Abstract
Background
The parietal foramen (PF) of the skull is a variable anatomic feature with important implications for venous drainage, infection, and injury. Its topography is clinically relevant for neurosurgeons for intracranial navigation and preoperative planning.
Methods
PF topography was investigated in a series of 440 head computed-tomography scans of Omani subjects at Sultan Qaboos University Hospital. The mean age of the patients was 52 ± 17 years and there were 160 males and 280 females. The topography features of the PF, including frequency, diameter, patency, and relative position in relation to the superior sagittal sinus (SSS), were recorded. Additionally, sex and laterality differences in PF parameters were analyzed using a Chi-square test.
Results
The overall prevalence of PF was 72.3% (318/440). The bilateral presence of PF was identified in 34% of skulls. Unilateral right-side prevalence was 18.2%, while left prevalence was 13.2% (p = 0.62). The prevalence of unilateral accessory PF on the right side was 1.8%, while it was 1.1% on the left (p = 0.69). PF within the sagittal suture/or intra-sutural PF was observed in 6.8% of skulls, with a frequency of 9.4% in men and 5.4% in women (p = 0.29). The diameter of the PF was 1.45 ± 0.74 mm on the right side, and 1.54 ± 0.99 mm on the left side (p = 0.96). There were 2% of incomplete PF. The PF was located over the SSS in 70.3% on the right side and 53.8% on the left side. No significant differences were observed between the PF topography parameters and sex or laterality.
Conclusion
The present study for the first time reports the baseline data of PF topography in a large sample of CT scans in the Arab population. The geography and race influence the PF topography differences. PF may be used as a reliable landmark of SSS. The morphological characteristics and distribution of PF reported in this study have clinical implications for imaging diagnosis, intracranial navigation of vascular disorders, and treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The parietal foramen (PF) topography is important for intracranial navigation for vascular disease diagnosis and treatment [2]. Knowledge of the prevalence of the PF and variability in its topography may have an impact on neurosurgical procedures, as the surgical maneuvers may rupture emissary veins, leading to epidural hemorrhage and sinus thrombosis [24]. Existing literature demonstrated that the anatomical features of PF vary in different ethnic groups with a range of 50–84.3% [15, 20, 23, 34, 38].
The PF is also called parietal emissary foramen. It is a small opening situated in the posterior aspect of the parietal bone, a few millimeters away from the sagittal suture (SS). A bony point between two PF over the sagittal suture is referred as obelion. It is an inconsistent opening, and when present, may be located unilaterally or bilaterally [37, 38]. Usually, they are 1–2 mm in size [25] and rarely, due to defects in parietal bone ossification, may reach 70 mm [24]. The delayed closure of both the parietal notch and the third fontanelle, or sagittal fontanelle, is responsible for the occurrence of PF-related anomalies, including obeliac bones, accessory parietal foramina (APF), parietal fissure, and enlarged parietal foramen (EPF) [36]. When a PF is present, it transmits valveless veins called ‘emissary veins’ (EVs) that connect extra-cranial scalp veins to the superior sagittal sinus (SSS). They allow the blood to flow bi-directionally from intracranial to extracranial spaces. This connection can be a potential route for infection from the scalp to brain in injuries and surgeries involving the parietal bone [8, 19]. Emissary veins also communicate with diploic veins in the spongy layer of the cranial bones [21]. Occasionally, PF transmits anastomotic arteries between the scalp arteries and branches of the middle meningeal arteries [26, 31]. The PF and its EVs serve as landmarks to identify the superior sagittal sinus (SSS) in imaging studies [31]. Thus, a comprehensive understanding of the PF anatomy and variations is important as it has clinical implications mainly related to venous drainage, infection, and injury.
The morphology of the PF, including its prevalence, symmetry, diameter, shape, and distance from the SS, has been studied using dry skulls or cadavers [15, 20, 23, 34, 38]. Only one imaging study could be found, with limited sample size of 123 head MRI [8], assessing the PF topography, despite its increasing importance in the radiological diagnosis of various vascular diseases and to avoid iatrogenic injuries during intracranial navigation. Furthermore, there are very few studies exploring the anatomical features of the PF in the Arab population [23, 28] and none based on CT/MRI images. This study aimed to evaluate the variations of the PF (occurrence, size, location, laterality, distance to SSS) in the Omani population, using head CT scans. We also analyzed PF differences based on sex and laterality.
Methods and study design
Study population
The present study was conducted at Sultan Qaboos University Hospital (SQUH) after receiving ethical approval from the Medical Research Ethics Committee (SQU-EC/499/2021). After applying strict inclusion and exclusion criteria, a total of 440 consecutive contrast-enhanced head CT scans of Omani patients aged ≥ 18 years who had visited the Department of Radiology and Molecular Imaging, SQUH during the period January 2019 to December 2020 were included in the study. The mean age of the patients was 52 ± 17 years (range 18–90 years). Most of the included contrast-enhanced scans were done as part of a metastatic workup, or in the context of suspected brain infections and tumors. Non-enhanced CT scans and patients with pathological changes in the calvaria and with intracranial conditions such as tumors, large acute or subacute infarcts, hemorrhage, or other causes of increased intracranial pressure were excluded. Patients with incomplete details, non-Omanis, and scans with significant artifacts were also excluded.
Acquisition protocol
Data collection
Thin axial CT images using a bone algorithm with a slice thickness of 1 mm and 3D reconstructions of the CT scans were used for the screening of PF in the cranial vault. Screening of CT scans was performed using the in-house Picture Archiving and Communication System (PACS) (Synapse PACS, Fujifilm Worldwide, version 5.7.102). The topographic features of the PF, including the presence or absence of the foramen, the side of the foramen, and the diameter of the foramen on the surface of the skull, were recorded. The presence of an additional foramen on each side is considered an APF. Each PF was screened for its intracranial and extracranial communication and grouped into incomplete and complete PFs. The distances between the medial margin of the PF on the right side and left side and the lateral margin of the SSS were measured in axial CT images using a brain window. All measurements were performed using tools available in the PACS. Patient demographic information such as age, sex, and clinical indications were recorded from electronic medical records. A senior radiology resident was involved in the data collection. The difficult cases were resolved by mutual consensus with a consultant radiologist. The observer inputted the data from each patient into a Microsoft Excel spreadsheet for further statistical analysis.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) (v24.0., IBM Corp., N.Y., USA) was used for the data analysis. The quantitative data were presented as mean ± SD while frequency (percentage) was used to express the qualitative variables. A Chi-square test or Fischer’s exact test, when applicable, was used to compare PF topography variables with sex and laterality. Statistically significance was considered when p < 0.05.
Results
PF prevalence
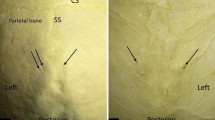
The overall prevalence of PF was 72.3% (318/440). The bilateral presence of PF was identified in 34% (150/440) of skulls. The unilateral right-side PF prevalence was 18.2% (80/440), while the left side was 13.2% (58/440) with no statistical significance (p = 0.62). The prevalence of unilateral APF on the right side was 1.8% (8/440), while on the left side it was 1.1% (5/440) with no statistical significance (p = 0.69). The sex differences in PF, APF, and intra-sutural PF prevalences are presented in Table 1. A bilateral PF was observed in 35.6% (57/160) and 33.2% (93/279) of male and female skulls, respectively. The unilateral right PF prevalence was observed in 16.3% (26/160) of skulls in men while 19.3% in women (54/280). On the other hand, the unilateral left PF was observed in 11.9% (19/160) and 13.9% (39/280) skulls in men and women, respectively. The unilateral right APF prevalence was observed in 0.6% (1/160) of skulls in men while 2.5% in women (7/280). The unilateral left APF was observed in 0.6% (1/160) and 1.4% (4/280) skulls in men and women, respectively. Intra-sutural PF was observed in 6.8% (30/440) of skulls with 9.4% (15/160) in men and 5.4% (15/280) in women (p = 0.77). There were no bilateral APFs among the study subjects. No statistically significant sex differences in PF, APF and intra-sutural PF prevalences were observed among the study subjects (Table 1). The 3D constructed CT images showing the variants of PF topography are provided in Fig. 1.
3D CT reconstructed images show variants of parietal foramina: A Bilateral small parietal foramina (arrows), B bilateral large parietal foramina (arrows), C unilateral right sided parietal foramen (arrow), D bilateral parietal foramina (solid arrows) and a right sided accessory foramen (dashed arrow), and E intra-sutural parietal foramen (arrow)
PF diameter and intracranial and extracranial communication
The diameter of the PF was measured at the surface of the skull, and it was 1.45 ± 0.74 mm on the right side, while it was 1.54 ± 0.99 mm on the left side. No statistical significances between the sides and sex with PF diameter were observed (Table 2). Two EPF (0.45%) with 6 mm diameter in size were observed. The presence of intracranial and extracranial communication or patency of foramen was checked in each skull. Incomplete PF without intracranial communication were identified in 2% (9/440) of skulls. Among incomplete foramina, 0.9% PF were on the right side and 1.1% PF on the left side.
Relative position of PF with SSS
The position of PF in relation to SSS is represented in Fig. 2. On the right side, 70.3% (162/230) of PFs were located directly over the SSS, while the remaining 29.6% (68/230) of PF were located within 11 mm lateral to the lateral margin of the SSS with a mean distance of 3.40 ± 2.29 mm. On the left side, 53.8% (117/208) of PFs were located on the SSS, while remaining 43.75% (91/208) of PF were located within 11 mm lateral to the lateral margin of the SSS with a mean distance of 4.42 ± 2.52 mm. No statistically significant sex and laterality differences were observed in mean distances of PF position from the lateral margin of the SSS (Table 3).
Axial CT images using bone algorithm from different patients show the position of the parietal foramina (arrows) relative to the superior sagittal sinus (outlined in blue). A The foramen is located directly over the superior sagittal sinus. B The parietal foramen is just lateral to the lateral margin of the superior sagittal sinus on the right side. C The left parietal foramen is located 5 mm lateral to the superior sagittal sinus
Discussion
In Omani subjects, the overall prevalence of PF is 72.3%, and it is present more frequently unilaterally than bilaterally, and less frequently in the midline. Very rarely, it is incomplete, and there is an additional accessory foramen. While ethnic differences exist in PF topography [29, 34, 38], exploring the PF prevalence specific to a geographic region is essentially important. The knowledge of PF variations is important to avoid and/or minimise the complications while performing neurosurgical procedures. Failure to recognize such variants during invasive procedures involving the PF may damage the emissary veins, anastomosing arteries, and neural structures. Iatrogenic injuries to vessels may lead to spontaneous hemorrhage and subsequent epidural hematoma and thrombosis [2, 24, 34]. Hence, a thorough understanding of the PF morphological differences is essential for successful surgeries. Despite enormous clinical significance, to date, limited studies have been conducted in the Middle East region. This study reports for the first time the PF topography in CT scans of the head as well as in the Omani population.
In the existing literature, the reported prevalence of PF is more than 50% in all populations [8, 11, 15, 17, 19, 20, 22, 23, 34, 38] (Table 4). A high PF prevalence of 84.3% in the Brazilian population [8], 86% in Thai population [18] and 85.7% in Ukraine population [29] was reported. Relatively, a low prevalence of PF was observed in Turkish (63%), African (62%), Polish (60%), Australian (56.2%), European (56%) and American (50%) populations [15, 20, 23, 34, 37, 38]. However, a recent study from Turkey reported a prevalence of 74.6% on the right side and 74.7% on the left side [14]. In the Omani population, the overall PF prevalence was 72.3%. This is close to the values reported in two different studies from India, with 77.1% and 71.5% of the total sample [11, 22]. However, in other Asian countries, such as Japan [19] and China [17], high prevalence rates of 80.3% and 82.86% were recorded.
In the present study, PF presence was observed unilaterally and bilaterally at similar frequencies. Contrary to these findings, in most of the reported studies, the bilateral prevalence was higher than the unilateral prevalence [2, 19, 22, 29]. On the other hand, in a few studies, the unilateral presence of PF was higher than the bilateral presence [4, 15, 18]. In a recent study, PF was observed to be more commonly found bilaterally (40.9%) than unilaterally (20.2%) in the African population, and more commonly unilaterally (29.5%) than bilaterally (24.2%) in the European population [34]. Very few studies discussed the sex differences in PF prevalence. In agreement with previous studies [3, 8], in the present study there were no statistically significant differences between sex and unilateral or bilateral PF prevalence. According to de Souza Ferreira et al. [8], these findings illustrate that in both sexes, the PF emissary veins involves equally to control pressure, temperature, and hemodynamics of the brain.
The PF is less commonly present in the SS. Previously, a low frequency of PF was reported in SS in European (5.3%), American (5%), Turkish (5%), African (3.6%), Chinese (2.5%), Indian (3.4%), and Japanese (0.7%) populations [15, 17, 19, 22, 34, 38]. Contrary to these findings, in Omani subjects, a relatively high prevalence of 6.8% PF was observed in the SS. Parietal bone ossification occurs from a single membranous center in the seventh to eighth weeks of fetal development. It is completed by the seventh fetal month. A parietal notch is formed as the ossification slows distinctly near the midline [6]. The delayed closure of both the parietal notch and the third fontanelle, or sagittal fontanelle, is responsible for the occurrence of PF-related anomalies, including obeliac bones, APF, parietal fissure, and enlarged parietal foramen (EPF). Furthermore, the lateral part of the primitive parietal notch persists as a small PF [35]. The differences in PF prevalence, symmetry, and midline locations observed among different studies could be attributed to ethnic and geographical differences [22] as well as variations in anterior fonticulus ossification patterns [10].
Rarely, an additional or accessory PF may be present in each parietal bone. The frequency of APF observed in the present study is lower than that reported in other studies from Brazilian samples (right side-8.5%; left side-11.8%) and Indian samples (13.8%) [8, 22]. The variability in APF is reported to be due to ossification pattern differences in the posterior fontanelle as well as ethnic variations [9, 10].
The diameter of PF is clinically important during intracranial navigation through PF to diagnose vascular diseases and perform surgical procedures. Generally, the mean diameter of the PF lies within a range of 1–2 mm [25]. The diameter of PF reported in different studies is presented in Table 4 [14, 17, 19, 23, 29, 34, 37, 38]. In the present study, the diameter of PF was similar on the right side and left side. The range of PF diameters was 1 mm to 6 mm. The mean size of PF observed in the present study is lower than that reported in European (1.9 ± 0.9 mm), African (1.8 ± 0.8 mm), Polish (2.0 ± 1.2 mm), American (1.8 mm) and Turkish (1.7 mm) populations [14, 34, 37]. In a recent study on Ukraine origin skulls, the reported PF diameters were 1.7 ± 0.6 mm (right side) and 2.7 ± 0.5 mm (left side) [29]. However, PF diameter in the Omani subjects is higher than that reported in the Chinese population (right side: 1.02 ± 0.72 mm; left side: 1.07 ± 0.67 mm [17] and Thailand population (right side: 1.23 ± 0.050 mm and left side: 1.18 ± 0.47) [18]. Although sexual dimorphism exists in the size of the skulls, significant differences between the sex or laterality and PF variability in terms of size have not been reported [8, 17]. However, in the Polish population, the diameter of PF in females is found to be statistically greater than that of males [37] while in Ukraine origin skulls, greater on the left side than the right side [29]. Similar to recently published studies [17, 18], in the present study the diameter of PF was not statistically significant between the sides or sexes.
Generally, a PF with a diameter > 5 mm is considered an enlarged parietal foramen (EPF) [31, 33]. EPF is considered a congenital defect of parietal bone development, formed from a large central defect due to parietal bone ossification failure [16]. Its prevalence is very rare, with a rate of 1 in 15,000 to 1 in 50,000 individuals [31], and it is transmitted by an autosomal dominant trait [36]. The EPF is seen in individuals with Saethre-Chotzen syndrome [30] and deletion of chromosome 11 [1]. It is known to result from mutations in the human homeobox genes MSX2 and ALX4, located at 11p11 and 5q34–35, respectively [12, 13]. Usually, they are asymptomatic and commonly prevalent in men [7]. In the present study, there were two EPF (0.45%) with a diameter of 6 mm. The observed prevalence of EPF is relatively high in Omani subjects, and further prospective studies are needed to explore the clinical symptoms of patients with EPF. It is noteworthy to mention that, EPF is an important anatomical variant to recognize as they can mimic some lytic skull lesions like Langerhans cell histiocytosis, metastasis and multiple myeloma [13, 25].
In addition to the EVs, nerves and anastomosing arteries enter the cranial cavity through the PF. Knowledge of the PF topography is clinically important while planning neurosurgical procedures. As neurosurgeons often utilize PF to reach intracranial structures, failure to recognize PF variants might injure emissary veins or arteries, resulting in spontaneous hemorrhage and sinus thrombosis [24]. PF variability is also crucial for radiologists when diagnosing some pathologies [2]. The vascular changes or pathophysiology at PF are known to be associated with various conditions such as the formation of dural arteriovenous fistula, air embolism, Moya–Moya disease, epidural and subdural hematomas, as well as defective cranial revascularization in surgical reconstructive procedures [38]. The transcranial puncture of the PF is used to treat dural fistula by enlarging its arterial anastomosis and supplying it to the vessels of the PF [5]. Additionally, in cases of parasagittal meningiomas, the exact location of EV in PF on radiological examination and its obstruction are essential to avoid intraoperative blood loss and injury to the SSS [5].
In surgeries of the cranial vault, the paramedian midline approach can potentially injure the SSS. The SSS is not a strict midline structure. In a cadaveric study by Tubbs et al. [32], the SSS in 80% of cases deviated either right or left at the lambda. Another study on cadaveric specimens and MRI images concluded that SSS can be displaced on either side of the midline and more towards the midline in the posterior part of the cranial vault [27]. The same study recommended any surgical incision with a 6 to 10 mm distance on either side of the midline for craniotomy procedures [27]. In the present study, more than 50% of PF both on the right and left sides are located on the SSS, while the remaining PF are located 11 mm from the margins of the SSS on both sides. These findings imply that the PF is a more reliable landmark for anticipating the SSS position during its course through the parietal lobe of the cerebrum and navigating this region than the SS or the midline of the calvarium, which is particularly beneficial in assisting surgeons in avoiding iatrogenic injuries during emergency surgeries.
The present study has the following limitations: In this retrospective study, only one investigator, who was blinded to the CT scans and trained in identifying the findings related to the PF topography, was involved. The study only aimed to explore the importance of PF in identifying the SSS as a reliable landmark. Future studies with a large sample are needed to explore the importance of PF in locating other intracranial vascular and parenchymal structures for neurosurgical procedures.
Conclusion
The present study, for the first time, investigated the radiological assessment of PF topography in a large sample of CT scans in the Arab population. The overall prevalence of PF was 72.3%. The prevalence of PF located over the SSS is relatively high. No significant differences were observed between the PF topography parameters and sex or laterality. The PF topography and variants reported in the present study are clinically relevant for neurosurgeons for imaging diagnosis and intracranial navigation of vascular diseases as well as treatment. Further, such knowledge aids neurosurgeons in the successful planning of surgical procedures involving the PF and in avoiding iatrogenic injuries to emissary veins and other important structures emerging from the PF. Prior knowledge about these variants helps radiologists in the diagnosis of various pathologies related to PF and differentiating it from other possible mimicking bone lesions and burr holes. PF may be used as a reliable landmark to identify the SSS position and surgically treat the dural fistulas.
Data availability
The anonymous raw data of the study showing the PF topography can be shared with readers and reviewers by the corresponding author.
References
Bartsch O, Wuyts W, Van Hul W et al (1996) Delineation of a contiguous gene syndrome with multiple exostoses, enlarged parietal foramina, craniofacial dysostosis, and mental retardation, caused by deletions in the short arm of chromosome 11. Am J Hum Genet 58:734–742
Berge JK, Bergman RA (2001) Variations in size and in symmetry of foramina of the human skull. Clin Anat 14:406–413
Berry AC (1975) Factors affecting the incidence of non-metrical skeletal variants. J Anat 120:519–535
Boyd G (1930) The emissary foramina of the cranium in man and the anthropoids. J Anat 65:108–121
Chapot R, Saint-Maurice JP, Narata AP et al (2007) Transcranial puncture through the parietal and mastoid foramina for the treatment of dural fistulas. Rep Four C J Neurosurg 106:912–915
Currarino G (1976) Normal variants and congenital anomalies in the region of the obelion. Am J Roentgenol 127:487–494
De Heer IM, Van Nesselrooij BP, Spliet W, Vermeij-Keers C (2003) Parietal bone agenesis and associated multiple congenital anomalies. J Craniofac Surg 14:192–196
de Souza Ferreira MR, Galvao AP, de Queiroz Lima PT et al (2021) The parietal foramen anatomy: studies using dry skulls, cadaver and in vivo MRI. Surg Radiol Anat 43:1159–1168
De Vis JB, Lu H, Ravi H, Hendrikse J, Liu P (2017) Spatial distribution of flow and oxygenation in the cerebral venous drainage system. J Magn Reson Imaging 47:1091–1098
Freire AR, Rossi AC, de Souza Oliveira VC, Prado FB, Ferreira Caria PH, Botacin PR (2013) Emissary foramens of the human skull: anatomical characteristics and its relations with clinical neurosurgery. Int J Morphol 31:287–292
Gangmei G, Devi HS, Daimei T et al (2018) Variations of parietal foramen in dried adult human skulls. ISOR J Dent Med Sci 17:26–29
Ghassibe M, Bernier V, Boon LM, Vikkula M (2006) A novel mutation in the MSX2 homeobox gene of a family with foramina parietalia permagna, headache and vascular anomaly. Eur J Pediatr 165:734–735
Griessenauer CJ, Veith P, Mortazavi MM et al (2012) Enlarged parietal foramina: a review of genetics, prognosis, radiology, and treatment. Childs Nerv Syst 29:543–547
Guzelad O, Ogut E, Yildirim FB (2023) Evaluation of the parietal foramen and its surgical importance in dry skulls: a cross-sectional morphometric study. Med Bull Haseki/Haseki Tip Bulteni 61(1):43–51
Keskil S, Gözil R, Calgüner E (2003) Common surgical pitfalls in the skull. Surg Neurol 59:228–231
Kortesis B, Richards T, David L, Glazier S, Argenta L (2003) Surgical management of foramina parietalia permagna. J Craniofac Surg 14:538–544
Liu D, Yang H, Wu J, Li JH, Li YK (2022) Anatomical observation and significance of the parietal foramen in Chinese adults. Folia Morphol (Warsz) 81:998–1004
Mahakkanukrauh C, Chitapanarux N, Kwangsukstith S, Navic P, Mahakkanukrauh P (2021) The morphometric study of parietal emissary foramen related with clinical implications in Thais. Int J Morphol 39(5):1283–1288
Mann RW, Manabe J, Byrd JE (2009) Relationship of the parietal foramen and complexity of the human sagittal suture. Int J Morphol 27:553–564
Milne N, Schmitt LH, Freedman L (1983) Discrete trait variation in Western Australian aboriginal skulls. J Hum Evol 12:157–168
Mortazavi MM, Tubbs RS, Riech S et al (2012) Anatomy and pathology of the cranial emissary veins: a review with surgical implications. Neurosurgery 70:1312–1319
Murlimanju BV, Saralaya VV, Somesh MS et al (2015) Morphology and topography of the parietal emissary foramina in South Indians: an anatomical study. Anat Cell Biol 48:292–298
Naidoo J, Luckrajh JS, Lazarus L (2021) Parietal foramen: incidence and topography. Folia Morphol 80:980–984
Piagkou M, Skotsimara G, Repousi E, Paraskevas G, Natsis K (2013) Enlarged parietal foramina: a rare finding in a female Greek skull with unusual multiple wormian bones and a rich parietal vascular network. Anat Sci Int 88:175–180
Reddy AT, Hedlund GL, Percy AK (2000) Enlarged parietal foramina: association with cerebral venous and cortical anomalies. Neurology 54:1175–1178
Reis CV, Deshmukh V, Zabramski JM et al (2007) Anatomy of the mastoid emissary vein and venous system of the posterior neck region: neurosurgical implications. Neurosurgery 61:193–201
Reis CV, Gusmão SN, Elhadi AM et al (2015) Midline as a landmark for the position of the superior sagittal sinus on the cranial vault: an anatomical and imaging study. Surg Neurol Int 6:121
Salama RM, Alsaykhan H, Farag AI, Salama R, Alsaykhan H, Farag A (2023) Osteological study of the parietal foramen in the adult human skull bones and its clinical and surgical correlations. Int J Morphol 1:2
Shmarhalov A, Vovk O, Ikramov V, Acharya Y, Vovk O (2022) Anatomical variations of the parietal foramen and its relations to the calvarial landmarks: a cross-sectional cadaveric study. Wiad Lek 75:1648–1652
Singh D, Raibagkar D (2011) Study of variation in atypical foramina of dry human skull. NJIRM 2:1–5
Tsutsumi S, Nonaka S, Ono H, Yasumoto Y (2016) The extracranial to intracranial anastomotic channel through the parietal foramen: delineation with magnetic resonance imaging. Surg Radiol Anat 38:455–459
Tubbs RS, Salter G, Elton S, Grabb PA, Oakes WJ (2001) Sagittal suture as an external landmark for the superior sagittal sinus. J Neurosurg 94:985–987
Tubbs RS, Smyth MD, Oakes WJ (2003) Parietal foramina are not synonymous with giant parietal foramina. Pediatr Neurosurg 39:216–217
van der Walt S, Hammer N, Prigge L (2023) Comparison between the parietal foramina observed in samples of african and european population groups. Int J Morphol 41(2):634–639
Wilkie AO, Tang Z, Elanko N et al (2000) Functional haploinsufficiency of the human homeobox gene MSX2 causes defects in skull ossification. Nat Genet 24:387–390
Wilkie AOM, Mavrogiannis LA (2012) Enlarged parietal foramina. Synonym. GeneReviews at GeneTests: Medical genetics information resource (database online). University of Washington, Seattle, pp 1997–2012
Wysocki J, Reymond J, Skarzyński H, Wróbel B (2006) The size of selected human skull foramina in relation to skull capacity. Folia Morphol (Warsz) 65:301–308
Yoshioka N, Rhoton AL Jr, Abe H (2006) Scalp to meningeal arterial anastomosis in the parietal foramen. Neurosurgery 58:123–126
Funding
The present study has no funding.
Author information
Authors and Affiliations
Contributions
SRS and EA-A: protocol/project development. AA-S: data collection. SRM: data analysis. SRS, SA-Q, YA-M and RK: manuscript writing and revision. EA-A, SRM and RK: manuscript editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
The present study has received institutional ethical approval from the Medical Research Ethics Committee (SQU-EC/ 499/2021).
Consent for publication
The present study has approval from the institutional ethics committee to conduct the study and publish the study findings.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al-Shuaili, A., Al-Ajmi, E., Mogali, S.R. et al. Computed-tomography evaluation of parietal foramen topography in adults: a retrospective analysis. Surg Radiol Anat 46, 263–270 (2024). https://doi.org/10.1007/s00276-023-03284-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-023-03284-8