Abstract
Purpose
This article reports a new variant of the persistent primitive trigeminal artery. This variant does not exist in Salas and Saltzman classifications.
Methods
We analyzed CTA images of a 39-year-old male patient using RadiAnt. This work had clinical and research purposes.
Results
A persistent primitive trigeminal artery arose from left internal carotid artery termination. Its course was atypical, superior, and lateral to the sella turcica. At its end, it joined a duplicated basilar artery. These morphological features are new compared to Salas and Saltzman’s variants.
Conclusion
Anatomists, radiologists, and neurosurgeons must know this new variant. Angiographic analysis of this variant will keep the patient safe and perform the surgery. This new variant deserves to be considered in Salas and Saltzman classifications as a new type.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cerebral vessels are subject to several variations. Among these variations, persistent carotid basilar anastomoses are very rare. The PPTA is the most common one [10] and the most cephalic [6]. We can observe several anomalies with it. They can be intracranial aneurysms, vascular occlusion, arteriovenous malformations, or tumors [2]. The angiographic evaluation of PPTA is necessary for a safe therapeutic decision [10]. We report a new variant of PPTA feeding AVM via a duplicated BA. To our knowledge, there is no similar previous report. We described this case according to the CARE checklist.
Case report
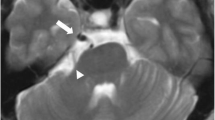
A 39-year-old male patient known for drug addiction presented to the neurology consultation for right-sided hemiparesis, headache, vomiting, and dizziness. On examination, he had right-sided hypotonic hemiplegia, abolished osteotendinous reflexes, a positive Babinski sign, Claude Bernard Horner syndrome, and dysarthria. The GCS was 15/15, and the NIHSS was 16. Brain CT and CTA revealed a cerebellar arteriovenous malformation with a hematoma in the left cerebellar peduncle as a complication. After stabilization in the intensive care unit for six days, the patient was discharged and referred to the neurosurgery department for further evaluation and treatment of his malformation. On the CTA, we suspected a duplication of BA formed by two arteries. One was anterior and the other posterior linked by their distal ends. At the level of the medulla oblongata, both VA fused to form the anterior artery. The posterior artery fed the AVM. Thus, we confirmed the BA duplication (Fig. 1a, b). The left VA was hypoplastic at its distal end (Fig. 1b). A large sinuous PPTA arose from left ICA termination. It ran above, outside, then behind the sella turcica; it terminates in the duplicated BA (Fig. 2a). This variant differs from Salas and Saltzman's descriptions. On both sides, the P1 segment was hypoplastic; both PCA arose from ipsilateral ICA. It formed a bilateral fetal posterior type of Willis ring (Fig. 2b). We observed a fenestration at the A1-A2 junction of the left ACA (Fig. 2b). Unfortunately, the endovascular approach was unavailable. Thus, neurosurgeons preferred the conservative option. Until this report, the patient had no rebleeding.
a Axial CTA images show that RVA and LVA fuse in front of medulla oblongata (asterix) to form the anterior artery (crossed arrow); the posterior artery (double crossed arrow) feeds the AVM nidus. b CTA arterial reconstruction in maximal intensity projection (MIP), bone removed, confirms these results and shows the link between the distal ends of the two arteries. The reconstruction confirms the BA duplication. The LVA is hypoplastic at its distal end (arrowheads). RVA = Right VA, LVA = Left VA
a In the left, sagittal CTA image shows that the PPTA arises from the left ICA termination (asterix) and runs above the sella turcica (arrowhead) before joining the posterior part of the duplicated BA (double crossed arrow). In the right side, axial CTA image shows that the PPTA runs outside then behind the sella turcica (arrowheads). b In the left, axial CTA image shows the bilateral fetal type of the Willis ring (arrows). In the right side, sagittal CTA image shows the fenestration at the A1-A2 junction of the left ACA (arrowhead)
Discussion
At the 8-mm embryonic stage, two longitudinal neural arteries supply the hindbrain. Four bilateral branches of ICA, called carotid-basilar anastomoses, feed these channels [7, 10]. The neural arteries fuse into the BA. Once the fetal PCA turns into PCOA, the carotid-basilar anastomoses regress. The BA fuse with PCOA to form the adult PCA. The reason for the persistence of PTA is still unknown [7]. It appears that the PPTA provides the blood flow necessary for brain development [2]. For individuals who have ICA occlusion or ICA hypoplasia, BA supplies the forebrain via the PPTA [2, 7].
Richard Quain was the first to describe the PPTA [3]. The PTA is the most common carotid basilar anastomoses to persist and accounts for 80–87% [3, 7, 10]. O'uchi and O'uchi evaluated 16,415 MRAs and reported a prevalence of 0.29% [1]. The literature reports a prevalence of 0.1–0.6% [3, 7, 10]. The PPTA can arise from the ICA or a common core with MHA [2, 10]. It originates from the petrous or cavernous segment anterior to the proximal dural ring [3, 10]. Salas used the origin and the relationship with the sella turcica and the abducens nerve as criteria. Thus, he classified the PPTA into intrasellar and laterosellar variants (Table 1) [2, 6, 10]. For other authors, the PPTA may run superior or inferior to the cranial nerves III, IV, and VI [1, 8]. Saltzman described three variants according to the basilar end (Table 1) [4, 7, 10]. In Chen's study, these variants account for 24%, 16%, and 60% [4]. Saltzman type III is the rarest one, with a prevalence of 0.34% in the O'uchi and O'uchi study [1]. The PPTA may supply the hindbrain, pituitary gland, and cerebellum tentorium [3]. It may go with fetal vessels such as FPCA, stapedial and ophthalmic arteries [8, 10]. BA and VA hypoplasia, fenestrated ACA, infraoptic A1, and ICA agenesis may also occur with PPTA [8]. Our variant has a different origin, course, and termination from what is known. It represents a new variant to add to Salas and Saltzman's classifications. In addition, we observed duplicated BA, hypoplastic left V4 segment, left A1-A2 fenestration, and bilateral FPCA associated with this variant. With only CTA, its environmental relationship and distribution were not evident. It needs further exploration using MRA sequences.
The literature reports an association between PPTA and Willis ring aneurysm [7]. Some authors suggest that this association occurs in 14–32% of individuals [10]. Other authors report that 3–4.2% of individuals with PPTA have a cerebral aneurysm. This prevalence is the same in the general population [1]. The aneurysm occurs due to wall weakness or hemodynamic stress [10]. It may involve the PCOA, the ACA, the MCA [3], the ACOA, the PCA, the cavernous ICA, the BA-SCA junction [2], or the PPTA itself (1% of cases) [8]. The PPTA aneurysm is often saccular and located in the PPTA trunk, at the PPTA-ICA or PPTA-BA junction [2, 3, 10]. Any selected surgical option needs an accurate hemodynamic analysis of the brain circulation. Thus, determining the PPTA Saltzman variant is necessary [10]. In Saltzman type 1, PPTA occlusion may lead to posterior fossa infarction. It is necessary to check the blood flow in the basilar system using a balloon occlusion test [5, 10]. In contrast, in Saltzman type 2, we can sacrifice the PPTA since the PCOA supplies the hindbrain [5]. In Saltzman type 3, there are several surgical risks. With a posterior approach, imprudence may lead to dramatic bleeding [10]. The occlusion of PPTA type 3a may lead to brainstem and cerebellum infarction [10]. If it is a type 3b, the occlusion will involve the internal auditory artery (branch of AICA) and lead to inner ear ischemia [10]. The PPTA occlusion may cause midbrain infarction or trigeminal nerve ischemia. It occurs when PPTA gives rise to pontine and trigeminal branches [10]. We can use the PPTA instead of the vertebrobasilar path to coil a posterior aneurysm. This option is the best when the vertebrobasilar system is hypoplastic, such as in Saltzman type 1 [2, 10]. In 2002, Ikushima reported a case of a BA-SCA junctional aneurysm. He used the PPTA path to deliver Guglielmi coils to the aneurysm with good outcomes. Schlamann reported the same experience in 2006 [1, 2]. A trigeminal-cavernous fistula may complicate a PPTA-ICA junctional aneurysm. If the basilar system is hypoplastic, the PPTA allows us to coil the fistula [10].
When vascular stenosis or thrombosis occurs, the presence of the PPTA is critical. If the thrombosis is below the PPTA, this last will have a detrimental effect. The microemboli may borrow the PPTA to pass in the basilar territory [4, 10]. But, in cervical ICA stenosis, the basilar system will supply the ICA one through the PPTA [10]. Although it is beneficial, it may lead to a carotid steal phenomenon. Thus, the PPTA will divert a large blood flow from the basilar territory to the carotid one. It results in basilar insufficiency [1, 4]. In Saltzman type 1, the blood flow decreases more in the posterior fossa with hindbrain stroke risk [4]. This PPTA type feeds the hindbrain, the midbrain, the temporal, and the occipital lobes. In that condition, ICA thrombosis will provoke extensive ischemia [1]. In Saltzman type 1, if the BA occlusion is proximal to the PPTA end, the PPTA will protect the distal territory [4]. Finally, we can realize a posterior thrombectomy via PPTA vice the hypoplastic BA [4, 10].
The PPTA runs along the cranial nerves III, IV, V, and VI. For this reason, it may provoke their palsy. Among 136 patients with trigeminal neuralgia, Bondt found 2.2% of ipsilateral PPTA. This prevalence exceeds that of the general population [1]. The PPTA provokes ophthalmoparesis more often by abducens nerve palsy. It occurs because of the higher frequency of the Salas lateral type. In this type, the abducens nerve is close to PPTA when entering Dorello’s canal [1]. The Salas medial type may also generate oculomotor nerve palsy by compression. The compression occurs by mass effect on the cavernous sinus [5]. Otherwise, it may occur at the roof, where the oculomotor nerve enters the cavernous sinus [1].
The literature reports four cases of cerebellar AVM fed by a PPTA. The patients presented with subarachnoid hemorrhage, trigeminal neuralgia, or brainstem ischemia. The surgeons treated the AVM with the conservative option, embolization with/without radiosurgery [9]. Our case is the fifth one; a cerebellar hematoma revealed the AVM. The novelty of our case lies in two points. The first is that hemorrhagic stroke revealed the AVM. The second is the new PPTA variant feeding the AVM through a duplicated BA. To our knowledge, no one has reported such a clinical form. The patient was a candidate for an endovascular approach. In lack of material, surgeons opted for the conservative option with good outcomes. In the last decade, the hybrid surgery option drew attention to its efficiency. First, the surgeon realizes a partial embolization to reduce the volume of the AVM. Second, he realizes a one-step resection guided by intraoperative angiography. More randomized trials are necessary to prove its superiority to traditional surgery [9]. The PPTA and the Moyamoya disease may coexist [2, 10]. The PPTA disappears at the same age when the vascular state looks like the Moyamoya form [10]. It may explain this association. It seems beneficial since the PPTA supplies the brain if ICA occlusion occurs [10]. It is possible to observe an association of PPTA and other anomalies. They include AVM of the corpus callosum and septum pellucidum [1], PHACE syndrome, Klippel-Feil syndrome [10], and neurofibromatosis type 1 [2].
It is rare to get an association of PPTA with skull base tumors. We mention meningioma, chordoma, pituitary adenoma, and hemangioblastoma [6]. Surgical management in that condition needs careful planning. It must be stricter if we choose a transsphenoidal or transclival approach [2, 6]. Shen proposed a preoperative algorithm using Salas and Saltzman classifications. If it is Salas lateral type, we do not need further angiography since it has no intrasellar course. But if it is a medial type, we must provide a complementary angiography. Thus, if it is a Saltzman type 1, there is no flow from PCOA. With careful dissection around PPTA, the surgeon avoid injuring it. Otherwise, it may cause hindbrain stroke. But, in Saltzman type 2, we can obstruct the PPTA since the PCOA provides enough blood flow. This obstruction allows us to reduce bleeding and expose the tumor better [6].
Conclusion
Even rare, PPTA has several clinical applications. Every anatomist, radiologist, and surgeon must know it. PPTA angiographic analysis guarantees patient safety and surgical performance and offers better results. We reported in this article a new variant of PPTA associated with duplicated BA. It deserves to be a new variant in the Salas and Saltzman classifications. Thus, this report offers new knowledge about the PPTA and opens the way for further studies.
Availability of data and materials
Not applicable.
Abbreviations
- ACA:
-
Anterior cerebral artery
- ACOA:
-
Anterior communicating artery
- AICA:
-
Anterior inferior cerebellar artery
- AVM:
-
Arteriovenous malformation
- BA:
-
Basilar artery
- CT:
-
Computed tomography
- CTA:
-
Computed tomography angiography
- FPCA:
-
Fetal posterior cerebral artery
- GCS:
-
Glasgow Coma Scale
- ICA:
-
Internal carotid artery
- MCA:
-
Middle cerebral artery
- MHA:
-
Meningohypophyseal artery
- MRA:
-
Magnetic resonance angiography
- NIHSS:
-
National Institutes of Health Stroke Scale
- PCA:
-
Posterior cerebral artery
- PCOA:
-
Posterior communicating artery
- PICA:
-
Posterior inferior cerebellar artery
- PPTA:
-
Persistent primitive trigeminal artery
- PTA:
-
Primitive trigeminal artery
- SCA:
-
Superior cerebellar artery
- VA:
-
Vertebral artery
References
Alcalá-Cerra G, Tubbs RS, Niño-Hernández LM (2012) Anatomical features and clinical relevance of a persistent trigeminal artery. Surg Neurol Int 3:111. https://doi.org/10.4103/2152-7806.101798
Azab W, Delashaw J, Mohammed M (2012) Persistent primitive trigeminal artery: a review. Turk Neurosurg 22:399–406. https://doi.org/10.5137/1019-5149.JTN.4427-11.1
Bechri H, Louraoui SM, Fikri M, El Fatemi N, El Maaqili MR, El Abbadi N (2020) Persistence of a trigeminal artery associated with a posterior meningeal artery aneurysm: case report and literature review. J Surg Case Rep 2:1–4. https://doi.org/10.1093/jscr/rjz389
Ferreira A, Coelho PS, Tedim Cruz VT (2019) Persistent trigeminal artery in a patient with posterior circulation stroke treated with rt-PA: case report. BMC Neurol 19:1–6. https://doi.org/10.1186/s12883-019-1492-2
Lam JJH, Bin Mohamed Shah MT, Chung SL, Ho CL (2018) Persistent primitive trigeminal artery associated with a cavernous carotid aneurysm: case report and literature review. J Radiol Case Rep 12:1–11. https://doi.org/10.3941/jrcr.v12i11.3500
Shen J, Tourje J, Chang EE, Mamelak AN, Wu AW (2016) Persistent trigeminal artery in endonasal resection of skull base tumors: a systematic review. J Neurol Surg B Skull Base 77:449–455. https://doi.org/10.1055/s-0036-1581066
Menshawi K, Mohr JP, Gutierrez J (2015) A functional perspective on the embryology and anatomy of the cerebral blood supply. J Stroke 17:144–158. https://doi.org/10.5853/jos.2015.17.2.144
Tubb RS, Verma K, Riech S, Mortazavi MM, Shoja MM, Loukas M, Curé JK, Żurada A, Cohen-Gadol AA (2011) Persistent fetal intracranial arteries: a comprehensive review of anatomical and clinical significance. J Neurosurg 114:1127–1134. https://doi.org/10.3171/2010.11.JNS101527
Wang L, Li J, Li Z, Chai S, Chen J, Xiong N, Yang B (2022) Hybrid surgery for coexistence of cerebral arteriovenous malformation and primitive trigeminal artery: a case report and literature review. Front Surg 9:1–6. https://doi.org/10.3389/fsurg.2022.888558
Wang Y, Yu J (2022) Clinical importance of the persistent primitive trigeminal artery in vascular lesions and its role in endovascular treatment. Front Neurol 13:1–10. https://doi.org/10.3389/fneur.2022.928608
Funding
No funding has been received by the author for preparing this work.
Author information
Authors and Affiliations
Contributions
MAA: Project development, data collection, management and analysis, manuscript editing and writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
There is no potential conflicts of interest.
Ethical approval
This case report has been performed in accordance with the ethical standards as laid down in the Declaration of Helsinki and the ethical standards of the ethical and deontological committee of the University of Oran 1 Ahmed Ben Bella.
Consent to participate
Not applicable.
Consent to publish
The data used were totally anonymized radiological images, so consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alnafie, M.A. New variant of persistent primitive trigeminal artery associated with duplicated basilar artery: a radiological case report. Surg Radiol Anat 45, 321–326 (2023). https://doi.org/10.1007/s00276-023-03082-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-023-03082-2