Abstract
Purpose
Few studies have explored the central retinal artery (CRA) using neuroimaging. Our study aimed to explore this using magnetic resonance imaging (MRI).
Methods
A total of 81 patients with intact orbital structures and visual function underwent thin-slice contrast MRI.
Results
The identified CRAs showed highly variable morphologies on both axial and sagittal images. On the axial images, the CRAs were detected in the right orbit in 11.1% and in the left orbit in 19.8%. The distance between the site of CRA branching from the ophthalmic artery to the posterior limit of the bulb was 18.8 ± 3.9 mm (12.8–24.6 mm) on the right and 18.9 ± 3.3 mm (14.6–26.7 mm) on the left. On the sagittal images, CRAs were detected on the right in 76.5% and on the left in 85.2%. The distance between the CRA branching site and the posterior limit of the bulb was 20.4 ± 3.8 mm (14.2–28.2 mm) on the right and 19.2 ± 3.7 mm (11.3–27.1 mm) on the left.
Conclusions
Thin-sliced, contrast sagittal MRI can be used to explore the proximal part of the CRA. In particular, serial sagittal imaging may be useful for detecting the CRAs and their relationship with relevant structures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
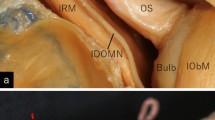
The central retinal artery (CRA) is commonly the first and one of the smallest branches of the ophthalmic artery. Its outer diameter is estimated to be 0.6 mm. The CRA is a terminal branch without anastomotic connections, and its loss results in blindness [5, 14]. The CRA commonly arises from the ophthalmic artery at the orbital apex, coursing on the inferior or inferomedial surface of the optic sheath for a distance, then penetrating the optic sheath, underlying subarachnoid space, and optic nerve. It then courses anteriorly in the central part of the nerve, and finally reaches the retina (Fig. 1).
Three dissected specimens of the right orbit, viewed from the anterolateral (a, c) and inferolateral (b) aspects, showing the course of the central retinal artery (arrows). It branches from the ophthalmic artery, coursing on the lower optic sheath as a tortuous vessel, then penetrating the optic sheath, underlying subarachnoid space, and optic nerve, and coursing anteriorly in the central part of the nerve to reach the retina. CilA ciliary artery, IObM inferior oblique muscle, IObN inferior oblique nerve, IRM inferior rectus muscle, LRM lateral rectus muscle, NCilN nasociliary nerve, ON optic nerve, OphA ophthalmic artery, OS optic sheath
The CRAs, which are relevant to visual function and have received much attention from clinicians, have been explored mainly in postmortem specimens [1, 6,7,8,9, 11, 12, 15, 16]. However, to the best of our knowledge, few studies have explored the CRAs with neuroimaging modalities [3, 13]. Due to its very fine blood flow compared to the ophthalmic artery from which it arises, it is difficult to delineate the CRAs even with catheter angiography, especially under physiological conditions [2, 10]. The present study aimed to explore CRAs using thin-slice contrast-enhanced magnetic resonance imaging (MRI).
Materials and methods
This retrospective study included 81 outpatients who underwent non-contrast and post-contrast MRI at the Medical Satellite Yaesu Clinic between April 2010 and May 2015. The patients presented with headaches, dizziness, tinnitus, hearing loss, sensory disturbances, or focal seizures. The population consisted of 38 men and 43 women, aged 50.4 ± 15.8 years (mean ± standard deviation; 18–76 years). Patients with visual impairment were excluded from the study. Initial examinations using axial T1- and T2-weighted imaging, T2 gradient echo, fluid-attenuated inversion recovery, diffusion-weighted sequences, and MR angiography confirmed that none of the patients had any signs of pathological conditions in their orbits, optic pathways, cerebral hemispheres and ventricles, cerebral vessels, ophthalmic arteries, cavernous sinuses, and cranial dura maters. The patients then underwent volumetric imaging with intravenous gadolinium infusion (0.1 m mol/kg) in the axial, coronal, and sagittal planes, involving the entire orbits and cavernous sinuses. The following parameters were adopted: repetition time 4.1 ms; echo time 1.92 ms; slice thickness 1 mm; interslice gap 0 mm; matrix 320 × 320; field of view 250 mm; flip angle 13°; and scan duration 7 min 25 s. All imaging sequences were performed using a 3.0-T MRI scanner (Achieva R2.6; Philips Medical Systems, Best, The Netherlands). Imaging data were transferred to a workstation (Virtual Place Lexus 64, 64th edition; AZE, Tokyo, Japan) and independently analyzed by two of the authors (S.T. and H.I.). The CRAs and relevant neurovascular and muscular structures were assessed on serial axial and sagittal images. A linear, small vessel arising from the ophthalmic artery at the orbital apex and coursing forward along the lower optic sheath was considered the CRA. In addition, the distance between the CRA branching site from the ophthalmic artery and the posterior limit of the bulb was measured on these images (Figs. 2 and 3). Due to their low performance in depicting the CRA, as confirmed in preliminary observations, coronal images were not used in this study.
Serial post-contrast axial T1-weighted magnetic resonance images (a-c) showing the central retinal artery (arrow) branching from the ophthalmic artery (dashed arrow) and the measurement of the distance between the branching site of the central retinal artery and posterior limit of the bulb (a, á). LRM lateral rectus muscle, MRM medial rectus muscle, OS optic sheath, TL temporal lobe. a (inferior) → c (superior)
Post-contrast sagittal T1-weighted magnetic resonance images showing the central retinal artery branching from the ophthalmic artery (a) and the measurement of the distance between the branching site of the central retinal artery and posterior limit of the bulb (b). ON optic nerve, IRM inferior rectus muscle, SRM superior rectus muscle, arrow central retinal artery, dashed arrow ophthalmic artery
In this article, pictures of cadaveric CRAs and the relevant neurovascular structures are presented. Dissections were performed by one of the authors (S.T.) at the Department of Neurological Surgery, University of Florida, Gainesville, FL, USA.
The study was conducted in accordance with the guidelines of our institution and those of the Medical Satellite Yaesu Clinic for human research. Written informed consent was obtained from all the patients prior to their participation in the study.
Results
Most of the CRAs were detected as enhancing linear, curvilinear, or tortuous structures coursing through the lower optic sheath as a single channel. These CRA segments, the proximal part of the CRAs defined in this study, had highly variable morphologies on both axial (Fig. 4) and sagittal (Fig. 5) images. In 2 of 162 orbits (1.2%), the CRA was found to branch into 2 channels (Fig. 5). On axial imaging, the CRAs were detected in 9 of 81 patients (11.1%) in the right orbit and 16 of 81 (19.8%) patients in the left orbit (Table 1). The distance between the site of CRA branching from the ophthalmic artery and the posterior limit of the bulb was 18.8 ± 3.9 mm (12.8–24.6 mm) on the right and 18.9 ± 3.3 mm (14.6–26.7 mm) on the left (Table 2). On sagittal imaging, the CRAs were detected on the right in 62 patients (76.5%) and on the left in 69 (85.2%) patients (Table 1). The distance between the CRA branching site and the posterior limit of the bulb was 20.4 ± 3.8 mm (14.2–28.2 mm) on the right and 19.2 ± 3.7 mm (11.3–27.1 mm) on the left (Table 2). All the identified CRA branching sites were located at the intraorbital part of the ophthalmic artery. The site of CRA penetration into the optic sheath and nerve, as well as the more distal CRA segment’s course in the central part of the optic nerve, was not identified in any of the 81 patients on both the axial and sagittal images. In one patient, the right ophthalmic artery was supplied by an anastomotic channel with the middle meningeal artery (Fig. 6).
Post-contrast axial T1-weighted magnetic resonance images of different patients showing variable anatomies of the central retinal artery (arrow) branching from the ophthalmic artery (dashed arrow). LRM lateral rectus muscle, MRM medial rectus muscle, ON optic nerve, TL temporal lobe. a, b left orbits, c–f right orbits
Post-contrast sagittal T1-weighted magnetic resonance images of different patients showing variable anatomies of the central retinal artery branching from the ophthalmic artery and coursing anteriorly along the optic nerve. Note that in b, the central retinal artery branches into two channels. IRM inferior rectus muscle, ON optic nerve, SRM superior rectus muscle, arrow central retinal artery, dashed arrow ophthalmic artery, a right orbit, b–d left orbits
Discussion
The CRAs are very small vessels with their outer diameter estimated to be 0.6 mm [5]. In this study, more than 75% of the CRAs were detected in the sagittal images, whereas they were identified in less than 20% of the axial images. In addition, the morphological variability of the CRAs was well demonstrated in previous investigations using cadaver specimens [1, 11, 16]. The difference in the detection rate in the two sectional planes may mainly derive from the typical CRA anatomy with its tortuous or curvilinear segment in the lower optic sheath, which can provide a greater chance of it being identified in the sagittal compared to the axial sections. In addition, in this study, the distance between the site of CRA branching from the ophthalmic artery and the posterior limit of the bulb was measured, adding novel anatomical data for avoiding interruption of the CRA. Previous studies measured the distance from the posterior limit of the bulb to the site of CRA penetration into the optic sheath, not to the CRA branching site [5, 8, 9, 11, 16].
A fraction of ophthalmic arteries is supplied by the external carotid system. In such cases, the CRA can be at risk of interruption during craniotomy; this also provides an alternative pathway during endovascular treatment of CRA occlusion [4, 17]. In the present study, only one patient had an ophthalmic artery supplied by the external carotid system. A previous study using phase-contrast MR angiography documented the ophthalmic arteries supplied by the external carotid system or in combination with the internal carotid artery were found in 9.2% of examined patients. In contrast, in the study, there were no patients detected the CRA arising from the ophthalmic artery [17]. Compared with MR angiography, contrast MRI may be more advantageous in detecting the CRAs.
This study has several limitations. The study population consisted of patients with an inhomogeneous age distribution and an uneven sex ratio. The patients were retrospectively evaluated and not randomly assigned. The CRAs assessments were based only on observations of contrast sagittal MRIs, and their flow patterns were not assessed. Low depiction rates of the CRAs that were confirmed, in the preliminary observations, on the contrast axial and sagittal images may mainly from the peculiar course along the optic sheath and small diameter of the arteries. Furthermore, the CRAs were only detected in the segment coursing along the lower surface of the optic sheath. Despite these limitations, we believe that our study with its simple methodology may show for the first time that MRI may be a worthwhile modality for exploring the CRAs. Evaluation in the setting of acute CRA occlusion may be the next step for investigation using contrast-enhanced MRI.
Conclusions
Thin-sliced, contrast sagittal MRI can be used to explore the proximal part of the CRAs. In particular, serial sagittal imaging may be useful for detecting the CRAs and their relationship with relevant neurovascular and muscular structures.
References
Baldoncini M, Campero A, Moran G, Avendaño M, Hinojosa-Martínez P, Cimmino M, Buosi P, Forlizzi V, Chuang J, Gargurevich B (2019) Microsurgical anatomy of the central retinal artery. World Neurosurg 130:e172–e187
Bracco S, Venturi C, Leonini S, Romano DG, Cioni S, Vallone IM, Gennari P, Galluzzi P, Hadjistilianou T, De Francesco S, Guglielmucci D, Tarantino F, Bertelli E (2015) Identification of intraorbital arteries in pediatric age by high-resolution superselective angiography. Orbit 34:237–247
Citrin CM (1986) High resolution orbital computed tomography. J Comput Assist Tomogr 10:810–816
Cohen JE, Moscovici S, Halpert M, Itshayek E (2012) Selective thrombolysis performed through meningo-ophthalmic artery in central retinal artery occlusion. J Clin Neurosci 19:462–464
Erdogmus S, Govsa F (2006) Topography of the posterior arteries supplying the eye and relations to the optic nerve. Acta Ophthalmol Scand 84:642–649
Erdogmus S, Govsa F (2007) Accurate course and relationships of the intraorbital part of the ophthalmic artery in the sagittal plane. Minim Invasive Neurosurg 50:202–208
François J, Fryczkowski A (1982) Functional importance of central retinal artery anastomoses in the anterior part of the optic nerve. Ophthalmologica 185:15–25
Kocabiyik N, Yalcin B, Ozan H (2005) The morphometric analysis of the central retinal artery. Ophthalmic Physiol Opt 25:375–378
Lee SH, Ha TJ, Lee JS, Koh KS, Song WC (2019) Topography of the central retinal artery relevant to retrobulbar reperfusion in filler complications. Plast Reconstr Surg 144:1295–1300
Louw L, Steyl J, Loggenberg E (2014) Imaging of dual ophthalmic arteries: identification of the central retinal artery. J Clin Imaging Sci 4:40
Merriam JC, Casper DS (2021) The entry point of the central retinal artery into the outer meningeal sheath of the optic nerve. Clin Anat 34:605–608
Perrini P, Cardia A, Fraser K, Lanzino G (2007) A microsurgical study of the anatomy and course of the ophthalmic artery and its possibly dangerous anastomoses. J Neurosurg 106:142–150
Raz E, Shapiro M, Shepherd TM, Nossek E, Yaghi S, Gold DM, Ishida K, Rucker JC, Belinsky I, Kim E, Mac Grory BM, Mir O, Hagiwara M, Agarwal S, Young MG, Galetta SL, Nelson PK (2022) Central retinal artery visualization with cone-beam CT angiography. Radiology 302:419–424
Rhoton AL Jr (2002) The orbit. Neurosurgery 51:S303–S334
Shoja MM, Harris A, Shoshani Y, Siesky B, Primus S, Loukas M, Tubbs RS (2012) Central retinal artery originating from the temporal short posterior ciliary artery associated with intraorbital external-to-internal carotid arterial anastomoses. Surg Radiol Anat 34:187–189
Tsutsumi S, Rhoton AL Jr (2006) Microsurgical anatomy of the central retinal artery. Neurosurgery 59:870–878 (Discussion 878)
Tsutsumi S, Yasumoto Y, Tabuchi T, Ito M (2012) Visualization of the ophthalmic artery by phase-contrast magnetic resonance angiography: a pilot study. Surg Radiol Anat 34:833–838
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
All the authors contributed equally to the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare regarding the materials or methods used in this study or the findings presented herein.
Ethical approval
All the procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all the participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tsutsumi, S., Ono, H. & Ishii, H. Central retinal artery delineation using magnetic resonance imaging. Surg Radiol Anat 44, 727–732 (2022). https://doi.org/10.1007/s00276-022-02947-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-022-02947-2