Abstract
Purpose
Motor deficits affecting anal sphincter control can severely impair quality of life. Peripheral nerve transfer has been proposed as an option to reestablish anal sphincter motor function. We assessed, in human cadavers, the anatomical feasibility of nerve transfer from a motor branch of the tibialis portion of the sciatic nerve to two distinct points on pudendal nerve (PN), through transgluteal access, as a potential approach to reestablish anal sphincter function.
Methods
We dissected 24 formalinized specimens of the gluteal region and posterior proximal third of the thigh. We characterized the motor fascicle (donor nerve) from the sciatic nerve to the long head of the biceps femoris muscle and the PN (recipient nerve), and measured nerve lengths required for direct coaptation from the donor nerve to the recipient in both the gluteal region (proximal) and perineal cavity (distal).
Results
We identified three anatomical variations of the donor nerve as well as three distinct branching patterns of the recipient nerve from the piriformis muscle to the pudendal canal region. Donor nerve lengths (proximal and distal) were satisfactory for direct coaptation in all cases.
Conclusions
Transfer of a motor fascicle of the sciatic nerve to the PN is anatomically feasible without nerve grafts. Donor nerve length was sufficient and donor nerve functionally compatible (motor). Anatomical variations in the PN could also be accommodated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Loss of voluntary control over the elimination of rectal content constitutes one of the most undesired conditions of human existence, causing marked decrease in quality of life and self-esteem. The multifactorial nature of anal incontinence (AI) makes it difficult to treat, especially in the presence of associated neuropathy. About half of incontinent patients have some degree of neural injury contributing to their symptoms [8, 31].
Currently, sacral neurostimulation is a major therapeutic focus for rectal sphincter motor dysfunction, yielding up to 90% success rates, in selected cases, when well indicated. However, several patients with AI are not good candidates for neurostimulation; thus lacking effective treatment options [17].
Nerve transfer (NT) is considered a paramount strategy in the treatment of shoulder, elbow, wrist and finger motor deficits caused by traumatic brachial plexus lesions when the primary repair is unachievable [11, 20]. In recent decades, several studies have assessed using NT to treat bladder and anorectal motor dysfunction, although most were anatomical feasibility or experimental trials in animals [4,5,6, 13, 14, 25,26,27]. Barbe et al. documented the anatomical feasibility of this model in human cadavers, using a femoral nerve branch as a donor to the vastus medialis muscle by perineal access [6]. While only few studies have evaluated feasibility of NT for reestablishing anal sphincter control in humans, clinical investigations have started to shed light on this technique to establish urethral sphincter control in patients with severe spinal cord lesions associated with neurogenic bladder [18]. For example, Livshits et al. proposed intercostal (T11-12) NT to S2 and S3 branches to treat neurogenic bladder symptoms in patients with spinal cord injuries, observing increased urodynamic parameters and bulbocavernosus, anal and cremaster reflex recovery [18].
Other studies have estabilished the feasibility of using NT to reinervate the urethral and anal sphincter muscles in animal models. In dogs, Ruggieri et al. demonstrated that re-innervating the bladder could be achieved via NT of spinal (coccygeal branches of the lumbosacral plexus) or peripheral nerves (genitourinary nerve) to sectioned sacral roots as late as three months after denervation [26, 27]. Also in dogs, these authors studied reinnervating urethral and anal sphincter muscles via NT of a sensory branch of the femoral nerve (saphenous nerve) to pudendal nerve (PN) technique [25]. Reinnervation was documented by retrograde immunofluorescence (fluorogold) and histopathological examination of specimens.
In rats, Dong et al. documented reinnervation of the rectum and anal sphincter, transferring autonomic nerves originating at L4 to somatic nerves from L5-S1 [12]. Reinnervation also was confirmed by fluorogold. The same authors recently published a further experimental study in rats describing rectal reinnervation via NT of the genitofemoral nerve to pelvic nerves [13]. In the most recent publication on this subject [4], anorectal function was restored by NT of anterior and posterior branches of L5 to anterior and posterior branches of S1.
Here, we investigated anatomical feasibility of nerve transfer from a motor fascicle from a specific branch of the tibial portion of the sciatic nerve that innervates the long portion of the biceps femoris muscle (LHBFM) to the PN, as a potential approach to reestablish the motor function of anal sphincter. Anatomical feasibility of this NT to reinnervate PN was tested via transgluteal access, using two distinct points of coaptation: proximal (technically easier) and distal (closer to the target muscle).
Materials and methods
This study was approved by the institutional ethics committee (n.60067316.6.0000.5258) and used formalinized anatomical specimens from the Department of Anatomy at the Biomedical Institute of the Federal University of Rio de Janeiro State (UNIRIO). All anatomical material was pre-inspected to ensure its integrity.
We studied 12 formalinized human cadavers (8 male and 4 female). A total of 24 specimens dissected from the gluteal region and proximal third of the posterior ipsilateral thigh.
Anatomical dissections were performed using microsurgical material, and a dissection microscope (D.F. Vasconcelos, São Paulo, Brazil) with 6–10 × magnification for intraneural dissections. The distances of interest were measured using rods and tapes graduated with pinpoint accuracy. Photographs of critical procedural steps were taken using a digital 12-megapixel camera with autofocus and optical image stabilization.
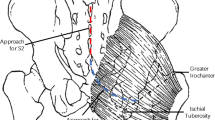
Dissections were performed with the cadavers in a prone position, using the following gluteal region anatomical landmarks: cranially, ipsilateral iliac crest; medially, buttocks groove; caudally, inferior gluteal folds. A wide incision access was used in the dissections to facilitate the anatomical study of the structures of interest in the gluteal region. This was necessary to identify and perform measurements of the chosen donor fascicle of the sciatic nerve.
The skin and subcutaneous tissue of the gluteal region were completely removed to expose fibers of the gluteus maximus muscle (GMM), which then sectioned in two parts, through an oblique incision following the direction of its fibers. Following retraction of the GMM, it was possible to identify the deep pelvic muscles (medius gluteus and piriformis muscles), thick fascia and tissue immediately superficial to the piriformis muscle (Fig. 1).
Overview demonstrating the anatomical references of dissection. MxGM maximus gluteus muscle, MdGM medius gluteus muscle, PM piriformis muscle, QFM quadratus femoris muscle, LHBFM long head of the biceps femoris muscle, SN sciatic nerve, PCNT posterior cutaneous nerve of the thigh, PB pudendal branch. Cr cranial, Lat lateral
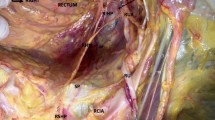
Through the dissection of the elements emerging from the greater sciatic foramen, we identified the sciatic nerve, the posterior cutaneous nerve of the thigh, the pudendal vein and pudendal artery, and the PN (Fig. 2).
Elements emerging from the greater sciatic foramen. It is possible to visualize the anatomical relationship between the sacrotuberal ligament and neural structures. PM piriformis muscle, STL sacrotuberous ligament sectioned, SN sciatic nerve, PCNT posterior cutaneous nerve of the thigh, PN pudendal nerve, PA pudendal artery, PV pudendal vein. Cr cranial, Lat lateral
We performed a median incision on the posterior surface of the thigh from the bottom gluteal fold to 2 cm proximal to the popliteal fossa, and the skin and subcutaneous tissue were retracted medially and laterally. The LHBFM was identified. We characterized the nerve branches originating from the tibial portion of the sciatic nerve that innervated this muscle, anteriorly to the adductor magnus muscle, crossed obliquely by the LHBFM in the posterior middle third of the thigh.
The sciatic nerve sheath was dissected, from its emergence in the greater sciatic foramen to the mid-third of the thigh, where we identified the first branch of the LHBFM (Fig. 3). The aforementioned branch was dissected in a retrograde manner (Fig. 4).
Dissected sciatic nerve and its branchs to the long head of the biceps femoris muscle (red arrows). PM piriformis muscle, LHBFM long head of the biceps femoris muscle, STL sacrotuberous ligament sectioned, SN sciatic nerve, PCNT posterior cutaneous nerve of the thigh, PN pudendal nerve, PA pudendal artery, PV pudendal vein. Cr cranial, Lat lateral
Dissected donor fascicle of the sciatic nerve. PM piriformis muscle, LHBFM long head of the biceps femoris muscle, STL sacrotuberous ligament sectioned, SN sciatic nerve, DF donor fascicle, PCNT posterior cutaneous nerve of the thigh folded, PN pudendal nerve, PA pudendal artery, PV pudendal vein. Cr cranial, Lat lateral
The PN was dissected from its emergence in the greater sciatic foramen, medially to the sciatic nerve until its passage anterior to the sacrotuberous ligament (gluteal portion). In continuity, the ligament was sectioned, what allowed the dissection and characterization of the PN, including the deep pelvic portion, until the pudendal canal.
We measured the donor's fascicles lengths (DFL): the segment of the donor's nerve (branch of the tibial component of the sciatic nerve to the LHBFM) between the caudal edge of the greater sciatic foramen to its most proximal insertion into the LHBFM; the proximal coaptations lengths (PCL): lengths of donors nerves necessaries for coaptations to the gluteal portions of the PN, immediately cranial to the sacrotuberous ligament; and the distal coaptations lengths (DCL): lengths of donors nerves necessaries for coaptation to the deep pelvic portion of the PN, immediately cranial to the pudendal canal (Fig. 5).
Proximal (blue star) and distal (red star) coaptations points and its anatomical relationship to the sacrotuberous ligament (green dashed line). PM piriformis muscle, STL sacrotuberous ligament sectioned, SN sciatic nerve, PCNT posterior cutaneous nerve of the thigh, PN pudendal nerve, PA pudendal artery, PV pudendal vein. Cr cranial, Lat lateral
Anatomical feasibility was tested mathematically by examining the ratio between the DFL and PCL, and DFL and DCL, and the potential for direct coaptation between its free end and the PN in its caudal-gluteal portion, along the cranial edge of the sacrotuberous ligament and in its cranial pelvic portion along with the cranial limit of the pudendal canal. Coaptation was performed with nylon 10.0, an atraumatic needle and glue (Fig. 6).
Example of feasibility assessment of proximal direct coaptation of the donor fascicle of the sciatic nerve to the pudendal nerve. MdGM medius gluteus muscle, PM piriformis muscle, STL sacrotuberous ligament sectioned, SN sciatic nerve, PCN posterior cutaneous nerve of the thigh folded, PN pudendal nerve, donor fascicle, PA pudendal artery, PV pudendal vein. Cr cranial, Med medial
GraphPad Prism version 5 (GraphPad Software, Inc, La Jolla, CA, USA) was used for statistical analysis. Wilcoxon’s paired non-parametric test was used to compare DFL, PCL and DLC lengths from the cadavers. The results were considered significant when p ≤ 0.05 with a 95% confidence interval.
Results
In our dissection, we identified three anatomical variations of the donor nerve fascicle (from the tibial portion of the sciatic nerve) as it innervates the LHBFM: (1) with two insertion points, corresponding a 71,4% of our specimens (n = 20), (2) with three points at 25% (n = 7) and (3) with four points in only 3,6% (n = 1). We also found three patterns of PN branches from the piriformis muscle to the pudendal canal region. Sixteen specimens had one-trunked pattern (57,2%), ten specimens presented two-trunked (35,7%), one of them being the inferior rectal nerve and the other a trunk with the genital nerve and perineal nerve as ramifications. Lastly, in two specimens, we observed a three-trunked pattern (7.1%), having each nerve an isolated path.
Anatomical measurements, ratios and calculated means are shown in Table 1. DFL ranged from 4.3 to 14.5 cm, averaging 10.2 cm. The average distance required for coaptation of the fascicle donor to the pudendal proximal location (PCL) was 4.8 cm (range 3.2–8.8 cm). The average distance required for coaptation of the fascicle donor to the pudendal distal location (DCL) was 3.0 cm (range 2.0—7.6 cm). The differences observed between DFL and donor fascicle sizes, as well as between DFL and DCL sizes were statistically significant (p < 0.0001) (Fig. 7).
Tests of coaptation (proximal and distal) were considered satisfactory for all the studied samples, since the ratio between the length of the fascicle and length of the proximal (DFL/PCL) and distal (DFL/DCL) coaptation was greater than 1.00 across all studied samples (Table 1).
Discussion
The pudendal nerve is involved in sensory and motor functions, and is responsible for innervating the anal sphincter complex (internal sphincter, external sphincter, and anus elevator muscles), the urethral sphincter and perineal muscles, and the skin of the perineum and genitalia. Despite its large sensory component [21], and the decrease in motor fibers with advancing age [7], the pudendal nerve has an important function in the complex continence mechanism. It is estimated that about half of the patients with AI have some degree of motor or sensitive denervation of the anal sphincter [7], which is a pervasive feature associated with worse therapeutic results.
Pudendal denervation by continuous compression or excessive stretching, such as those that accompany chronic constipation or after successive births, are closely related to worse therapeutic results since it is difficult to determine the exact location of the injury. The absence of a defined lesion location and partial integrity of the injured nerve have led to the use of therapeutic electrical neurostimulation (sacral or tibial) for idiopathic AI. It stimulates axonal regeneration and promotes central and peripheral neuroplasticity [30]. However, it is well known that electrical neuromuscular stimulation has disadvantages related to high costs, involuntary muscular contractions, fatigue, selective recruitment of fibers, and the potential for pain and discomfort [9].
Studies of NT using retrograde immunofluorescence markers, such as FluoroGold, have documented the growth of axonal structures from the donor to recipient nerve [12, 28], indicating that the reinnervation achieved via this technique reaches far beyond merely reestablishing nerve impulse conduction. NT has the capacity to stimulate the re-composition of damaged neural structures in the receptor nerve, preserving, in a more efficient way, the targeted muscular tissue [10]. Specifically, in AI, the mechanisms involved in pudendal neuropathy could favor good results with NT. With partial denervation, nervous impulses, even transmitted with low intensity, continue to stimulate the target muscle. Thereby, muscular atrophy is limited and easily reversible, relative to totally denervated muscles, in which muscular degeneration is progressive and permanent. Thus, NT therapy could be an attractive therapeutic option for AI, even when symptoms are chronic. Partial integrity of the nerve fiber would act as a matrix for the growth of new neuronal elements from the donor's nerve, allowing more organized and efficient regeneration.
Gustafson et al. [15], while studying a PN segment placed between the sacrospinous ligament and pudendal canal (deep perineal extra-pelvic portion), documented median length and diameter measurements of this nerve of 26 and 3,2 mm, respectively, proving the feasibility of surgical procedures like NTs or device implants. Based on these observations, we opted to evaluate the anatomical feasibility of transferring PN in two distinct segments: (a) the deep perineal point suggested by Gustafson et al. (i.e., the perineal portion or distal coaptation point); and (b) the cranial position (i.e., gluteal portion or proximal coaptation point). These segments may enable better visualization of adjacent anatomical structures, favoring the surgical technique and facilitating coaptations, since these regions entail higher-caliber fascicles [15]. More distal coaptations [6], despite being more proximal to the target areas, would present technical difficulties due to the extremely small caliber of the neural structures involved.
Another advantage of the coaptations we studied is that, in these segments, coaptations to pudendal branches (inferior rectal, perineal and genital) could be accomplished in isolation, including the independent sacral variant [15] and even at anatomical variations of the PN branches, guided by selective intraoperative electrical stimulation. In line with the research performed by Mahakkanukrauh et al. [19], we identified three different patterns of the PN branches, all of which would allow NT. NT to more distal ramifications of PN, particularly in its distal perineal segment [6], could even result in neural coaptations involving restricted motor areas and/or have a little overall impact on continence, as with the independent sacral variant [15].
In recent research, Agarwal et al. stated that an NT from motor fascicles from the sciatic nerve to the PN is feasible, through an anatomic study in five specimens [2]. These authors’ methods used measurements based on the distance between the two nerves and their diameters. Our study, however, demonstrated feasibility of two different points of coaptations related to the gluteal and perineal portions of the PN, in a larger sample size.
The neural structure we selected as a donor in this study was one of the sciatic nerve motor fascicles, responsible for the innervation of the LHBFM. That the donor and recipient nerves are localized in the gluteus region with little distance between them, and surgical access is already known and devoid of joint movements, was considered favorable to the proposed transfer.
Some authors have detailed the innervation of the LHBFM, indicating the presence of one or two branches of the tibial portion of the sciatic nerve, with two or three motor fixation points in the LHBFM. Most report the presence of a unique branch (in 60–82% of cases) related to two branches [3]. Conversely, the pattern found in all our dissections mimicked the results of Rab et al. [24], who observed across their entire sample (n = 30) a single trunk divided into two smaller fascicles, fixated by two or three motor points in the LHBFM. On the other hand, eight percent of our sample had more than one fascicle, which allowed us to select the fascicle more proximal to the corresponding donor muscle.
Selective transfer of the fascicle more proximal to the LHBFM could permit maintenance of innervation of this muscular portion by the remaining, more distal fascicle. That more than one sciatic nerve fascicle innervates this portion of the muscle in a staggered manner could, perhaps, provide security during coaptations that require longer donors.
The innervation pattern totally distinct between the two heads of the BFM (short and long) and the synergistic action of this muscle with the other tuber-ischiatic muscles could compensate for eventual, isolated denervation of the long head.
During the dissections, it was possible to identify a pattern in the disposition of the origin of the donor fascicle, which happened through a fascicular group located in an anteromedial position in the sciatic nerve trunk near the inferior border of the piriformis muscle. Despite frequent anatomic variations in the arrangement of the sciatic nerve, relative to the piriformis muscle, described in the literature [23], none of these findings negatively influence the proposed NT.
In all the simulations we performed, the donor nerve’s length was sufficient for direct coaptation, in both proposed segments (proximal and distal), without requiring graft interposition. These characteristics were statistically proven by the high mathematical differences observed when comparing the DFL lengths to DCL and PCL lengths from the same cadaver, as well as by calculating the ratio between donor fascicle length and coaptation distances at PN (proximal and distal), all of them greater than 1. The absence of associated anthropometric measures was a factor that limited the comparative detailed analysis of anatomical measures in this study.
No comparative studies have been published assessing physiological similarities (sensory versus motor) of transferred nerves for reestablishing rectal/sphincter function. The only similar study involved an attempt at bladder reinnervation [14], in which better results were obtained using a motor (femoral) than sensory (genitofemoral) donor nerve. The donor fascicle we used was exclusively motor.
The transgluteal surgical access to PN is already used for the treatment of compressive neuropathy in this region. PN decompression is traditionally performed using a small transgluteal incision, allowing sacrotuberous ligament sectioning [22]. Thus, using this modified access may provide simultaneous visualization of sciatic nerve and PN since its emergence is in the larger ischiatic foramen (infra-piriformis) until it enters PN into pudendal canal [22].
Denervation by continuous compression or by excessive stretching, such as those that accompany chronic constipation or denervations after successive births, are closely related to worse therapeutic results since it is difficult to determine the exact location of the injury. A modified transgluteal access could provide visualization of the greatest part of the PN’s perineal portion (recipient), favoring, simultaneously, trunk and peripheral interventions, as well as possible interventions in the independent lower rectal branch perineal trajectory.
Intraoperative electroneuromyography could prevent over-extensive transgluteal incisions, combining incisions adequate to view the proximal third of the thigh, just as it would permit better visualization of the coopted segment.
Interventions distal to the sacrotuberous ligament would also become feasible, with sectioning of this and the sacrospinous ligament. Even though Hibner et al. [16] warned about the risk of pelvic destabilization, most biomechanical studies have failed to identify greater clinical consequences after simultaneously sectioning the sacrotuberous and sacrospinous ligaments, since pelvic stabilization is maintained primarily by the stronger and more resistant anterior and posterior sacroiliac ligaments [1].
The cadaveric study of the sciatic nerve’s fascicle anatomy, performed by Sladjana et al., demonstrated a variation from 27 to 70 fascicles (1 to 4 fascicles by square millimeter), being more numerous in specimens with younger age [29]. To the best of our knowledge, there are no similar studies evolving the PN at the English literature. The paucity of studies involving NT in the lower limbs, especially its practical use reestablishing sphincter function, added to the absence of previous knowledge about the fascicular anatomy of the sciatic nerve in its proximal portion, are obstacles to the present analysis. Nonetheless, our results suggest the anatomical feasibility of NT involving a sciatic nerve fascicle that innervates LHBFM, to the PN as a treatment option for AI with a pudendal neuropathy component. This approach has the following potential advantages:
Donor length is sufficient for transfer to various segments of the gluteal and perineal portions of the PN, even when anatomical variations exist.
-
Isolated transfer to branches of the PN and accessory rectal branch is possible.
-
The donor and recipient nerves could be identified by transgluteal modified access.
-
It allows study and transfer.
-
Any resulting loss of motor function should be compensated for by synergistic muscles.
-
Corresponding cerebral representations the donor and recipient nerves are proximal.
Conclusions
Transfer of a nerve fascicle to the LHBFM from the sciatic nerve to the PN is anatomically feasible, without requiring nerve grafts.
References
Abdelfattah A, Moed BR (2014) Ligamentous contributions to pelvic stability in a rotationally unstable open-book injury: a cadaver study. Injury 45(10):1599–1603
Agarwal P, Sharma D, Wankhede S, Jain PC, Agrawal NL (2019) Sciatic nerve to pudendal nerve transfer: anatomical feasibility for a new proposed technique. Indian J Plast Surg 52(02):222–225
An XC, Lee JH, Im S, Lee MS, Hwang K, Kim HW, Han S-H (2010) Anatomic localization of motor entry points and intramuscular nerve endings in the hamstring muscles. Surg Radiol Anat 32(6):529–537
Bao B, Fu K, Zheng X, Wei H, Luo P, Zhu H, Zhu X, Li X, Gao T (2018) Novel method for restoration of anorectal function following spinal cord injury via nerve transfer in rats. J Spinal Cord Med. https://doi.org/10.1080/10790268.2018.1444542
Barbe MF, Brown JM, Pontari MA, Dean GE, Braverman AS, Ruggieri MR (2011) Feasibility of a femoral nerve motor branch for transfer to the pudendal nerve for restoring continence: a cadaveric study. J Neurosurg Spine 15(5):526–531
Barbe MF, Ruggieri MR (2011) Innervation of parasympathetic postganglionic neurons and bladder detrusor muscle directly after sacral root transection and repair using nerve transfer. Neurourol Urodyn 30(4):599–605
Bharucha AE, Daube J, Litchy W, Traue J, Edge J, Enck P, Zinsmeister AR (2012) Anal sphincteric neurogenic injury in asymptomatic nulliparous women and fecal incontinence. Am J Physiol Gastrointest Liver Physiol 303(2):G256–G262
Bharucha AE, Rao SSC (2014) An update on anorectal disorders for gastroenterologists. Gastroenterology 146(1):37-45.e2
Bickel CS, Gregory CM, Dean JC (2011) Motor unit recruitment during neuromuscular electrical stimulation: a critical appraisal. Eur J Appl Physiol 111(10):2399–2407
Brown JM, Shah MN, Mackinnon SE (2009) Distal nerve transfers: a biology-based rationale. Neurosurg Focus 26(2):E12
de Cardoso MM, de Gepp RA, Mamare E, Guedes-Correa JF (2019) Results of phrenic nerve transfer to the musculocutaneous nerve using video-assisted thoracoscopy in patients with traumatic brachial plexus injury: series of 28 cases. Oper Neurosurg 17(3):261–267
Dong C, Gao W, Jia R, Li S, Shen Z, Li B (2013) Reconstruction of anorectal function through end-to-side neurorrhaphy by autonomic nerves and somatic nerve in rats. J Surg Res 180(2):e63-71
Dong C, Zhu P, Xie Z, Fan Z, Dong Z (2018) Reinnervation of the rectum with transfer of the genital branch of the genitofemoral nerve to the pelvic nerve in rats. J Neurosurg Spine 28(5):562–567
Gomez-Amaya SM, Barbe MF, Brown JM, Lamarre NS, Braverman AS, Massicotte VS, Ruggieri MR (2015) Bladder reinnervation using a primarily motor donor nerve (femoral nerve branches) is functionally superior to using a primarily sensory donor nerve (genitofemoral nerve). J Urol 193(3):1042–1051
Gustafson KJ, Zelkovic PF, Feng AH, Draper CE, Bodner DR, Grill WM (2005) Fascicular anatomy and surgical access of the human pudendal nerve. World J Urol 23(6):411–418
Hibner M, Castellanos ME, Drachman D, Balducci J (2012) Repeat operation for treatment of persistent pudendal nerve entrapment after pudendal neurolysis. J Minim Invasive Gynecol 19(3):325–330
Hollingshead JRF, Dudding TC, Vaizey CJ (2011) Sacral nerve stimulation for faecal incontinence: results from a single centre over a 10-year period. Colorectal Dis 13(9):1030–1034
Livshits A, Catz A, Folman Y, Witz M, Livshits V, Baskov A, Gepstein R (2004) Reinnervation of the neurogenic bladder in the late period of the spinal cord trauma. Spinal Cord 42(4):211–217
Mahakkanukrauh P, Surin P, Vaidhayakarn P (2005) Anatomical study of the pudendal nerve adjacent to the sacrospinous ligament. Clin Anat 18(3):200–205
de Mendonça CM, Gepp R, Correa JFG (2016) Outcome following phrenic nerve transfer to musculocutaneous nerve in patients with traumatic brachial palsy: a qualitative systematic review. Acta Neurochir 158(9):1793–1800
Nyangoh Timoh K, Bessede T, Lebacle C et al (2017) Levator ani muscle innervation: anatomical study in human fetus. Neurourol Urodyn 36(6):1464–1471
Ploteau S, Perrouin-Verbe M-A, Labat J-J, Riant T, Levesque A, Robert R (2017) Anatomical variants of the pudendal nerve observed during a transgluteal surgical approach in a population of patients with pudendal neuralgia. Pain Physician 20(1):E137–E143
Pokorný D, Jahoda D, Veigl D, Pinskerová V, Sosna A (2006) Topographic variations of the relationship of the sciatic nerve and the piriformis muscle and its relevance to palsy after total hip arthroplasty. Surg Radiol Anat 28(1):88–91
Rab M, Mader N, Kamolz LP, Hausner T, Gruber H, Girsch W (1997) Basic anatomical investigation of semitendinosus and the long head of biceps femoris muscle for their possible use in electrically stimulated neosphincter formation. Surg Radiol Anat 19(5):287–291
Ruggieri MR, Braverman AS, Bernal RM, Lamarre NS, Brown JM, Barbe MF (2011) Reinnervation of urethral and anal sphincters with femoral motor nerve to pudendal nerve transfer. Neurourol Urodyn 30(8):1695–1704
Ruggieri MR, Braverman AS, D’Andrea L, Betz R, Barbe MF (2008) Functional reinnervation of the canine bladder after spinal root transection and genitofemoral nerve transfer at one and three months after denervation. J Neurotrauma 25(4):401–409
Ruggieri MR, Braverman AS, D’Andrea L, McCarthy J, Barbe MF (2008) Functional reinnervation of the canine bladder after spinal root transection and immediate somatic nerve transfer. J Neurotrauma 25(3):214–224
Ruggieri MR, Braverman AS, D’Andrea L, Simpkiss B, Kozin SH, Pontari MA, Betz R, Barbe MF (2006) Functional reinnervation of the canine bladder after spinal root transection and immediate end-on-end repair. J Neurotrauma 23(7):1125–1136
Sladjana UZ, Ivan JD, Bratislav SD (2008) Microanatomical structure of the human sciatic nerve. Surg Radiol Anat 30(8):619–626
Socolovsky M, Malessy M, Lopez D, Guedes F, Flores L (2017) Current concepts in plasticity and nerve transfers: relationship between surgical techniques and outcomes. FOC 42(3):E13
Thomas C, Lefaucheur J-P, Galula G, de Parades V, Bourguignon J, Atienza P (2002) Respective value of pudendal nerve terminal motor latency and anal sphincter electromyography in neurogenic fecal incontinence. Neurophysiol Clin/Clin Neurophysiol 32(1):85–90
Funding
This research did not receive any funding.
Author information
Authors and Affiliations
Contributions
AP: protocol/ project development, dissection, data collection or management, data analysis, manuscript writing/editing. RSB: dissection, data collection or management, manuscript writing/editing. DANB: dissection, data collection or management, manuscript writing/editing. RKAF: protocol/ project development, data collection or management, data analysis, manuscript writing/editing. FG: protocol/ project development, data collection or management, data analysis, manuscript writing/editing.
Corresponding author
Ethics declarations
Conflicts of interests
There are no conflicts of interests.
Ethics approval
This study has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) and was approved by our institutional ethics committee (n.60067316.6.0000.5258).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Povedano, A., Brown, R.S., Barbosa, D.A.N. et al. Anatomical feasibility of peripheral nerve transfer to reestablish external anal sphincter control – cadaveric study. Surg Radiol Anat 43, 785–793 (2021). https://doi.org/10.1007/s00276-020-02635-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-020-02635-z