Abstract
Variations in the anatomy of inferior vena cava (IVC) may have important clinical implications. In-depth knowledge of its embryology and variations are of fundamental importance to prevent any potential medical complications related to anatomic variations of the IVC. In this article, we described a previously unreported, to the best of our knowledge, a variation of IVC. In the case we presented, the IVC was seen almost completely encircling the abdominal aorta. We decided to call this anatomic variation as “a sling of a normal right IVC around the abdominal aorta”. Cross-sectional imaging is a prompt and highly reliable method to evaluate IVC anatomy and may have significant clinical importance to prevent any potential complications related to IVC during surgery or interventional radiology procedures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introductıon
Inferior vena cava (IVC) is the fundamental pathway for venous return from the lower extremity, pelvis and kidneys. The complex sequential development during embryogenesis may lead to anatomical variations of the IVC. Among these variants, duplicated IVC, left-sided IVC, azygos continuation of the IVC, retroaortic left renal vein, circumaortic left renal vein, double IVC with retroaortic right renal vein and hemiazygos continuation of the IVC may be counted [2, 6]. Although the anatomic variations of IVC have an overall reported prevalence of 0.5% [5], it can be up to 5% in young patients with spontaneous deep venous thrombosis [3].
On cross-sectional imaging studies, the diseases affecting IVC and its anatomic variations may be easily overlooked, especially to inexperienced eyes. Duplicated IVC, left-sided IVC, and circumaortic left renal vein may be misdiagnosed as pathologically enlarged lymph nodes and retroperitoneal masses [2]. As the anatomic variations of IVC may be easily confused with several disease processes on imaging and its variations may have a significant impact on surgical and minimally invasive interventions, a thorough knowledge of its embryology, anatomy and diseases affecting it should be well-known to imagers to prevent potentially devastating clinical ramifications.
Case report
A 29-year old male patient was referred for follow-up liver magnetic resonance imaging (MRI) study for surgically and percutaneously treated hydatid liver cyst from an outside center.
The MRI scan (Table 1) confirmed the absence of left liver lobe due to surgical removal and a ventral abdominal wall hernia related to surgery. There was no evidence of active disease in the abdomen.
An incidental note is made of the IVC coursing behind the abdominal aorta (AA) and crossing to the left side of the abdomen. After this first curve, the IVC was not seen to ascend to the chest on the right side of the abdomen and rather it was seen to cross back to the right side of the AA to its usual course to the heart through the liver parenchyma. There was no evidence for neither AA compression to IVC nor an endoluminal thrombus. The caudal portion of IVC could not be detected as the study was limited to the liver.
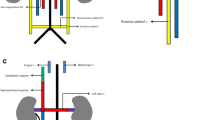
For better outlining this unusual IVC anatomy, a computed tomography (CT) venography was planned. On the CT venography (Table 2), the iliac veins were seen to preserve their usual anatomy with subsequent formation of the IVC on the right side of the AA. Around the level of the renal hila, IVC again was visualized crossing to the left side of the abdomen coursing behind the abdominal aorta, with a subsequent acute bend to the right side of the abdomen to its usual location after almost completely encircling the AA (Fig. 1). The left renal vein was seen to join the IVC at this acute bend, with subsequent joining of the right renal vein as IVC starts to ascend toward the liver (Fig. 2). Again there was no evidence of an endoluminal thrombus or mass in the retroperitoneum.
a Axial venous phase contrast enhanced CT image demonstrates the IVC (arrow) crossing to the left side of the abdomen by coursing behind the abdominal aorta (arrowhead). b 3D cinematic rendering VRT (volume rendering technique) image shows the route of the IVC, passing behind and encircling the abdominal aorta (arrows). c Curved MPR image demonstrates the IVC (arrow) almost completely encircling the abdominal aorta (dashed arrow)
Dıscussıon
Due to its long course through the several parts of the abdomen, IVC has a complex embryological anatomy with multiple steps in a set order [10]. The abnormal anatomic variants of IVC is well-known for a long time. Abernethy first described a congenital mesocaval shunt and azygos continuation of the IVC in a 10-month-old infant with polysplenia and dextrocardia in 1793 [1].
As of its complex formation, several clinically silent or significant abnormalities may happen in utero. The formation of IVC happens during the 4th–8th weeks of gestation. Three sets of paired veins; the posterior cardinal veins, the subcardinal veins, and the supracardinal veins are present at this stage and they regress as the final IVC forms [8]. Initially, a pair of posterior cardinal veins appear during the early stages of the embryologic development and subsequently subcardinal veins start to develop. As the posterior cardinal veins start to gradually regress, supracardinal veins become the dominant venous structures. In the meantime, the left subcardinal and supracardinal veins also undergo involution. Suprarenal portion of the IVC is derived from the right subcardinal vein, whereas the infrarenal portion develops from the right supracardinal vein. The renal segment of IVC is formed as the anastomoses between the right and left subcardinal veins coalesce [7,8,9]. Variant IVC anatomy is a result of abnormal persistence or regression of these fetal vessels.
As mentioned above, due to its complex and multisegmental embryogenesis, IVC is subject to several developmental abnormalities [4]. The readers may refer to several excellent papers regarding these variants for further information [2, 9, 10].
To the best of our knowledge, the variant anatomy that we observed in this patient has not been reported elsewhere before. We are not sure about the exact embryological pathogenesis of this anatomy but we think that this interesting anatomy may be related to abnormal embryogenesis of the formation of the renal segment of IVC. Although it is speculative, we tend to consider the abnormal union of the right and left subcardinal veins as the main culprit.
Knowing anatomic variations of IVC as these abnormalities may have important clinical consequences. Despite the fact that most of these variations are clinically silent, as in the presented case, these abnormalities have a significant potential to impact patient care. Abnormally located IVC may be misdiagnosed as a retroperitoneal mass or lymphadenopathy or an abnormal course with an acute bend, as in our patient, may give rise to even a caval rupture, a potentially fatal process, during endocaval interventions. Endocaval interventions are very common in modern medical practice, such as IVC filter replacement or thrombolysis with pharmacologic, mechanical or pharmacomechanical means. Correct knowledge of the IVC anatomy before these procedures is crucially important for proper procedural planning to avoid potential complications. Knowledge of renal vein anastomoses may also be important for kidney surgeries.
Cross-sectional imaging, especially CT venography, is extremely helpful for evaluating IVC anatomy. With the advent of state-of-art CT technology, IVC can now be imaged in a very rapid fashion with minimal radiation burden to the patient, owing to the recent developments in dose reduction technology.
As our last words, we tend to name this previously unreported, to the best of our knowledge, abnormality of IVC as “a sling of a normal right IVC around the abdominal aorta”.
References
Abernethy J (1793) Account of two instances of uncommon formation in the viscera of the human body. Philos Trans R Soc 83:59–66. https://doi.org/10.1098/rstl.1793.0010
Bass JE, Redwine MD, Kramer LA, Huynh PT, Harris JH Jr (2000) Spectrum of congenital anomalies of the inferior vena cava: cross-sectional imaging findings. Radiographics 20(3):639–652. https://doi.org/10.1148/radiographics.20.3.g00ma09639 (PMID: 10835118)
Chee YL, Culligan DJ, Watson HG (2001) Inferior vena cava malformation as a risk factor for deep venous thrombosis in the young. Br J Haematol 114(4):878–880. https://doi.org/10.1046/j.1365-2141.2001.03025.x (PMID: 11564079)
Edwards EA (1951) Clinical anatomy of lesser variations of the inferior vena cava; and a proposal for classifying the anomalies of this vessel. Angiology 2(2):85–99. https://doi.org/10.1177/000331975100200201 (PMID: 14819704)
Koc Z, Ulusan S, Oguzkurt L, Tokmak N (2007) Venous variants and anomalies on routine abdominal multi-detector row CT. Eur J Radiol 61(2):267–278. https://doi.org/10.1016/j.ejrad.2006.09.008 (PMID: 17049792)
Malaki M, Willis AP, Jones RG (2012) Congenital anomalies of the inferior vena cava. ClinRadiol 67(2):165–171. https://doi.org/10.1016/j.crad.2011.08.006 (PMID: 22070941)
Maxwell EV, Erwin GS (1928) Four cases of anomalous inferior vena cava with an explanation of their developmental origin. J Anat 62:184–197 (PMID: 17104183)
McClure CFW, Butler EG (1925) The development of the vena cava inferior in man. Am J Anat 35(3):331–383. https://doi.org/10.1002/aja.1000350302
Shin DS, Sandstrom CK, Ingraham CR, Monroe EJ, Johnson GE (2019) The inferior vena cava: a pictorial review of embryology, anatomy, pathology, and interventions. AbdomRadiol (NY) 44(7):2511–2527. https://doi.org/10.1007/s00261-019-01988-3 (PMID: 30937506)
Spentzouris G, Zandian A, Cesmebasi A, Kinsella CR, Muhleman M, Mirzayan N et al (2014) The clinical anatomy of the inferior vena cava: a review of common congenital anomalies and considerations for clinicians. ClinAnat 27(8):1234–1243. https://doi.org/10.1002/ca.22445 (PMID: 25042045)
Author information
Authors and Affiliations
Contributions
ADK: manuscript writing, data collection, and image collection. SAD: manuscript editing, image collection, and preparation. DA: manuscript editing, data collection, and image collection. MNO: manuscript editing, data collection, and image collection. MK: manuscript editing, data collection, and image collection.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karaosmanoglu, A.D., Ardali Duzgun, S., Akata, D. et al. A sling of a normal right inferior vena cava around the abdominal aorta. Surg Radiol Anat 43, 1391–1394 (2021). https://doi.org/10.1007/s00276-021-02693-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-021-02693-x