138). Although there was no association between hemolysis and corticosteroid use in the 17 patients with SAA/PNH (P = 0.335) and the 125 patients with classical PNH (P = 0.061), corticosteroid was more frequently used in the 81 AA/PNH patients with hemolysis than in those without hemolysis (P = 0.004). In addition, AA/PNH patients with hemolysis showed significantly higher incidence of corticosteroid use compared with SAA/PNH patients with hemolysis (P = 0.004).
TE events according to the PNH subgroups
Among the 53 patients with TE (18.8 %), 19 patients (35.8 %) experienced multiple TE events. Overall TE events were 80 in our cohort. One event in 34 patients, two events in 13 patients, three events in 4 patients, and four events in 2 patients were reported. In PNH subgroups, TE events were reported in 12.5 % of patients with PNH/SAA (n = 3), 14.6 % of patients with PNH/AA (n = 14), and 22.2 % of patients with classic PNH (n = 36) with no significant differences between the subgroups (Fig. 1c). Cumulative incidence of the first TE was analyzed between the combined PNH-cytopenia subgroup (PNH/SAA and PNH/AA) and the classic PNH subgroup and demonstrated that there was no statistically significant difference between the subgroups (P = 0.402, Fig. 2).
Analysis of known risk factors for TE in the PNH-cytopenia subgroup (n = 120) showed that elevated hemolysis (LDH levels ≥1.5 times ULN), hemoglobinuria, and abdominal pain were more frequently observed in the patients with a history of TE (Table 2). However, only abdominal pain was reported in significantly more patients with TE compared with those without TE (P = 0.027), although the differences between patients with and without TE for the other two risk factors did approach statistical significance (P = 0.060 and 0.055, respectively). In patients with classic PNH (n = 162), statistically significant risk factors for TE were LDH levels ≥1.5 times ULN (P = 0.009), abdominal pain (P = 0.016), chest pain (P < 0.001), and dyspnea (P = 0.002, Table 3). Granulocyte clone size was not found to be a significant risk factor for TE in either the PNH-cytopenia subgroup or classic PNH subgroup. In addition, high PNH clone size (>50 %) also had no impact on the development of TE in both the PNH-cytopenia subgroup and classic PNH subgroup.
Survival in three PNH subgroups
Among 282 enrolled patients, 39 patients (13.8 %) died during the median follow-up time from diagnosis of 6.6 years (range 0–28.8 years). Six patients (25.0 %) with PNH/SAA, 12 (12.5 %) with PNH/AA, and 21 (13.0 %) with classic PNH died. There was no statistically significant difference between the subgroups in overall mortality rate (P = 0.096). However, overall survival was significantly lower in the PNH/SAA subgroup than in the classic PNH subgroup: the hazard ratio for PNH/SAA versus classical PNH was 2.6 with a 95 % confidence interval of 1.048, 6.473 (P = 0.033, Fig. 3). Among the 37 patients who received allogeneic HSCT, 6 patients (16.2 %) died due to treatment-related mortality (one patient in PNH/SAA subgroup, one in PNH/AA subgroup, and four in classic PNH subgroup).
The most common cause of death was infection or critical bleeding related with underlying cytopenia in the PNH/SAA subgroup (n = 5, 83.3 %) and the PNH/AA subgroup (n = 6, 50.0 %). Two patients with PNH/SAA died from bleeding complications (one intracerebral hemorrhage and one pulmonary hemorrhage). However, TE was the most common cause of death in the classic PNH subgroup (n = 5, 23.8 %, Table 4).
Clonal evolution from preceding AA to PNH
Of the 93 patients with a history of AA, 33 patients (35.5 %) had received IST prior to the diagnosis of PNH. Fourteen (42.4 %) of these patients had received treatment with anti-thymocyte globulin (ATG) and cyclosporin A (CsA), 10 (30.3 %) with CsA only, and 9 (27.3 %) with ATG only. The median time from diagnosis of AA to diagnosis of PNH was 3.8 years (range, 0.1–7.5 years). Of these patients, 16 patients (48.5 %) were included in the classic PNH subgroup and 17 (51.5 %) in the PNH-cytopenia subgroup (6 PNH/SAA and 11 PNH/AA). For the remaining 60 patients who did not receive IST, the median time from diagnosis of AA to PNH was 2.3 years (range, 0.1–26.8 years) and 6 patients (10.0 %) were included in the PNH/SAA subgroup, 23 (38.3 %) in the PNH/AA subgroup, and 31 (51.7 %) in the classic PNH subgroup (Fig. 4). There were no differences in the pattern of evolution into the three PNH subgroups based on prior administration of IST (P = 0.524). There were no differences between patients who had or had not received IST in median PNH clone size at the diagnosis of PNH (49.3 vs. 48.7 %, respectively; P = 0.571) and incidence of TE (18.2 vs. 11.7 %, respectively; P = 0.386).
Discussion
We identified that the median granulocyte PNH clone sizes and the incidence of elevated hemolysis (LDH levels ≥1.5 times ULN) at the time of diagnosis were significantly lower in patients with PNH/SAA compared with classic PNH. Although there was no difference in the incidence of TE between these two subgroups, patients with PNH/SAA showed significantly poorer survival largely due to the high incidence of infection or critical bleeding related to the underlying SAA. There was no patient death as a result of TE in PNH/SAA patients. Among the patients with PNH-cytopenia, symptoms of abdominal pain were associated with a significant increase in the incidence of TE with trends for an increased incidence seen in patients with LDH levels ≥1.5 times ULN. Therefore, the identification of the PNH/SAA subgroup among the patients with PNH-cytopenia should be crucial for the decisions of treatment.
According to the results of the South Korean National PNH Registry, approximately half of newly diagnosed PNH patients had concomitant characteristics of AA [5]. Based on the risk of hemolytic PNH symptoms and infection, newly diagnosed PNH patients can be further classified according to the presence and severity of cytopenia. SAA is usually defined as (i) BM cellularity <25 % and (ii) at least two of the following three peripheral blood cytopenias: ANC <0.5 × 109/L, platelet count <20 × 109/L, and anemia with corrected reticulocytes <1 % [18]. However, reticulocytes could not be used for the evaluation of exact functional status of RBC production in the current study because PNH patients usually show increased reticulocyte counts due to increased hemolytic destruction of RBCs. In a French study, 224 patients (48.2 %) with at least two peripheral blood cytopenias (Hb ≤10 g/dL, ANC ≤1.0 × 109/L, and platelet count ≤80 × 109/L) were classified as PNH-AA subcategory [8]. In this study, we used similar criteria for the PNH/AA subgroup and applied more stringent criteria to define patients with PNH/SAA. Although the French study reported that overall survival of patients with PNH-AA was similar to that for patients with classic PNH [8], their PNH-AA cohort did not differentiate patients based on severity of cytopenia which our data has shown that patients with PNH/SAA have significantly inferior survival compared with patients with classic PNH. In our study, relatively low number of patients who received specific treatment for BM failure such as IST (n = 1, 4.2 %) or allogeneic HSCT (n = 3, 12.5 %) might be related with poor survival in the PNH/SAA subgroup. Moreover, patients with PNH/SAA had a significantly higher incidence of history of RBC transfusions, lower granulocyte clone sizes, and lower median LDH levels above ULN. Therefore, further classification of PNH-cytopenia patients into PNH/SAA subgroup and PNH/AA subgroups using our suggested criteria would be appropriate.
Although allogeneic HSCT is the only curative therapy for PNH [19, 20], eculizumab, a monoclonal antibody against complement protein C5, has been used for controlling the intravascular hemolysis in PNH and has been reported a dramatic reduction of complications related to PNH [13, 14]. In addition, PNH patients receiving eculizumab have been reported to have survival times similar to that of the general population [21]. Although eculizumab can be effective in controlling intravascular hemolysis even in PNH patients with BM failure, it cannot improve impaired hematopoiesis related to underlying BM failure [20, 22]. Therefore, allogeneic HSCT should be recommended in PNH patients with life-threatening cytopenias because complications of pancytopenia represent a more imminent cause of morbidity and mortality [19]. Because recent results of HLA-matched relative HSCT using fludarabine-based reduced-intensity conditioning regimen showed excellent treatment outcomes (87.8 % of 6-year survival) [16], reduced-intensity HSCT should be recommended for PNH/SAA patients if a suitable donor is available. IST would be considered in PNH/SAA patients with no suitable donor, old age, or co-morbidities such as TE [23, 24]. Eculizumab could also be considered in hemolytic PNH patients if significant improvement of cytopenias is observed following IST in patients with PNH/SAA. Therefore, treatment strategy should be individualized in patients with PNH-cytopenia based on severity of cytopenia, age, availability of transplant donor, co-morbidities, etc. Our suggested criteria for PNH/SAA or PNH/AA can be used as a tool to select appropriate PNH patients who may benefit from HSCT or IST. However, an individualization of treatment strategies using these criteria still requires more robust investigations.
Retrospective investigations into clonal evolution in this study showed that 93 PNH patients with a prior diagnosis of AA were included in each of the three PNH subgroups irrespective of if they had received IST or not. Although AA patients with PNH clones have been known as a high probability of responding to IST [24, 25], approximately half of our PNH patients with prior AA had clonal evolution to classic PNH regardless of IST. Therefore, the regular monitoring of PNH clones should be recommended in patients with AA, even if they had not received IST.
According to the results from the International PNH Registry, PNH patients with larger clones are more likely to suffer TE events [12]. In addition, some studies have reported the clinical importance of high PNH clone size (>50 %) in relationship to risk of TE [26, 27]. Although patients with PNH/SAA showed significantly lower incidence of high PNH clone size (>50 %), there were no differences in the incidences of TE across the three PNH subgroups in our study. Considering the pathogenesis of TE in patients with PNH [28], the development of TE is multifactorial including platelet activation, toxicity of free hemoglobin, nitric oxide depletion, absence of other glycosylphosphatidylinositol-linked proteins such as urokinase-type plasminogen activator receptor, and endothelial dysfunction. Because these multiple mechanisms have played a role in the development of TE in PNH, all PNH patients may have a risk of TE regardless of PNH clone size. We previously reported that clone size alone cannot explain the development of TE nor predict the risk of TE in patients with PNH [5]. In addition, we also suggested that PNH patients with LDH levels ≥1.5 times ULN had a higher risk of TE [5]. Although the percentages of patients with elevated hemolysis at diagnosis was significantly lower in the PNH/SAA subgroup than in the classic PNH subgroup (P = 0.049), the PNH-cytopenia patients (both PNH/AA and PNH/SAA) with LDH levels ≥1.5 times ULN had a trend toward an increased incidence of TE compared with those without elevated hemolysis. We had a limitation for validating the relationship between increased hemolysis and risk of TE in the PNH/SAA subgroup because of the small number of patients included in the PNH/SAA subgroup. In addition, LDH levels, which can undergo considerable fluctuation according to the degree of hemolysis, were only captured at the time of diagnosis of PNH. Therefore, the relationship between LDH/hemolysis and risk of TE in patients with PNH-cytopenia should be evaluated in future prospective studies.
Although clinical symptoms such as chest pain or dyspnea were associated with increased risk of TE only in patients with classic PNH, abdominal pain was a significant risk factor for TE in both patients with PNH-cytopenia and patients with classic PNH (P = 0.027 and P = 0.016, respectively). It might be considered as a meaningful result because clinical symptom related with TE (abdominal pain) was a risk factor even in patients with PNH-cytopenia. Therefore, we recommend the evaluation of serum LDH level and the regular monitoring of symptoms related to PNH for expecting the risk of TE in patients with PNH-cytopenia.
The retrospective nature of this study meant that we could not evaluate the exact percentage of clonal evolution to PNH in patients with AA. In addition, hematologic parameters and results of BM study at the time of diagnosis of preceding AA were not available. Therefore, we were not able to observe the exact natural course of the patients with AA who eventually transform or evolve into PNH. However, this study demonstrated the diverse clonal evolution pattern from AA to three different subgroups of PNH regardless of IST in a large PNH cohort. Therefore, we can propose that regular evaluations should be made for the presence of PNH clones in patients with AA. In addition, our results could be useful to discriminate the PNH/SAA from a more general PNH-cytopenia patient population.
In conclusion, the PNH/SAA subgroup showed distinct clinical manifestations such as higher requirements of RBC transfusion, lower incidence of elevated hemolysis, and smaller PNH clone sizes compared with the classic PNH subgroup. Because a substantial proportion of TE and poor survival were reported in the PNH/SAA subgroup, different treatment strategies such as IST or allogeneic HSCT should be considered on an individual patient basis. Therefore, our further classification of PNH-cytopenia provided a rationale for an individualized approach in the treatment of PNH patients. In addition, the evaluation of serum LDH level and the regular monitoring of symptoms related to PNH for expecting the risk of TE should be required in patients with PNH-cytopenia as well as classic PNH. We also recommend the regular monitoring for PNH clone in all AA patients regardless of IST because patients with AA can evolve to three different subgroups of PNH.
References
Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV (1995) Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med 333(19):1253–1258. doi:10.1056/NEJM199511093331904
Parker CJ (2012) Paroxysmal nocturnal hemoglobinuria. Curr Opin Hematol 19(3):141–148. doi:10.1097/MOH.0b013e328351c348
Socie G, Mary JY, de Gramont A, Rio B, Leporrier M, Rose C, Heudier P, Rochant H, Cahn JY, Gluckman E (1996) Paroxysmal nocturnal haemoglobinuria: long-term follow-up and prognostic factors. French Society of Haematology. Lancet 348(9027):573–577
Nishimura J, Kanakura Y, Ware RE, Shichishima T, Nakakuma H, Ninomiya H, Decastro CM, Hall S, Kanamaru A, Sullivan KM, Mizoguchi H, Omine M, Kinoshita T, Rosse WF (2004) Clinical course and flow cytometric analysis of paroxysmal nocturnal hemoglobinuria in the United States and Japan. Medicine (Baltimore) 83(3):193–207
Lee JW, Jang JH, Kim JS, Yoon SS, Lee JH, Kim YK, Jo DY, Chung J, Sohn SK (2013) Clinical signs and symptoms associated with increased risk for thrombosis in patients with paroxysmal nocturnal hemoglobinuria from a Korean Registry. Int J Hematol 97(6):749–757. doi:10.1007/s12185-013-1346-4
Brodsky RA (2008) Paroxysmal nocturnal hemoglobinuria: stem cells and clonality. Hematology Am Soc Hematol Educ Program:111–115. doi:10.1182/asheducation-2008.1.111
Pu JJ, Brodsky RA (2011) Paroxysmal nocturnal hemoglobinuria from bench to bedside. Clin Transl Sci 4(3):219–224. doi:10.1111/j.1752-8062.2011.00262.x
de Latour RP, Mary JY, Salanoubat C, Terriou L, Etienne G, Mohty M, Roth S, de Guibert S, Maury S, Cahn JY, Socie G, French Society of H, French Association of Young H (2008) Paroxysmal nocturnal hemoglobinuria: natural history of disease subcategories. Blood 112(8):3099–3106. doi:10.1182/blood-2008-01-133918
Parker C, Omine M, Richards S, Nishimura J, Bessler M, Ware R, Hillmen P, Luzzatto L, Young N, Kinoshita T, Rosse W, Socie G, International PNHIG (2005) Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood 106(12):3699–3709. doi:10.1182/blood-2005-04-1717
Pu JJ, Mukhina G, Wang H, Savage WJ, Brodsky RA (2011) Natural history of paroxysmal nocturnal hemoglobinuria clones in patients presenting as aplastic anemia. Eur J Haematol 87(1):37–45. doi:10.1111/j.1600-0609.2011.01615.x
Raza A, Ravandi F, Rastogi A, Bubis J, Lim SH, Weitz I, Castro-Malaspina H, Galili N, Jawde RA, Illingworth A (2014) A prospective multicenter study of paroxysmal nocturnal hemoglobinuria cells in patients with bone marrow failure. Cytometry B Clin Cytom 86(3):175–182. doi:10.1002/cyto.b.21139
Schrezenmeier H, Muus P, Socie G, Szer J, Urbano-Ispizua A, Maciejewski JP, Brodsky RA, Bessler M, Kanakura Y, Rosse W, Khursigara G, Bedrosian C, Hillmen P (2014) Baseline characteristics and disease burden in patients in the International Paroxysmal Nocturnal Hemoglobinuria Registry. Haematologica 99(5):922–929. doi:10.3324/haematol.2013.093161
Hillmen P, Young NS, Schubert J, Brodsky RA, Socie G, Muus P, Roth A, Szer J, Elebute MO, Nakamura R, Browne P, Risitano AM, Hill A, Schrezenmeier H, Fu CL, Maciejewski J, Rollins SA, Mojcik CF, Rother RP, Luzzatto L (2006) The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med 355(12):1233–1243. doi:10.1056/NEJMoa061648
Kim JS, Lee JW, Kim BK, Lee JH, Chung J (2010) The use of the complement inhibitor eculizumab (Soliris(R)) for treating Korean patients with paroxysmal nocturnal hemoglobinuria. Korean J Hematol 45(4):269–274. doi:10.5045/kjh.2010.45.4.269
Peffault de Latour R, Schrezenmeier H, Bacigalupo A, Blaise D, de Souza CA, Vigouroux S, Willemze R, Terriou L, Tichelli A, Mohty M, de Guibert S, Marsh JC, Passweg J, Yves Mary J, Socie G (2012) Allogeneic stem cell transplantation in paroxysmal nocturnal hemoglobinuria. Haematologica 97(11):1666–1673. doi:10.3324/haematol.2012.062828
Pantin J, Tian X, Geller N, Ramos C, Cook L, Cho E, Scheinberg P, Vasu S, Khuu H, Stroncek D, Barrett J, Young NS, Donohue T, Childs RW (2014) Long-term outcome of fludarabine-based reduced-intensity allogeneic hematopoietic cell transplantation for debilitating paroxysmal nocturnal hemoglobinuria. Biol Blood Marrow Transplant 20(9):1435–1439. doi:10.1016/j.bbmt.2014.05.012
Brodsky RA, Young NS, Antonioli E, Risitano AM, Schrezenmeier H, Schubert J, Gaya A, Coyle L, de Castro C, Fu CL, Maciejewski JP, Bessler M, Kroon HA, Rother RP, Hillmen P (2008) Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood 111(4):1840–1847. doi:10.1182/blood-2007-06-094136
Marsh JC, Ball SE, Cavenagh J, Darbyshire P, Dokal I, Gordon-Smith EC, Keidan J, Laurie A, Martin A, Mercieca J, Killick SB, Stewart R, Yin JA, British Committee for Standards in H (2009) Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol 147(1):43–70. doi:10.1111/j.1365-2141.2009.07842.x
Brodsky RA (2009) How I treat paroxysmal nocturnal hemoglobinuria. Blood 113(26):6522–6527. doi:10.1182/blood-2009-03-195966
Brodsky RA (2010) Stem cell transplantation for paroxysmal nocturnal hemoglobinuria. Haematologica 95(6):855–856. doi:10.3324/haematol.2010.023176
Kelly RJ, Hill A, Arnold LM, Brooksbank GL, Richards SJ, Cullen M, Mitchell LD, Cohen DR, Gregory WM, Hillmen P (2011) Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood 117(25):6786–6792. doi:10.1182/blood-2011-02-333997
Lee JL, Lee JH, Lee JH, Choi SJ, Kim S, Seol M, Lee YS, Chi HS, Park CJ, Kim WK, Lee JS, Lee KH (2003) Allogeneic hematopoietic cell transplantation for paroxysmal nocturnal hemoglobinuria. Eur J Haematol 71(2):114–118
Scheinberg P, Young NS (2012) How I treat acquired aplastic anemia. Blood 120(6):1185–1196. doi:10.1182/blood-2011-12-274019
Kulagin A, Lisukov I, Ivanova M, Golubovskaya I, Kruchkova I, Bondarenko S, Vavilov V, Stancheva N, Babenko E, Sipol A, Pronkina N, Kozlov V, Afanasyev B (2014) Prognostic value of paroxysmal nocturnal haemoglobinuria clone presence in aplastic anaemia patients treated with combined immunosuppression: results of two-centre prospective study. Br J Haematol 164(4):546–554. doi:10.1111/bjh.12661
Sugimori C, Chuhjo T, Feng X, Yamazaki H, Takami A, Teramura M, Mizoguchi H, Omine M, Nakao S (2006) Minor population of CD55-CD59- blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood 107(4):1308–1314. doi:10.1182/blood-2005-06-2485
Hall C, Richards S, Hillmen P (2003) Primary prophylaxis with warfarin prevents thrombosis in paroxysmal nocturnal hemoglobinuria (PNH). Blood 102(10):3587–3591. doi:10.1182/blood-2003-01-0009
Moyo VM, Mukhina GL, Garrett ES, Brodsky RA (2004) Natural history of paroxysmal nocturnal haemoglobinuria using modern diagnostic assays. Br J Haematol 126(1):133–138. doi:10.1111/j.1365-2141.2004.04992.x
Hill A, Kelly RJ, Hillmen P (2013) Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood 121(25):4985–4996. doi:10.1182/blood-2012-09-311381, quiz 5105
Acknowledgments
This study was presented in the form of a poster presentation at the 52nd annual meeting of the American Society of Hematology, Orlando, FL, December 4–7, 2010. This work was supported by the Korean Society of Hematology.
Conflict of interest
All authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jin Seok Kim and Jun Ho Jang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kim, J.S., Jang, J.H., Yoon, SS. et al. Distinct subgroups of paroxysmal nocturnal hemoglobinuria (PNH) with cytopenia: results from South Korean National PNH Registry. Ann Hematol 95, 125–133 (2016). https://doi.org/10.1007/s00277-015-2511-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-015-2511-z