Abstract
Objective
This study aimed to peruse anatomic features of the cranial aperture of the optic canal (CAOC) for obtaining an extended morphometric dataset in children.
Methods
Computed tomography images of 200 children were included in this retrospective work to analyze the shape, location and diameters of the CAOC.
Results
The CAOC area, width and height were observed as 17.53 ± 2.80 mm2, 6.12 ± 0.84 mm, and 4.35 ± 0.64 mm, respectively. The angle of the optic canal in axial plane was found as 39.28 ± 5.13°, while in sagittal plane as 16.01 ± 6.76°. The distance between the CAOC and the midsagittal line was 7.17 ± 1.48 mm. The CAOC was measured as 54.04 ± 5.23 mm and 42.55 ± 3.28 mm away from the anterior and lateral boundary of the anterior skull base, respectively. The CAOC shape was described as the tear-drop (186 foramina, 46.5%), triangular (156 foramina, 39%), oval (47 foramina, 11.8%), and round (11 foramina, 2.8%).
Conclusion
The depth, angle and diameter measurements belonging to the CAOC were changing according to its shape or demographic data (e.g., sex and age). Therefore, preoperative radiologic evaluation containing the shape, location and size of the CAOC should be considered by multidisciplinary operating teams in terms of surgical interventions such as implant positioning.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The optic canal’s proximal or cranial aperture (CAOC), transmits the ophthalmic artery, sympathetic fibers (around the ophthalmic artery), and optic nerve between the middle cranial base and orbital cavity [26, 41]. Due to traumatic (e.g., fracture) or pathologic lesions (e.g., craniopharyngioma, optic nerve compression, optic nerve meningioma, optic glioma, mucocele, and ophthalmic aneurysm) located around the CAOC, surgeons perform different surgical procedures such as the endoscopic endonasal transsphenoidal approach, pterional approach, subfrontal approach, and supraorbital approach [7, 8, 19, 35, 38, 44, 47]. The sellar region including the CAOC has an extremely complex structure leading to technical challenge in application of those approaches, but morbidity and mortality rates decrease owing to the developing technology (e.g., intraoperative image-guidance/neuronavigation), increasing anatomical knowledge, and novel surgical methods [12, 14, 18, 27, 29]. Despite these positive advances, one of the main criticisms is that the vast majority of information in the literature (e.g., surgical approach analysis, morphometric evaluations, and exhaustive anatomical definitions) is obtained from limited age groups (mostly adults) [3,4,5, 9]. In this context, further investigation conducted on children may be useful for surgeons to avoid complications (e.g., the optic nerve or ophthalmic artery injury).
Some pathologic lesions such as craniopharyngioma, optic glioma, and optic nerve compression are diagnosed in childhood [8, 9, 38]. Unintentional damage of the optic nerve is one of the common complications during the treatment of those lesions [7, 9, 19]. Considering the differences of pediatric patients compared to adult cases (e.g., management, types or metastasis pattern of tumoral lesions, and also anatomic features of the skull base) [13, 39, 45, 46], safe dissection route in children should be known in detail by surgeons [9, 28, 31, 40]. However, the majority of existing articles provided information based on adult subjects [3, 9]. This situation may create difficulties for surgeons familiar with adult data (depth, angle and diameter measurements) to ensure surgical orientation in children (e.g., implant positioning) [9]. In this context, similar guidelines with adult reference measurements should be adapted to children to position the patient, to choose the appropriate approach, and to treat lesions [9, 13, 39, 45, 46]. An extended morphometric dataset focused on characteristic features of the CAOC in pediatric patients may be helpful for otolaryngologist, neurosurgeons, and ophthalmologist to better enlighten safe dissection zone. The present work aimed to peruse the growth pattern of the CAOC in pediatric subjects between the ages of one and 20.
Materials and methods
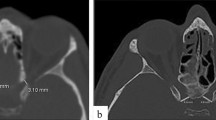
The ethical approval was obtained from the review committee of Mersin University for the present retrospective computed tomography (CT) study (2020/412). The inclusion criteria of the work were planned as follows: (a) 200 (100 boys and 100 girls) pediatric patients aged from one to 20 years, (b) ten patients, five boys and girls, for all ages, (c) patients without fractures, infectious diseases, tumoral lesions, vascular disorders, syndromic or genetic malformations, (d) patients with good quality CT images, and (e) files of child patients who admitted to the hospital between January 1, 2019 and December 30, 2019. In this context, the files (which contained information about hospital admission date, anamnesis data, complaints, sex, age, radiological images, diagnostic info, management procedures, and hospital discharge date) of 4592 pediatric patients (2859 boys and 1733 girls) with different grievances (blow to head, dizziness, falling from high, headache, etc.) in 2019 were reviewed retrospectively. The exclusion criteria consisted of the following: (a) patients after 200 (100 boys and girls) randomly chosen pediatric subjects aged from one to 20 years, (b) patients after 10 (five boys and girls) randomly chosen child subjects for all ages, (c) patients over 20 years, (d) patients with fractures in craniofacial bones (e.g., orbit or skull base), (e) patients with syndromic, vascular or genetic malformations (e.g., aneurysms or Crouzon syndrome), (f) patients with infectious diseases, (g) patients with tumoral lesions (e.g., craniopharyngioma), and (h) patients with low quality CT images. The raw data from CT scans (64-slice scanner, Aquillion 64, matrix: 512 × 512, FOV: 240 mm, pixel size: 0.46 mm, 0.5 mm thick slices, 230 mA, 120 kV, 0.3 mm interval, Toshiba Medical Systems Tokyo, Japan) were reformatted in different planes to form three-dimensional multiplanar reconstruction images. All measurements were performed by the same neuroradiologist (BT) once in different images (sagittal, coronal and axial planes). In 200 randomly chosen pediatric subjects (sex: 100 boys and girls, age: 10.50 ± 5.77 years) according to the inclusion and exclusion criteria, the following parameters were measured (Figs. 1, 2):
-
The height (vertical diameter) of the CAOC in coronal plane on CT.
-
The width (horizontal diameter) of the CAOC in coronal plane on CT.
-
The surface area of the CAOC in coronal plane on CT.
-
The distance (Dis-to-AB) between the CAOC (the frontmost point) and the anterior boundary of the anterior skull base in axial plane on CT.
-
The distance (Dis-to-LB) between the CAOC (the most lateral point) and the lateral boundary of the anterior skull base in axial plane on CT.
-
The distance (Dis-to-MSL) between the CAOC (the most medial point) and the midsagittal line in axial plane on CT.
-
The angle (Ang-in-AP) between the optic canal and sagittal horizontal line (i.e., the midline axis) in axial plane on CT.
-
The angle (Ang-in-SP) between the optic canal and sagittal horizontal line (i.e., the line parallel to the ground) in sagittal plane on CT.
-
The CAOC shape in coronal plane on CT.
The normality control of the morphometric values belonging to the CAOC parameters was checked with Shapiro–Wilk test. One-way ANOVA (post-hoc Bonferroni test) was used to examine the changes in the CAOC parameters during the transition from 1 to 20 years (Table 1), and from infancy period to postpubescent period (patients aged between 0 and 2 years: infancy group, patients aged between 3 and 5 years: early childhood group, patients aged between 6 and 9 years: later childhood group, patients aged between 10 and 13 years: prepubescent group, and patients aged between 14 and 20 years: postpubescent group) (Table 2) [5, 43]. Moreover, those tests were utilized to display the alterations of the parameters relative to the CAOC shape (Table 3). Right–left (the paired test) or boys–girls comparisons (the independent test) were carried out with Student’s t test (Table 4). Correlations between the depth, angle and diameter measurements of the CAOC were analyzed with Pearson correlation coefficient test (Table 5). The relation of the CAOC shape with child growth periods (between infancy period to postpubescent period) were evaluated with Chi square test (Table 6). The regression equations and scatter plots related to the CAOC parameters were obtained via the simple linear regression test. The “p < 0.05” was set as threshold during statistical evaluations.
Results
The numerical values related to the CAOC parameters were presented as mean data ± standard deviations in tables due to the normal distribution. Our findings were as follows:
-
All parameters (depth, angle and diameter measurements) varied depending on growth in childhood (Table 1). The CAOC height, Ang-in-AP, and Ang-in-SP were decreasing during the transition from 1 to 20 years, while the CAOC width, Dis-to-AB, Dis-to-LB, and Dis-to-MSL were increasing (p < 0.001) (Table 1).
-
The CAOC height, CAOC width, Ang-in-SP, and Dis-to-MSL did not alter from prepubescent period, while the Ang-in-AP did not vary from early childhood period. The surface area of the CAOC displayed irregular alteration between child age groups. The Dis-to-LB did not vary from the age of the six (i.e., later childhood period), while the Dis-to-AB were increasing proportionally with age between infancy and postpubescence (Table 2).
-
The CAOC shape were described as the tear-drop (186 foramina, 46.5%), triangular (156 foramina, 39%), oval (47 foramina, 11.8%), and round (11 foramina, 2.8%) (Figs. 3, 4).
-
The CAOC shape in boys was observed as the triangular (86 foramina, 43%) > tear-drop (83 foramina, 41.5%) > oval (27 foramina, 13.5%) > round (four foramina, 2%), while in girls as the tear-drop (103 foramina, 51.5%) > triangular (70 foramina, 35%) > oval (20 foramina, 10%) > round (seven foramina, 3.5%).
-
The CAOC shape in right sides was found as the triangular (89 foramina, 44.5%) > tear-drop (81 foramina, 40.5%) > oval (24 foramina, 12%) > round (six foramina, 3%), while in left sides as the tear-drop (105 foramina, 52.5%) > triangular (67 foramina, 33.5%) > oval (23 foramina, 11.5%) > round (five foramina, 2.5%).
-
The measured parameters except that the CAOC area (p = 0.223) and Dis-to-LB (p = 0.225) varied according to the shapes of the CAOC (Table 3).
-
The parameters other than the Ang-in-SP (p = 0.348), Ang-in-AP (p = 0.730), and Dis-to-MSL (p = 0.118) were greater in boys than girls. In terms of sides, the parameters did not display significant differences (Table 4).
-
Correlation findings between the measurements are given in Table 5. Correlations did not found between the vertical and horizontal diameters (p = 0.052, r = 0.097), and between the Ang-in-SP and Ang-in-AP (p = 0.125, r = 0.077) (Table 5).
-
The CAOC shape distribution by child growth periods displayed that the age affected the shape (p < 0.001) (Table 6).
-
The CAOC height was smaller than the width (p < 0.001).
-
The Ang-in-SP was smaller than the Ang-in-AP (p < 0.001).
-
The Dis-to-AB was greater than the Dis-to-LB (p < 0.001).
-
The regression equations for the CAOC width (y = 5.760 + 0.035× years), the CAOC height (y = 4.626—0.026×years), the CAOC area (y = 17.678—0.014× years), the Ang-in-SP (y = 22.218—0.592×years), the Ang-in-AP (y = 41.085—0.172×years), the Dis-to-AB (y = 46.457 + 0.723×years), the Dis-to-LB (y = 40.247 + 0.220×years), and the Dis-to-MSL (y = 5.701 + 0.141 × years) were calculated (Figs. 5, 6).
Discussion
The anatomic features of the CAOC in association with neighboring structures such as the anterior clinoid process, prechiasmatic sulcus, optic strut may be important for surgeons during the management of tumoral entities such as meningioma and craniopharyngioma using transcranial or transnasal approaches [8, 18, 21, 29, 38]. For example, Beer-Furlan et al. used the pterional port approach for removal of the bones surrounding the optic nerve (anterior clinoid process and optic strut) to obtain 270° nerve decompression [2]. However, Shibata et al. used the endonasal transsphenoidal technique (endoscopically) to make urgent optic nerve decompression in an infant with craniopharyngioma [38]. Guthikonda et al. identified the prechiasmatic sulcus as four different types (a: wide-flat, b: wide-steep, c: narrow-flat, and d: narrow-steep), and suggested that the narrow-steep type was more suitable for the pterional approach, while the wide-flat type for the subfrontal approach [18]. Moreover, they stated that the narrow prechiasmatic sulcus was more suitable for transcranial procedures, whereas the wide sulcus for transnasal procedures [18]. Beger et al. studied on the prechiasmatic sulcus in pediatric cases aged from one to 20 years, and observed that the sulcal length (antero-posterior length of the sulcus) did not alter after birth, but the sulcal angle (the angle between the planum sphenoidale and prechiasmatic sulcus) was decreasing from the postpubescent period [5]. In the light of all these clinical comments, we thought that this morphometrically expanded study focused on the CAOC in children might be beneficial for multidisciplinary operating teams to understand alterations in its morphology in children aged between infancy to postpubescence periods, to avoid unintentional damage of neurovascular structures (e.g., the optic nerve), to guess its location and diameter in different age groups, and to fathom the difference of the sellar region in children from adults.
The CAOC area, width and height in this radiologic study were found as 17.53 ± 2.80 mm2, 6.12 ± 0.84 mm, and 4.35 ± 0.64 mm, respectively. The existing papers related to these parameters are given in Table 7, which displayed that limited studies focused on the CAOC anatomy in children were available in the literature [33]. Prado et al. stated that the diameter of the CAOC (3.76/3.79 mm for fetuses, 4.67/4.72 mm for children, and 5.24/5.43 mm for adults) were increasing from prenatal period to adulthood [33]. Our average values obtained from children aged with 10.50 ± 5.78 years were within the mean data range given for adults in previous articles (4.45–19.36 mm2 for the area, 4.59–7.38 mm for the width, and 3.60–5.17 mm for the height) [1, 6, 16, 20, 23, 26, 28, 33, 34, 36, 37, 40, 42]. The CAOC height and width did not change from the age of 10 (prepubescent period), while its area displayed irregular alteration between child growth periods. The existing papers (Table 7) displayed that the numerical data related to the CAOC in previous studies were quite different. Possible reasons for this situation were listed by Beger as follows: (a) measurement technique (e.g., CT and digital caliper), (b) demographic differences (e.g., sex and sides), and (c) specimen selection (e.g., pediatric or adult patients) [3]. Kalthur et al. observed that the diameters measured by caliper were greater than measured by CT [23]; however, Berlis et al. stated that the measurements obtained from CT and caliper were similar [6]. Kalthur et al. reported that the diameters in the left (4.51 ± 0.79 mm for the width, and 3.54 ± 0.71 mm for the height) were smaller than in the right (4.68 ± 0.86 mm for the width, and 3.67 ± 0.82 mm for the height) [23]. Kumar et al. found that the height in the left (4.82 ± 0.77 mm) was smaller than in the right (5.14 ± 0.73 mm) [26]. Similar to the other studies [3, 20, 33, 34, 36], we did not find significant differences between right and left sides. In addition, Kalthur et al. observed that the diameters in females (4.24 ± 0.67 mm for the width, and 3.51 ± 0.82 mm for the height) were smaller than in males (4.75 ± 0.83 mm for the width, and 3.63 ± 0.74 mm for the height) [23]. In this study, the parameters including the area, height and width of the CAOC were greater in boys than girls. Area calculation method might be one of the reasons that trigger the difference between studies [36, 42]. The area in the study of Tao et al. (4.45 ± 0.46 mm2, using computer morphometric analysis system) was smaller than in the study Radunovic et al. (19.36 ± 1.87 mm2, using “width×height×π” formula) [36, 42].
The Ang-in-AP and Ang-in-SP in this study were found as 39.28 ± 5.13° and 16.01 ± 6.76°, respectively. The existing articles related to the angles are given in Table 8, which showed that limited studies focused on the CAOC anatomy in children were available [21]. The Ang-in-AP in this work was compatible with the value (39.5°) of Harwood-Nash’s work focused on children [21]. Our average values obtained from children were within the mean data range given for adults in previous articles (29.56–45.32° for the Ang-in-AP, and 10.27–18.2° for the Ang-in-SP) [6, 11, 15, 16, 21, 22, 31, 32, 40, 48]. Guthikonda et al. suggested that the position of the nerve might change due to the optic canal disruption caused by tumoral lesions; in such cases, the safest way of intervention in avoiding iatrogenic injury might be the normal course of the nerve [18]. The Ang-in-SP did not alter from the age of 10 (prepubescent period), while the Ang-in-AP did not vary from the age of three (early childhood period). The Ang-in-SP in infants (24.87 ± 5.74°) was almost twice that of adolescents (12.81 ± 5.53° for postpubescent period), and in addition, the Ang-in-AP in infants was statistically greater than the other age groups. On the other hand, the Dis-to-MSL, Dis-to-AB, and Dis-to-LB in this study were found as 7.17 ± 1.48 mm, 54.04 ± 5.23 mm, and 42.55 ± 3.28 mm, respectively. Our measurement related to the Dis-to-MSL was compatible with the previous measurements (at mean range: 6.48–7.48 mm) (Table 8) [11, 16, 31, 48]. The orbital depth measurements (i.e., the Dis-to-AB) were presented between 39–50 mm in the previous articles [1, 10, 24, 25, 30, 31]. Selection of different reference points during the depth measurements may be the reason that trigger the difference between studies (Table 9) [9]. Chang et al. measured orbital floor depth (the distance between the inferior orbital rim and the optic canal’s inferomedial rim) in children aged between 3 months and 18 years using magnetic resonance imaging views, and found that the depth was increasing rapidly until 6 years, and it seemed to reach almost adult size at 13 years [9]. The Dis-to-MSL did not alter from the age of 10 (prepubescent period), while the Dis-to-LB did not change from the age of six (later childhood period). In addition, the Dis-to-AB were increasing in the transition from infancy period to postpubescent period. In a nutshell, these angular and locational changes in childhood should be kept in mind by surgeons in terms of interventions such as implant positioning.
The CAOC shapes were described as the tear-drop in 186 cases (46.5%), triangular in 156 cases (39%), oval in 47 cases (11.8%), and round in 11 cases (2.8%). The existing papers containing shape classifications are given in Table 10, which showed that shape definitions were quite different between the studies. The CAOC was described as elliptical in all skulls by Akdemir et al. and Maniscalco and Habal, as oval by Govsa et al. and Purohit and Singh, and as round by Slavin et al. and Kumar et al. [1, 16, 26, 28, 34, 40]. In the study of Beger, the CAOC was identified as round (28%) and oval (72%) [3]. The shape of the CAOC was defined by Guseva and Denisov as triangular, polygonal, rhomboidal, horizontal–oval, and round [17]. Demographic information such as population, small-scale research, or imperfect techniques may be the reasons that trigger the difference in shape description between studies [16, 17, 28, 34]. Beger observed that the width of the CAOC in fetuses (round shape: 1.48 ± 0.34 mm, oval shape: 1.97 ± 0.61 mm) were varying according to the CAOC shape [3]. Except that the CAOC area and Dis-to-LB, the other parameters in this study varied according to the shapes of the CAOC. Moreover, the distribution of the CAOC shapes by child growth periods displayed that its shape was affected by age. Knowledge related to microsurgical anatomy of the CAOC may be useful for surgeons to prevent unintentional damage of neurovascular structures (the optic nerve, ophthalmic artery, etc.).
Conclusion
The CAOC height, CAOC width, Ang-in-SP, and Dis-to-MSL did not change from the age of 10 (prepubescent period). The Dis-to-LB did not change from the age of six (later childhood period), while the Dis-to-AB were increasing in the transition from infancy period to postpubescent period. In addition, the Ang-in-AP did not vary from the age of three (early childhood period). The depth, angle and diameter measurements belonging to the CAOC were changing according to the CAOC shape or demographic data (age and sex). From these perspectives, preoperative radiologic evaluation containing the shape, location and size of the CAOC may be beneficial for multidisciplinary operating teams during surgical interventions such as implant positioning.
References
Akdemir G, Tekdemir I, Altın L (2004) Transethmoidal approach to the optic canal: surgical and radiological microanatomy. Surg Neurol 62:268–274. https://doi.org/10.1016/j.surneu.2004.01.022
Beer-Furlan A, Evins AI, Rigante L, Burrell JC, Anichini G, Stieg PE, Bernardo A (2014) Endoscopic extradural anterior clinoidectomy and optic nerve decompression through a pterional port. J Clin Neurosci 21:836–840. https://doi.org/10.1016/j.jocn.2013.10.006
Beger O (2020) Assessment of the optic foramen shape and size in human fetuses. J Craniofac Surg 31:2021–2024. https://doi.org/10.1097/SCS.0000000000006610
Beger O, Taghipour P, Çakır S, Hamzaoğlu V, Özalp H, Kara E, Vayisoğlu Y, Dağtekin O, Dağtekin A, Bağdatoğlu C, Öztürk AH, Talas DÜ (2020) Fetal anatomy of the optic strut and prechiasmatic sulcus with a clinical perspective. World Neurosurg 136:e625–e634. https://doi.org/10.1016/j.wneu.2020.01.125
Beger O, Ten B, Balcı Y, Çakır S, Özalp H, Hamzaoğlu V, Vayisoğlu Y, Dağtekin A, Bağdatoğlu C, Talas DÜ (2020) A computed tomography study of the prechiasmatic sulcus anatomy in children. World Neurosurg 141:e118–e132. https://doi.org/10.1016/j.wneu.2020.05.023
Berlis A, Putz R, Schumacher M (1992) Direct and CT measurements of canals and foramina of the skull base. Brit J Radiol 65:653–661. https://doi.org/10.1259/0007-1285-65-776-653
Caporlingua A, Prior A, Cavagnaro MJ, Winston G, Oliveira DL, Sadwhani SD, Arias GA, Schwalb JN, Akhbari M, Evins AI (2019) The intracranial and intracanalicular optic nerve as seen through different surgical windows: endoscopic versus transcranial. World Neurosurg 124:522–538. https://doi.org/10.1016/j.wneu.2019.01.122
Cares H, Bakay L (1971) The clinical significance of the optic strut. J Neurosurg 34:355–364. https://doi.org/10.3171/jns.1971.34.3.0355
Chang JT, Morrison CS, Styczynski JR, Mehan W, Sullivan SR, Taylor HO (2015) Pediatric orbital depth and growth: a radiographic analysis. J Craniofac Surg 26:1988–1991. https://doi.org/10.1097/SCS.0000000000001974
Cheng A, Lucas P, Yuen H, Lam D, So K (2008) Surgical anatomy of the Chinese orbit. Ophthalmic Plast Reconstr Surg 24:136–141. https://doi.org/10.1097/IOP.0b013e31816704f5
Cheng Y, Liu M, Zhang S, Tian Y, Song D, Li Y, Luo Q (2013) Optic canal (OC) and internal carotid artery (ICA) in sellar region. Surg Radiol Anat 35:797–801. https://doi.org/10.1007/s00276-013-1193-2
Gagliardi F, Donofrio CA, Spina A, Bailo M, Gragnaniello C, Gallotti AL, Elbabaa SK, Caputy AJ, Mortini P (2016) Endoscope-assisted transmaxillosphenoidal approach to the sellar and parasellar regions: an anatomic study. World Neurosurg 95:246–252. https://doi.org/10.1016/j.wneu.2016.08.034
Gil Z, Constantini S, Spektor S, Abergel A, Khafif A, Beni-Adani L, Leonor T, DeRowe A, Fliss D (2005) Skull base approaches in the pediatric population. Head Neck 27:682–689. https://doi.org/10.1002/hed.20226
Giovannetti F, Mussa F, Priore P, Scagnet M, Arcovio E, Valentini V, Genitori L (2018) Endoscopic endonasal skull base surgery in pediatric patients. A single center experience. J Craniomaxillofac Surg 46:2017–2021. https://doi.org/10.1016/j.jcms.2018.09.013
Goldberg RA, Hannani K, Toga AW (1992) Microanatomy of the orbital apex: computed tomography and microcryoplaning of soft and hard tissue. Ophthalmology 99:1447–1452. https://doi.org/10.1016/S0161-6420(92)31785-3
Govsa F, Erturk M, Kayalioglu G, Pinar Y, Ozer M, Ozgur T (1999) Neuro-arterial relations in the region of the optic canal. Surg Radiol Anat 21:329–335. https://doi.org/10.1007/BF01631334
Guseva Y, Denisov S (2006) Structure of the optic canal in human ontogenesis. Ann Anat 188:103–116. https://doi.org/10.1016/j.aanat.2005.05.007
Guthikonda B, Tobler WD Jr, Froelich SC, Leach JL, Zimmer LA, Theodosopoulos PV, Tew JM Jr, Keller JT (2010) Anatomic study of the prechiasmatic sulcus and its surgical implications. Clin Anat 23:622–628. https://doi.org/10.1002/ca.21002
Güler TM, Yılmazlar S, Özgün G (2019) Anatomical aspects of optic nerve decompression in transcranial and transsphenoidal approach. J Craniomaxillofac 47:561–569. https://doi.org/10.1016/j.jcms.2019.01.027
Hart CK, Theodosopoulos PV, Zimmer LA (2009) Anatomy of the optic canal: a computed tomography study of endoscopic nerve decompression. Ann Otol Rhinol Laryngol 118:839–844. https://doi.org/10.1177/000348940911801203
Harwood-Nash D (1970) Axial tomography of the optic canals in children. Radiology 96:367–374. https://doi.org/10.1148/96.2.367
Hayashi N, Masuoka T, Tomita T, Sato H, Ohtani O, Endo S (2004) Surgical anatomy and efficient modification of procedures for selective extradural anterior clinoidectomy. Minim Invasive Neurosurg 47:355–358. https://doi.org/10.1055/s-2004-830121
Kalthur S, Periyasamy R, Kumar S, Gupta C, D’souza AS, (2015) A morphometric evaluation of the optic canal: Comparative study between computerized tomographic study and direct anatomic study. Saudi J Med Med Sci 3:204. https://doi.org/10.4103/1658-631X.161997
Karampatakis V, Natsis K, Gigis P, Stangos N (1998) Orbital depth measurements of human skulls in relation to retrobulbar anesthesia. Eur J Ophthalmol 8:118–120. https://doi.org/10.1177/112067219800800212
Katsev DA, Drews RC, Rose BT (1989) An anatomic study of retrobulbar needle path length. Ophthalmology 96:1221–1224. https://doi.org/10.1016/S0161-6420(89)32748-5
Kumar A, Tripathi A, Jain S, Khare S, Kaushik RK, Kausar H, Arora S (2019) Anatomical and morphometric study of optic foramen in north indian population. Natl J Clin Anat 8:053–056. https://doi.org/10.1055/s-0039-1689079
Locatelli M, Di Cristofori A, Draghi R, Bertani G, Guastella C, Pignataro L, Mantovani G, Rampini P, Carrabba G (2017) Is complex sphenoidal sinus anatomy a contraindication to a transsphenoidal approach for resection of sellar lesions? Case series and review of the literature. World Neurosurg 100:173–179. https://doi.org/10.1016/j.wneu.2016.12.123
Maniscalco JE, Habal MB (1978) Microanatomy of the optic canal. J Neurosurg 48:402–406. https://doi.org/10.3171/jns.1978.48.3.0402
Mazzatenta D, Zoli M, Guaraldi F, Ambrosi F, Fustini MF, Pasquini E, Asioli S, Zucchelli M (2020) Outcome of endoscopic endonasal surgery in pediatric craniopharyngiomas. World Neurosurg 134:e277–e288. https://doi.org/10.1016/j.wneu.2019.10.039
Mcqueen CT, Diruggiero DC, Campbell JP, Shockley WW (1995) Orbital osteology: a study of the surgical landmarks. Laryngoscope 105:783–788. https://doi.org/10.1288/00005537-199508000-00003
Öztürk A, Bozbuga M, Bayraktar B, Arı Z, Sahinoglu K, Polat G, Gürel I (1999) Surgical anatomy and morphometric analysis of the optico-chiasmatic apparatus, optic canal and sphenoid ridge. Okajimas Folia Anat Jpn 75:319–322. https://doi.org/10.2535/ofaj1936.75.6_319
Park S-J, Yoo J-N, Yoo M-S, Heo Y-C (2017) A study on double angle of optic foramen in the rhese method. J Korean Soc Radiol 11:313–319. https://doi.org/10.7742/jksr.2017.11.5.313
Prado PA, Ribeiro EC, De Angelis MA, Smith RL (2007) Biometric study of the optic canal during cranial development. Orbit 26:107–111. https://doi.org/10.1080/01676830600987540
Purohit BJ, Singh PR (2016) An osteologic study of cranial opening of optic canal in Gujarat region. J Clin Diagn Res 10:AC08-AC11. https://doi.org/10.7860/JCDR/2016/22110.8929
Puzzilli F, Ruggeri A, Mastronardi L, Agrillo A, Ferrante L (1999) Anterior clinoidal meningiomas: report of a series of 33 patients operated on through the pterional approach. Neuro Oncol 1:188–195. https://doi.org/10.1093/neuonc/1.3.188
Radunovic M, Vukcevic B, Radojevic N, Vukcevic N, Popovic N, Vuksanovic-Bozaric A (2019) Morphometric characteristics of the optic canal and the optic nerve. Folia Morphol 78:39–46. https://doi.org/10.5603/FM.a2018.0065
Rajeswaran SA, Mohanraj KG, Babu KY (2019) An osteologic study of the cranial opening of optic canal in dry human skulls. Drug Invent Today 12:2051–2053. https://doi.org/10.7860/JCDR/2016/22110.8929
Shibata T, Tanikawa M, Sakata T, Mase M (2018) Urgent optic nerve decompression via an endoscopic endonasal transsphenoidal approach for craniopharyngioma in a 12-month-old infant: a case report. Pediatr Neurosurg 53:182–187. https://doi.org/10.1159/000487086
Shlomi B, Chaushu S, Gil Z, Chaushu G, Fliss DM (2007) Effects of the subcranial approach on facial growth and development. Otolaryngol Head Neck Surg 136:27–32. https://doi.org/10.1016/j.otohns.2006.07.019
Slavin KV, Dujovny M, Soeira G, Ausman JI (1994) Optic canal: microanatomic study. Skull Base Surg 4:136–144. https://doi.org/10.1055/s-2008-1058965
Standring S, Borley N, Collins P, Crossman A, Gatzoulis M, Healy J (2008) Gray’s anatomy: the anatomical basis of clinical practice. Elsevier, London
Tao H, Ma Z, Dai P, Jiang L (1999) Computer-aided three-dimensional reconstruction and measurement of the optic canal and intracanalicular structures. Laryngoscope 109:1499–1502. https://doi.org/10.1097/00005537-199909000-00026
Ten B, Beger O, Direk MÇ, Balcı Y, Çiçek F, Özalp H, Hamzaoğlu V, Temel G, Vayisoğlu Y, Bağdatoğlu C, Talas DÜ (2020) Radiologic analysis of the location, shape and size of the external aperture of the carotid canal in children. Surg Radiol Anat 42:749–759. https://doi.org/10.1007/s00276-020-02448-0
Vohra ST, Escott EJ, Stevens D, Branstetter BF (2011) Categorization and characterization of lesions of the orbital apex. Neuroradiology 53:89–107. https://doi.org/10.1007/s00234-010-0712-7
Wasserzug O, DeRowe A, Ringel B, Fishman G, Fliss DM (2018) Open approaches to the anterior skull base in children: review of the literature. J Neurol Surg B Skull Base 79:42–46. https://doi.org/10.1055/s-0037-1621739
Weninger WJ, Müller GB (1999) The parasellar region of human infants: cavernous sinus topography and surgical approaches. J Neurosurg 90:484–490. https://doi.org/10.3171/jns.1999.90.3.0484
Yilmazlar S, Saraydaroglu O, Korfali E (2012) Anatomical aspects in the transsphenoidal–transethmoidal approach to the optic canal: an anatomic–cadaveric study. J Craniomaxillofac Surg 40:e198–e205. https://doi.org/10.1016/j.jcms.2011.10.008
Zhang H, Liu X, Cheng Y, Zhang S, Wang C, Cui D, Li Y, Fu Y, Wang Y (2013) A new method of locating the optic canal based on structures in sella region: computed tomography study. J Craniofac Surg 24:1011–1015. https://doi.org/10.1097/SCS.0b013e318287d228
Acknowledgements
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
BT, OB, KE, EK, DÜT: Project development, Data collection, Data analysis, Manuscript writing, Manuscript editing. SSA, ECH, FÇ, PT, YV: Data analysis, Manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ten, B., Beger, O., Esen, K. et al. Anatomic features of the cranial aperture of the optic canal in children: a radiologic study. Surg Radiol Anat 43, 187–199 (2021). https://doi.org/10.1007/s00276-020-02604-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-020-02604-6