Abstract
Purpose
Degenerative foraminal stenosis of the cervical spine can lead to cervicobrachial neuralgias. Computed tomography (CT)-scan assists in the diagnosis and evaluation of foraminal stenosis. The main objective of this study is to determine the bony dimensions of the cervical intervertebral foramen and to identify which foraminal measurements are most affected by degenerative disorders of the cervical spine. These data could be applied to the surgical treatment of this pathology, helping surgeons to focus on specific areas during decompression procedures.
Methods
A descriptive study was conducted between two groups: an asymptomatic one (young people with no evidence of degenerative cervical spine disorders) and a symptomatic one (experiencing cervicobrachial neuralgia due to degenerative foraminal stenosis). Using CT scans, we determined a method allowing measurements of the following foraminal dimensions: foraminal height (FH), foraminal length (FL), foraminal width in its lateral part ((UWPP, MWPP and IWPP (respectively Upper, Medial and Inferior Width of Pedicle Part)) and medial part (UWMP, MWMP and IWMP (respectively Upper, Medial and Inferior Width of Medial Part)), and disk height (DH). Foraminal volume (FV) was calculated considering the above data. Mean volumes were measured in the asymptomatic group and compared to the values obtained in the symptomatic group.
Results
Both groups were made up of 10 patients, and a total of 50 intervertebral discs (100 intervertebral foramina) were analyzed in each group. Comparison of C4C5, C5C6 and C6C7 levels between both groups showed several significant decreases in foraminal dimensions (p < 0.05) as well as in foraminal volume (p < 0.001) in the symptomatic group. The most affected dimensions were UWPP, MWPP, UWMP, MWMP and FV. The most stenotic foraminal areas were the top of the uncus and the posterior edge of the lower plate of the overlying vertebra.
Conclusion
Using a new protocol for measuring foraminal volume, the present study refines the current knowledge of the normal and pathological anatomy of the lower cervical spine and allows us to understand the foraminal sites most affected by degenerative stenosis. Those findings can be applied to foraminal stenosis surgeries. According to our results, decompression of the foramen in regard of both uncus osteophytic spurs and inferior plate of the overlying vertebra might be an important step for spinal nerves release.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The cervical spine consists of a mobile segment that suffers from early degeneration, starting in the third decade of life [24]. Degenerative changes in the lower cervical spine narrow the intervertebral foramen resulting in a compression of spinal nerves, clinically associated with cervicobrachial neuralgias and significant morbidity. Despite the widespread of this pathology, there are still missing data regarding the areas most affected by cervical degeneration within the intervertebral foramen.
Due to of its effectiveness in analyzing bone structure, Computed-Tomography (CT)-Scan is one of the most important imaging examinations in the diagnosis and evaluation of foraminal stenosis, and is now often part of the preoperative assessment of this pathology. The most recent advances in spinal imaging techniques suggest that we could propose a reliable method for spinal foraminal measurements [3, 16, 21]. However, only a few studies have analyzed the bony anatomy of the lower cervical spine using CT-Scan [3, 6, 16, 21]. Most of the time, the cervical intervertebral foramen is divided in 2 parts: a pedicular part laterally and a recess part medially. Apart from these descriptions, the role of the intervertebral foramen and the impact of cervical degeneration on its dimensions remain unknown, with most studies focusing on the intervertebral disc as the starting point for spinal nerve compression [13, 19, 22]. Moreover, this foramen can be assimilated to a tunnel in which spinal nerve could be compressed everywhere, so that the most reliable parameter that should be studied in the foramen might be its volume. To our knowledge, we did not find any study focusing on this parameter, as most of the studies only analyze foraminal dimensions at a specific location.
The main purpose of our study was to determine a CT-scan-based volume measurement protocol leading to a mapping of the bony anatomy of the pedicle part of the intervertebral foramen of the lower cervical spine in a young asymptomatic population and to compare these results with those obtained in a population suffering from cervicobrachial neuralgia. Those data might provide a better knowledge of the normal foraminal dimensions and could help surgeons to focus on specific areas while performing spinal decompression procedures.
Material and methods
Study design
We designed a retrospective study comparing two groups. On one hand, foraminal measurements were performed on a "healthy population" composed of 10 patients aged from 19 to 29 years old who underwent a cervical CT scan with bone reconstruction for an extra neural indication between December 2016 and March 2017. On the other hand, the "neuralgic population" made out of 10 patients who underwent an anterior cervical disc fusion for cervicobrachial neuralgias in the spine unit of the Bordeaux University Hospital between December 2017 and March 2018. In this group, foraminal stenosis was defined by the concordance between the clinical neuralgia observed by the surgeon and the foraminal stenosis on CT scan noted by the radiologist.
Exclusion criteria
Patients were excluded from the healthy group if there was any clinical or radiological suspicion of cervical pathology or if they had a history of spinal pathology.
Patients with symptomatic foraminal stenosis were excluded from the neuralgic group if they had a history of cervical spine fracture, cervical spine surgery, cervicarthrosic myelopathy, polymalformative syndrome and congenital cervical spine anomaly.
Data collection
Clinical data were collected retrospectively using the patients' medical records. Age, gender and Body Mass Index (BMI) were collected for every patient. Since BMI appears to be more associated with the development of disc disease than height alone, this parameter was chosen to compare populations. Images of each examination (DICOM (Digital Imaging and Communication in Medicine) format) were burned onto an anonymized CD (Compact Disc). Level, disc height, width, height and length of the foramen were measured using a three-dimensional Multiplanar Reconstruction (3DMPR) with orthogonal reconstructions in bone window and the software "HOROS" version 3.1.1. All length measurements were expressed in millimeters (mm), and volume in mm3. Precision was made to the nearest thousands. In order to accurately determine every radiological parameter, two observers performed the measurements twice, and the average value was used for statistical analysis.
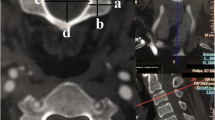
Determination of the plane of the intervertebral foramen (Fig. 1)
In order to determine the plane of the intervertebral foramen, the 3DMRP center was put in the center of the pedicle of the superior vertebrae, with an axial plan parallel to the underlying vertebrae superior endplate. Rotation was controlled so that the sagittal axis was parallel to the pedicle axis (Fig. 1a). After locating the center of the overlying pedicle, sagittal, coronal and axial axis were kept the same, but were placed in the center of the intervertebral foramen (Fig. 1b).
Definition of radiological measurements
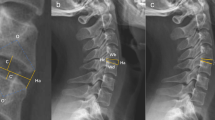
Disc Height (DH) was defined as the craniocaudal distance between the 2 vertebral bodies on the sagittal reconstruction when the 3DMPR center was placed in the center of the interbody space and axial plan was parallel to the underlying vertebrae superior endplate (Fig. 2).
Foraminal height (FH) was defined by the distance between bony component on sagittal reconstruction passing through the middle of the foramen (Fig. 3).
Foraminal length (FL) was defined by the maximal length of the orthogonal vector of the underlying pedicle in the axial plane (meaning the distance between the most medial and the most lateral point of the foramen).
Foraminal width (FW) was measured at 2 locations: its lateral part (pedicle) and its medial part (recess). For the pedicle part, width measurements were defined among a vector perpendicular to the axis of the foramen (Fig. 4), and were performed at 3 levels: the upper part (up to the overlying vertebra inferior endplate), the middle part (up to the top of the uncus of the underlying vertebra) and the inferior part (up to underlying vertebrae superior endplate). Those parts were respectively named upper width of pedicle part (UWPP), middle width of pedicle part (MWPP) and inferior width of pedicle part (IWPP). For the medial part, the same measurements were performed in a plane parallel to the medial edge of the overlying pedicle (Fig. 5), and were named upper width of medial part (UWMP), middle width of medial part (MWMP) and inferior width of medial part (IWMP).
Calculation of the volume of the pedicle part of the intervertebral foramen
In order to calculate foraminal volume (FV), the pedicle part of the foramen was considered as an elliptical cylinder. Area of the ellipse (AE) was calculated according to the following formula π x a x b with a and b being respectively the half of the major axis and the half of the minor axis. Applied to the foramen, major axis is foraminal height (2a = FH) and minor axis is foraminal width (2b = FW). Finally, foraminal volume was calculated using the following formula: FV = π x (FH/2) x ((UWPP + MWPP + IWPP)/ 3)/2) x FL.
Statistical analysis
Intra and inter-rater reliability was assessed by calculation of intraclass correlation coefficients (ICC) based on analysis of variance [9]. A comparative analysis was conducted in order to determine whether there were significant differences between those 2 groups. A Shapiro test was used to check that the results followed a normal distribution. A F-test was then used to check that the variances of our groups were not significantly different. If the p-value of the Shapiro test was greater than 0.05, a Wilcoxon test was performed. If the p-value of the Shapiro test was less than 0.05 and the p-value of the F-test was greater than 0.05, a Welch test was performed. If the p-value of the Shapiro test and the F-test were less than 0.05, a Student test was used.
Results
A total of 50 intervertebral discs and 100 intervertebral foramina were analyzed in each group. 23 foramina were stenosed and 77 were considered healthy among the neuralgic population. Our results focus on the main levels of the lower cervical spine affected by degenerative pathology, namely C4C5, C5C6 and C6C7. Reproducibility analysis revealed an ICC > 0.9 for intra- and inter-observer reproducibility.
Preliminary analysis on healthy population
The control group was made up of 5 men and 5 women whose mean age was 24 years old (min: 19, max: 29). More characteristics of this population are given in Table 1. The dimensions and volume of the intervertebral foramina were measured for each level and the average values were considered as a reference. Those results are shown in Table 2.
Analysis of the neuralgic population
The neuralgic population was also made out of 5 men and 5 women, with a respective mean age at surgery 51.6 years old (min: 43, max: 61). More characteristics are given in Table 3. Of the 100 foramens measured, 23 were considered stenotic (2 at C3-C4 level, 4 at C4-C5 level, 10 at C5-C6 level and 7 at C6-C7 level) after both clinical and radiologic expertise.
Operative findings and follow-up
We did not find any compressive osteophytes arising from the articular facets. All of these patients had resolution or improvement of symptoms at the first post-operative follow-up visit (usually 3 months after surgery).
Comparison of foraminal measurements between stenotic foramen in the neuralgic group and foramen in the healthy group
After statistical analysis, our results showed a significant decrease in FV, DH and all parameters related to the intervertebral foramen (except FL) for all levels analyzed in the neuralgic group, except for MWPP and IWPP at C4C5 level (p < 0.005) (Table 4).
We found an overall volume loss of 59% at C4C5 level, 45% at C5C6 level and 34% at C6C7 level in symptomatic operated patients.
Comparison of foraminal measurements between stenosed foramen and normal foramen in the neuralgic group
At C4-C5 level, we found a significant decrease in FV, FH, UWPP, MWPP, UWMP and IWMP (p < 0.001), in MWMP and DH (p < 0.005) and a non-significant decrease in IWPP (Table 5).
At C5-C6 level, a significant decrease in FV, DH, UWPP, MWPP, UWMP, MWMP and IWMP (p < 0.001) was shown with no significant decrease in FH and IWPP (Table 5).
At C6-C7 level, FV, DH, UWPP, MWPP, UWMP, MWMP and IWMP decrease significantly (p < 0.001), such as IWPP (p < 0.005). However, we did not find a significant decrease in FH (Table 5).
At every level, no significant variation in FL was observed.
Discussion
This work allows us to propose an accurate mapping of the anatomical dimensions of the cervical intervertebral foramen in healthy subjects as well as in patients suffering from cervicobrachial neuralgias due to foraminal stenosis.
The first imaging-based anatomical observations of intervertebral foramen were performed in the late 1980s, with Czervionke et al. describing that the overall growth of foraminal dimensions increases in the cranio-caudal direction (becoming more important in the cervico-thoracic junction), and that their shape was ovoid (height greater than the width) [4]. In the same period, measurements of maximum lengths, heights and widths of this intervertebral foramen were performed in a cadaveric study and were shown to be increasing from C3 to C7 [6]. A few years later, it was shown that the length of the intervertebral foramen varies with the width of the pedicles [21]. Based on both these cadaveric and imaging findings, the different foraminal measurements might increase the closer the cervical level studied is to the cervicothoracic junction [4, 6, 21], as it is shown in our work. Our results also showed a smooth increase of FV along a craniocaudal gradient. Even if the literature does not agree with the increase of the foraminal surfaces according to the craniocaudal gradient, those results seems reliable to our mind because FL is a major component of FV and it increases according to this same gradient [6, 17, 21]. Furthermore, most of the other value obtained in our study are consistent with those reports [4, 6, 21]. Nevertheless, although the length of the foraminal reported in Ebraheim's work is very close to the one we found, they found a slightly greater width and height of the foraminal than ours [6]. Two factors might explain this phenomenon, since the populations differed in age (with subjects being over 60 years old in the cadaveric study) and measurements were made considering ligamentary structures (whereas we only considered bone for our measurements).
Anatomically, the vertebral canal in cervical portion has a triangular shape with an anterior base, so that it has a lateral tunnel-shaped extension with a medial base at the entrance of the intervertebral foramen [23]. Our results showing larger widths in the recess part of the foramen than in its pedicular part are an agreement with this particular anatomy [10, 23]. In a more recent study, two widths at the medial entrance of the intervertebral foramina of C3 and C7 were measured on anatomical specimens [16]. One was located in front of the lower endplate (equivalent for UWMP) and one was in front of the upper endplate (equivalent for IWMP). Value reported were slightly higher than ours, which can be explained by the fact that our measurements were performed strictly perpendicular to the axis of the foramen thanks to the use of orthogonal planes, whereas the authors measured the widths in the sagittal plane. Indeed, the length and direction of the intervertebral foramen varied with the width and direction (axis) of the pedicles [21], and our method is based on the fact that the axis of the foramen is perpendicular to the pedicles. Furthermore, it was shown that an acquisition oriented perpendicular to the axis of the intervertebral foramen allowed higher precision in the diagnosis of cervical foraminal stenosis [18].
Even if compression of the spinal nerves frequently occur at the entrance zone of the intervertebral foramen [21], we consider that the spinal nerve can be compressed anywhere while it passes through the foramen, and thus we believe that the most reliable parameter that should be studied in the foramen may be its volume. Despite the importance of the foraminal volume in analyzing cervical intervertebral foramina, we did not find any study focusing on this parameter, as most of the studies only analyze foraminal dimensions at a specific location. It was however estimated by Yang et al. who studied the influence of the size of interbody grafts on the volume and area of the foramen [25]. Using a 3D laser scan, they identified 2 areas in the foramen, one at its medial entrance and the other at its lateral exit. According to them, the intervertebral foramen consisted of the tunnel between those 2 areas. Even though our calculation methodology is different from that of the authors, the volume calculated in both studies was that of a cylinder with an elliptical base. No others studies concerning foraminal volume were found in the literature to compare our results. As a result, the present study is one of the first to propose a reliable and reproductible protocol for measuring foraminal volume.
Nowadays, cervicobrachial neuralgias are more and more common, with more and more patients undergoing surgical treatment for that condition. During surgery for cervical foraminal stenosis, clinical outcome can be improved by either direct decompression (direct removal of osteophytic bone spurs compressing spinal nerves) or indirect decompression (with the interbody implant indirectly increasing the size of the intervertebral foramen) [26]. Even if direct decompression of the foramen appears to be essential in this surgery for many authors [2, 20], there is still a lack of data regarding the exact cervical sites most involved in radicular pain. Since many studies have shown that the C4-C5, C5-C6 and C6-C7 levels are the most vulnerable to degenerative stenosis [1, 5, 8, 11, 15], we focused on these 3 particular levels to compare values between groups. Our results show that the most decreased foraminal measurements were the width facing the inferior plate of the overlying vertebra (UWPP, UWMP), the width facing the top of the uncus (MWPP, MWMP) and FV. The course of the cervical spinal nerves in the intervertebral foramen was described in an anatomical study [21]. In this study, the authors concluded that spinal nerves were compressed most of the time because of unco-vertebral arthritic changes in its pedicle portion, which might correspond anatomically with the decrease in UWPP, UWMP, MWPP and MWMP observed in our study. According to our results, foraminal decompression must combine both decompression of uncus osteophytic spurs and the inferior plate of the overlying vertebra in order to maximize pain relief.
Our study has some strengths. The fact that we first screened healthy patients helped us to focus on normal anatomy and this is, according to us, the best way to understand pathological anatomy. Furthermore, CT scan is the gold standard for bone tissue analysis. It allowed us to precisely identify bone limits of the foramen and perform measurements with values consistent with the data in the literature [7, 12, 14]. The use of orthogonal planes allowed us to position ourselves perfectly in the plane of the foramen in all reconstructions, and it was shown that an acquisition oriented perpendicular to the axis of the intervertebral foramen allowed higher precision in the diagnosis of cervical foraminal stenosis [15].
We also admit that our method of assessing foraminal volume has several limitations. Measurement time is relatively long, leading to difficulties in comparing large cohorts, and there is, to our knowledge, no software available that could be helpful saving time. In addition, volume is calculated and depends on multiple measured parameters, which increases its variability. Due to the design of this study, there is a risk of selection bias in patient selection. Furthermore, our study only analyses the container (the foramen) and volume measurements of the foramen contents (the root and the fat) were not performed. Finally, with only 10 patients in each group and measurements obtained from 100 intervertebral foramen of varying levels, our work needs to be further investigated in a larger cohort to confirm our results.
Conclusion
Thanks to a new protocol of foraminal volume measurement, the present study allows us to refine current knowledge regarding normal and pathological anatomy of the lower cervical spine in order to understand the foraminal sites most affected by cervical degeneration. Those findings can be applied to foraminal stenosis surgeries and guide the surgeon in his procedure. Based on our results, we suggest that decompression of the foramen in regard of both uncus osteophytic spurs and inferior plate of the overlying vertebra seems to be an important step for spinal nerves release (in addition to direct decompression of the uncus and indirect decompression by interbody distraction).
Availability of data and material
Data transparency: P. Coudert had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Data are available on request to the corresponding author.
Code availability
Not applicable.
Abbreviations
- 3D MPR:
-
Three-dimensional multiplanar reconstruction
- AE:
-
Area of the ellipse
- BMI:
-
Body Mass Index
- CD:
-
Compact disc
- CT:
-
Computed tomography
- DH:
-
Disc height
- DICOM:
-
Digital imaging and communication in medicine
- FH:
-
Foraminal height
- FL:
-
Foraminal length
- FV:
-
Foraminal volume
- FW:
-
Foraminal width
- ICC:
-
Intraclass correlation coefficients
- IWMP:
-
Inferior width of medial part
- IWPP:
-
Inferior width of pedicle part
- MRI:
-
Magnetic resonance imaging
- MWMP:
-
Middle width of medial part
- MWPP:
-
Middle width of pedicle part
- SD:
-
Standard deviation
- UWMP:
-
Upper width of medial part
- UWPP:
-
Upper width of pedicle part
References
Boijsen E (1954) The cervical spinal canal in intraspinal expansive processes. Acta Radiol 42:101–115
Brigham CD, Tsahakis PJ (1995) Anterior cervical foraminotomy and fusion. Spine 20:766–770
Cinotti G, De Santis P, Nofroni I et al (2002) Stenosis of lumbar intervertebral foramen: anatomic study on predisposing factors. Spine 27:223–229
Czervionke LF, Daniels DL, Ho PS et al (1998) Cervical neural foramina: correlative anatomic and MR imaging study. Radiology 169:753–759
De Palma AF, Rothman RH (1970) The intervertebral disc. WB Saunders, Philadelphia
Ebraheim NA, An HS, Xu R et al (1996) The quantitative anatomy of the cervical nerve root groove and the intervertebral foramen. Spine 21:1619–1623
Freedman BA, Hoffler CE, Cameron BM et al (2015) A comparison of computed tomography measures for diagnosing cervical spinal stenosis associated with myelopathy: a case-control study. Asian Spine J 9:22–29
Friedenberg ZB, Miller WT (1963) Degenerative disc disease of the cervical spine: a comparative study of asymptomatic and symptomatic patients. J Bone Joint Surg Am 45:1171–1178
Landis JR, Koch GG (1997) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Moore KL (2001) Anatomie médicale, aspects fondamentaux et applications cliniques. De Boeck Superieur ed, 1st edn, Paris
Murphey F, Simmons JC, Brunson B (1973) Ruptured cervical discs, 1939 to 1972. Clin Neurosurg 20:9–17
Naji P, Alsufyani NA, Lagravère MO (2014) Reliability of anatomic structures as landmarks in three-dimensional cephalometric analysis using CBCT. Angle Orthod 84:762–772
Oda J, Tanaka H, Tsuzuki N (1998) Intervertebral disc changes with aging of human cervical vertebra. From the neonate to the eighties. Spine 13:1205–1211
Park JY, Hwang JT, Oh KS et al (2014) Revisit to scapular dyskinesis: three-dimensional wing computed tomography in prone position. J Shoulder Elbow Surg 23:821–828
Polston DW (2007) Cervical radiculopathy. Neurol Clin 25:373–385
Rühli FJ, Müntener M, Henneberg M (2006) Human osseous intervertebral foramen width. Am J Phys Anthropol 129:177–188
Ruofu Z, Huilin Y, Xiaoyun H et al (2008) CT evaluation of cervical pedicle in a Chinese population for surgical application of transpedicular screw placement. Surg Radiol Anat SRA 30:389–396
Shim JH, Park CK, Lee JH et al (2009) A comparison of angled sagittal MRI and conventional MRI in the diagnosis of herniated disc and stenosis in the cervical foramen. Eur Spine J 18:1109–1116
Singh K, Masuda K, Thonar EJ-MA et al (2009) Age-related changes in the extracellular matrix of nucleus pulposus and anulus fibrosus of human intervertebral disc. Spine 34:10–16
Sonntag VKH, Vishteh AG (2001) Anterior cervical discectomy. Neurosurgery 49(4):909–912
Tanaka N, Fujimoto Y, An HS et al (2000) The anatomic relation among the nerve roots, intervertebral foramina, and intervertebral discs of the cervical spine. Spine 25:286–291
Taylor TK, Melrose J, Burkhardt D et al (2000) Spinal biomechanics and aging are major determinants of the proteoglycan metabolism of intervertebral disc cells. Spine 25:3014–3020
Vital JM, Lavignolle B, Pointillart V et al (2004) Cervicalgie commune et névralgies cervicobrachiales. EMC Rhumatol Orthopédie 1:196–217
Wong JJ, Côté P, Quesnele JJ et al (2014) The course and prognostic factors of symptomatic cervical disc herniation with radiculopathy: a systematic review of the literature. Spine J Off J North Am Spine Soc 14:1781–1789
Yang R, Ma M, Huang L et al (2018) Influences of different lower cervical bone graft heights on the size of the intervertebral foramen: multiple planar dynamic measurements with laser scanning. Lasers Med Sci 33:627–635
Yoshihara H (2017) Indirect decompression in spinal surgery. J Clin Neurosci 44:63–68
Acknowledgements
Amaury Lainé for grammar and spelling check. The work described has not been published before and it is not under consideration for publication anywhere else. Its publication has been approved by all co-authors, as well as by the responsible authorities at the institute where the work has been carried out.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
PC: protocol development, data collection, management and analysis. GL: data analysis, manuscript writing and editing. VP: manuscript editing. CD: manuscript Editing. LB: manuscript editing. JMV: manuscript editing. BB: data analysis, manuscript editing. OG: project development, protocol development, data management, manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval (include appropriate approvals or waivers)
Because of its retrospective design and in accordance with the requested opinion of the ethical committee of the Bordeaux University Hospital, no ethics committee approval is required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Coudert, P., Lainé, G., Pointillart, V. et al. Tomodensitometric bone anatomy of the intervertebral foramen of the lower cervical spine: measurements and comparison of foraminal volume in healthy individuals and patients suffering from cervicobrachial neuralgia due to foraminal stenosis. Surg Radiol Anat 44, 883–890 (2022). https://doi.org/10.1007/s00276-022-02941-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-022-02941-8