Abstract
Purpose
The importance of the infraorbital canal in the growth of the maxilla and associated mid-facial region has significance for innervation of this region as well as the associated dentition, yet little is known about the development of the canal. An analysis of its dimensions and morphology during the late prenatal and early postnatal periods was thus undertaken. The aim of this study was to describe changes in the morphology, size and branching pattern of the infraorbital canal during the late prenatal and early postnatal stages of human growth.
Methods
Fifty human fetal and neonatal maxillae were analyzed. The sample included 27 late prenatal individuals (30 gestational weeks and birth) and 23 early postnatal individuals (birth and 1 year). Maxillae were scanned using a Nikon XTH 225 L micro-CT unit and analyzed using VG studiomax v3.2. Measurements included the maximum width, height and surface area of each foramen associated with the infraorbital canal and the total length of the canal, bilaterally.
Results
All the measurements of the canal were greater in the early postnatal group than in the late prenatal group, while the walls and branching pattern of the canal were better developed in the postnatal group. Bone development occurred within the walls as development proceeded. Variations in the branching pattern of the canal were found.
Conclusion
The morphology of the infraorbital canal reflected the developmental stage of associated structures such as the dentition, maxillary sinus and orbit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The human infraorbital canal serves an important role in the development and growth of the maxilla. While the role of the canal as a conduit for neurovascular elements is well defined, little information exists as to how its development and growth may influence the development and growth of the maxilla. As the infraorbital canal is an important landmark for anesthesia in dental interventions, oto-rhino-laryngology and maxillofacial procedures, precise knowledge of the changes in its morphology and subsequent distribution of the contained neurovascular structures is of clinical importance [4, 10, 14, 17].
The late prenatal and early postnatal periods of human growth are of particular importance given the functional changes which occur within the maxillofacial region during this stage of life. The onset of masticatory forces during this period is associated with the development of the dentition which is initiated by the development of nervous tissue at specific sites [8]. Progression of these nerve paths facilitates the development of structures such as the infraorbital canal [8].
The growth of structures forming the boundaries of the infraorbital canal may also influence its development and orientation [5, 7, 20, 26]. The canal’s course may be influenced by the width of the developing orbit and palate, the maxillary sinus, as well as the development of the associated dentition [7, 20, 26]. Furthermore, any deviation in the direction of the infraorbital canal may result in deviation in the process of eruption of the maxillary canines, or cause canine-premolar transposition [7].
Information from adult populations restricts the depth of inferences that may be made regarding the role of early development and growth of the canal on the maxilla in cases of both normal and abnormal growth patterns. While knowledge of the infraorbital nerve distribution is important for bilateral infraorbital nerve block for early repair of facial clefts in neonates, and in pre- and post-operative management of pain in the repair of facial clefts [1, 5, 28], few descriptions of the infraorbital neurovascular distribution in neonates are available. Thus, knowledge of the developmental patterns of the infraorbital canal will assist in interpreting the normal and aberrant development of the innervation and vascular networks supplying the maxillary dentition and the mid-facial region [11, 15, 29]. In addition, studying the growth of the infra-orbital canal across the perinatal stages may provide increased comprehension of existing growth relationships the canal and it’s contained neurovascular structures may share with adjacent anatomical spaces such as the maxillary sinuses. Thus, the purpose of this study was to investigate the changes in the morphology of the infraorbital canal specifically during the functionally intricate late prenatal and early postnatal periods.
Materials and methods
Specimens from the School of Anatomical Sciences, University of the Witwatersrand’s Pediatric Collection and the Raymond A. Dart Collection of Human Skeletons were used in this study. These Collections have historically comprised both donated and unclaimed bodies, but more recently have transitioned to a purely donated population [18]. All bodies fall under the National Health Act, 2004 of the Republic of South Africa [24]. Ethics clearance to utilize cadavers and skeletonized specimens for this study was obtained from the University of the Witwatersrand’s Human Research Ethics Committee (HREC-medical, W-CJ-140604-1).
Sample
A total of 50 fetal and neonatal maxillae of intact human skulls were assessed. Thirty-two cadavers were sourced from the Pediatric Collection and a further 18 skulls were obtained from the Raymond A. Dart Collection of Human Skeletons. Maxillae with any evidence of congenital abnormalities, general damage, pathologies or surgical intervention were excluded from this study.
The sample ranged in age between 30 gestational weeks and 1 year postnatal. Age estimations utilized the methods of Alqahtani et al. [3], Hansen et al. [12] and Lubchenco et al. [19]. The sample was subdivided into a late prenatal (30 gestational weeks to birth) and an early postnatal (birth to 1 year) age group based on the contrasting biomechanical demands of the two periods of growth [21].
Methods
All maxillae were scanned at the MIXRAD (Micro-focus X-ray Radiography and Tomography) facility at the South African Nuclear Energy Corporation (NECSA), using a Nikon XTH 225L micro-CT X-ray unit (Nikon Metrology, Leuven, Belgium). The scanning parameters were optimized for the highest spatial resolution by conducting region of interest scans. The scanning conditions ranged between 150 kV/148 µm and 150 kV/100 µm to compensate for the varying sample sizes. A 0.25 mm thick copper filter was used to optimize X-ray penetrations through the specimens [13]. The specimens were secured in place by mounting them in a polystyrene mold. Scanning of the specimen in 360° was facilitated by placing the mounted specimen on a rotating sample manipulator. The maximum magnification improved the visualization of the structures that were analyzed. Nikon CTPro software (Nikon Metrology, Leuven, Belgium) was utilized to reconstruct the scans of the specimens. The scans were then imported into VGStudio Max v2.3 (Volume graphics GmbH, Heidelberg, Germany) as volume files [13].
Subsequent to volume file reconstruction, a surface determination was applied to all scans prior to the alignment of the slices to a particular reference plane and the insertion of markers. The surface determination allowed for the clear selection of skeletal landmarks on the skull. In order to standardize the orientation of the micro-CT slices, a vertical reference plane was inserted in relation to the nasal aperture by selecting three fiducial points on the skull, namely the nasion and the two piriform curvatures. The coronal slices were then aligned to the vertical reference plane (Fig. 1) while the transverse slices were aligned to the Frankfort horizontal plane.
A reference plane (red grid) inserted in relation to the nasal aperture of an early postnatal individual. The plane was inserted in the coronal plane by selecting three fiducial points on the skull. (Fiducial points: nasion (ns) (most anterior point of the frontonasal suture, intersecting with the internasal suture) and the piriform curvature (pc) (the most infero-lateral points of the piriform aperture)
Measurements
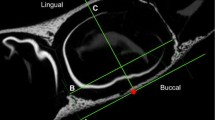
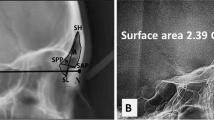
In the assessment of the dimensions of the infraorbital canal, an “internal” and an “external” infraorbital foramen were defined bilaterally. In order to undertake specific measurements, the infraorbital foramen proper was termed the “external” foramen for the purposes of this study, while the “internal” infraorbital foramen, situated at the internal opening of the canal was defined to measure the length of the canal. Both the “internal” and “external” infraorbital foramina were identified through serial visual scanning of the region. The openings were determined as the position where all boundaries of the foramina were evident. A horizontal reference plane was established (Fig. 2a), parallel to the horizontally aligned palatine processes of the maxilla, to assist in the assessment of the maximum width of each foramen. The maximum width of each foramen was measured parallel to the horizontal reference plane. The maximum width, maximum height and surface area of the “internal” and “external” infraorbital foramina, respectively, were measured bilaterally in the coronal plane (Fig. 2b). The maximum width of each foramen was measured from its most lingual to its most buccal point in the horizontal plane, while the maximum height of the foramen was measured from the most superior to the most inferior point, perpendicular to the maximum width (Fig. 2b). The surface area of each foramen was calculated using the VG studiomax software. For the measurement of the length of the canal between the “external” and “internal” foramina, all slices were aligned to the midpoint of the “external” foramen in the sagittal plane (Fig. 2c). In cases where the canal had an internal angulation in its floor, a marker was inserted at the point where the angle of the canal changed (Fig. 2d).
Measurements of the infraorbital canal. a Coronal section indicating the horizontal reference plane (yellow), inserted by selecting a minimum of three reference points along the most horizontal plane of the nasal floor, where the triangular median palatal process fuses with the secondary palate. b Coronal section indicating the maximum height (A) and width (B) of the right “external” infraorbital foramen of a late postnatal individual. Note that all the boundaries of the foramen are evident. c and d Sagittal view of a left infraorbital canal in early postnatal individuals. c The canal does not have an internal angulation. The length of the infraorbital canal was thus measured between the midpoints of the “external” (C) and “internal” (D) infraorbital foramina. d An internal angulation in the floor of the canal is evident. The measurements were taken from the internal angulation marker (F) to the midpoint of the “internal” (G) and “external” (E) infraorbital foramina, respectively, and then summed to determine the total length of the canal. [Note that the position of the boundaries of the “internal” and “external” infraorbital foramina was determined in the coronal plane]
The assessment of the morphology of the walls of the canal was standardized by aligning the transverse slices to the mid-point of the height of the “internal” foramina. The sagittal slices were aligned to the mid-point of the width of the “internal” foramina. The integrity of the walls of the canal were classified as being absent (no bone is present at the posterior end of the canal), patchy (the wall consists of bony islets and may show signs of completion in certain areas, but is still incomplete) or complete (definitive bony walls without any defect) (Fig. 3).
Integrity of walls of the infraorbital canal. a and b Sagittal view indicating the roof and floor of the right infraorbital canal in early postnatal individuals. a Note that the roof of the canal is absent posteriorly (as indicated by the yellow arrows). b The roof is complete along the entire length of the canal. c and d Transverse view of the infraorbital canal in a late prenatal (c) and early postnatal (d) individual. The integrity of both the medial and lateral wall of the infraorbital canal in the late prenatal individual (c) have bony islets (indicated by yellow arrows), while the integrity of the lateral and medial wall of the infraorbital canal in the early postnatal individual (d) are complete
In order to comprehensively study the branching pattern (i.e. the secondary branches and their origin from the main infraorbital canal) of the canal bilaterally within the maxilla, the infraorbital region of interest was expanded beyond the canal to include its associated branches to the orbit, maxillary sinus and dentition. This was achieved by aligning the slices to a coronal plane. Utilizing three-dimensional extraction, each branch of the canal was traced from its point of origin to the specific region of the maxilla where it terminated. The number of branches, their frequency of occurrence and the structure/s to which they led were recorded. When possible, each specific tooth to which the branch of the infraorbital canal led was noted. In cases where the branches of the canal did not completely extend to a specific tooth, the region where the canal was located was classified as “heading towards either the anterior or posterior dentition” or as “undifferentiated”.
Data analysis
All statistical analysis was performed with the use of PAST (Paleontological Statistics) (Paleontological Association, London, UK) and SPSS (Statistical Package for the Social Sciences) v.25 (IBM, Armok, NY, USA) statistical programs. To assess if the data was parametric in nature, a Shapiro–Wilk’s test was performed. A paired sample “t”-test compared all measurements on the left and right side. As the data was found to be parametric and no significant differences were observed between left and right, descriptive statistics (mean and standard deviation) were calculated for the mean value (left and right combined) of each measurement of the infraorbital canal.
A multivariate analysis of variance (MANOVA) with a Tukey’s post hoc test allowed for the assessment of variance between dependent (measurements) and independent variables (age). A value of p ≤ 0.05 was considered to be statistically significant. Qualitative data, such as the branching pattern of the infraorbital canal and frequency of occurrence of each branch directed towards a specific structure/s, was assessed using contingency and frequency tables.
A Lin’s concordance correlation co-efficient of reproducibility was conducted on 10% of the sample [2]. Values ranging between 0.8 and 1 were considered as acceptable levels of repeatability.
Results
A high level of inter-observer repeatability (74–100%) was observed across all the measurements. While the potential influence of asymmetry in the human form is acknowledged, the assessment of differences between left and right measurements indicated no significant differences and thus measurements were combined.
A general increase in the size of all the dimensions of the infraorbital canal in the early post-natal group was observed compared to the late prenatal group (Table 1). No significant differences (p ≤ 0.05) were observed between the age groups in terms of the measurements of the dimensions of the “internal” foramen and length of the canal. However, the mean surface area (p ≤ 0.024) and the maximum width (p ≤ 0.052) of the “external” foramen were significantly larger than in the late prenatal group.
A single infraorbital canal extending from the “internal” infraorbital foramen to the “external” infraorbital foramen was observed bilaterally in all individuals. The infraorbital canal coursed in an antero-medial direction. The anterior portion of the canal was directed medially and inferiorly and emerged on to the external surface of the skull as the “external” infraorbital foramen. An internal angulation in the sagittal plane was observed in the floor of the infraorbital canal in only 14 individuals (late prenatal: n = 9/27; early postnatal: n = 5/23). An accessory “external” foramen was observed lateral to the main infraorbital foramen in only one late prenatal individual (5%) on the left side of the skull (Fig. 4).
The integrity of the walls of a late prenatal (b and d) and an early postnatal (a and c) individual. a and b Sagittal view indicating the roof and floor of the left infraorbital canal. Note that the roof of the infraorbital canal in the late prenatal individual (b) is absent along the posterior third of the canal (yellow arrows), while the roof of the infraorbital canal in the early postnatal individual (a) is complete. c and d Transverse view indicating the medial and lateral walls of the left infraorbital canal. Note that the bone forming the walls of the infraorbital canal in the late prenatal individual (b and d) has a consistent appearance, while the bone surrounding the infraorbital canal in the early postnatal individual (a and c), has a trabeculated appearance. (Infraorbital canal is indicated by a green star)
The bony floor of the infraorbital canal appeared to be thick and well developed throughout its entire length in both age groups. The roof of the infraorbital canal was much thinner and in some cases patchy, particularly in the late prenatal group. A fully formed roof was present in only 10 prenatal (18.5%) and 6 postnatal (13.0%) canals (Fig. 5a). In the remaining individuals (68.5%), the absence of the roof extended between 25 and 50% of the posterior length of the canal, creating an infraorbital groove posteriorly (Fig. 5b). No noticeable difference in the degree of ossification was seen between the lateral and medial walls of the canal. The bone surrounding the canal had a homogenous appearance in the prenatal group and showed trabeculae in the early postnatal group across all surfaces (Fig. 5c, d).
Variations in branches and branching patterns of the canal were observed bilaterally within the same specimen as well as between different specimens (Table 2). The number of branches of the infraorbital canal varied between zero and three. The origin of these branches was dependent on the number of branches that were present. The most frequently noted branch in the prenatal group was a single branch directed towards the anterior dentition (anterior undifferentiated; 23.9%) (Table 2, variation 2a). The second most frequent branch was a single branch terminating at the canine (10.87%) and maxillary sinus (10.87%), respectively (Table 2, variations 2c and 2d), followed by two branches heading towards the anterior dentition (anterior undifferentiated; 8.7%) (Table 2, variation 6a). Similar to the prenatal group, the most frequently seen branch in the postnatal group was a single branch heading towards the anterior dentition (anterior undifferentiated; 14.8%), while the second and third most frequently seen branches were two branches terminating at the 1st premolar/1st deciduous molar (12.96%) and a single branch terminating at the 1st premolar/1st deciduous molar (11.11%), respectively (Table 2). A significant decrease in the incidence of the types of branching pattern (p ≤ 0.05) were observed in the early postnatal group when compared to the late prenatal, except for the increased (p ≤ 0.015) pattern found in the right undifferentiated anterior dentition branch.
Discussion
The development of the infraorbital canal and its branching pattern are clinically relevant within the context of the repair of cleft lip and palate and the administration of nerve blocks during pediatric maxillofacial surgery and surgical procedures of the maxillary dentition [10]. In addition, the growth and development of the canal is equally important in understanding the early growth of the mid-facial region of the skull [20].
All the dimensions of the infraorbital canal were found to be larger in the postnatal group when compared to the prenatal group in the current study. However, only the surface area of the external foramina and their width were statistically significantly larger. By the postnatal stage, the width of the “external” infraorbital foramen appears to have achieved approximately 64–69% of the width of the foramen in an adult population [9]. Moss and Greenberg [20] showed that bone modeling and remodeling in the area of the infraorbital canal and “external” infraorbital foramen occurs as a result of biomechanical forces of surrounding structures such as the growth of palatal and dental structures. These craniofacial changes may explain the larger surface area and width of the “external” infraorbital foramen in the postnatal group in the current study.
As the maxillary tuberosity grows, the length of the infraorbital canal is increased by appositional growth at the posterior end of the canal [20]. Although previous studies [1, 10, 15] have measured the length of the infraorbital canal, it should be noted that the inner point of measurement of the canal was not clearly defined and thus may cause some bias when the length of the canal is compared between studies. Therefore an “inner foramen” was defined in the current study to better delineate the length measurement. The mean length of the infraorbital canal in the current study in both the prenatal and postnatal groups is over double the length measured by Farah and Faruqi [10] in fetuses between the ages of 16–34 weeks. This may be due to a difference in the definition of the position of the “internal” infraorbital foramen in the two studies. The length of the canal in the present study equates to only 21–27% of the average length in an adult population [1, 14] indicating that major growth in length must occur postnatally.
In the present study, an internal angulation in the floor of the canal, was present in 14 of the immature individuals. An angulation between the infraorbital canal and the infraorbital groove has been documented by Hwang et al. [14] in adults. However, the angulation in the floor of the infraorbital canal in this study, may be indicative of the initiation of a change in growth in this region resulting in a declivity in the anterior portion of the canal as seen in adults [6, 20]. Although the exact cause of the internal angulation is unknown, the anterior and vertical growth of the cranium or the development of the maxillary dentition may affect the direction of the canal [7, 22].
Ossification of the canal walls was more advanced in the postnatal group than in the prenatal group, but variations occurred in both age groups. The roof of the canal was found to be complete in more prenatal than postnatal individuals. The absence of a roof along the posterior end of the canal (creating a groove) in some individuals marks the region of the infraorbital fissure in the floor of the orbit [20]. Considering that the roof of the infraorbital canal is formed by the floor of the orbit [25], the integrity and thinness of the roof of the canal may be a reflection of the development of the orbit during the perinatal stages. The presence of a well-developed canal floor, however, is not seen in all adult individuals as cases where the infraorbital nerve bulges into the maxillary sinus have been reported [29, 30]. Nunez-Castruita et al. [22] found that the lateral wall of the maxillary sinus (medial boundary of the canal) in embryos had a progressive ossification pattern. A variable degree of ossification along the medial and lateral wall is expected during the prenatal stages of development, as growth and bone deposition is still progressing [22]. Although the exact developmental stage at which closure of the canal begins is still unknown [5, 20], it is evident from this study that closure of the walls of the canal has already commenced at the late prenatal stages of development. Bone modeling and remodeling in the area of the infraorbital canal and the “external” infraorbital foramen are said to occur as a result of the growth forces exerted by surrounding structures such as the palate and the dentition [20] and may thus play an important role in the degree of ossification of the walls of the canal.
Variations in the number of branches and branching pattern of the canal bilaterally within the same specimen, as well as between different specimens, were found in this study. Similar variations were observed in prenatal studies of the maxillary nerve by Pearson [23] and Schwartz and Langdon [27]. Iwanaga et al. [16] reported an additional palpebral branch of the infraorbital nerve emanating from the superior surface of the infraorbital canal, innervating the lower eyelid. Although description of branches originating from the superior surface of the infraorbital canal are rare [16], in the present study a branch heading towards the orbit was observed in three individuals. A high incidence of a right undifferentiated branch in the prenatal group when compared to the postnatal group was also found. Branches of the canal in the prenatal group are still developing and have not yet reached their specific targets. More branches were found to be “undifferentiated” when compared to the postnatal group. Assessing the branching pattern of the infraorbital canal relative to the developing dentition is important, as the developmental fields of each tooth class are determined by the branching patterns of the anterior, middle and posterior superior alveolar nerves coursing through the infraorbital canal and its secondary branches during the prenatal development of the maxilla [27]. Knowledge of these branching patterns is valuable in pediatric patients who require maxillofacial surgery, particularly in cleft lip and palate repair as well as regional block anesthesia [5, 16, 28], where access to the maxillary sinuses may be required.
Limitations
The cross-sectional nature of the study limits the depth of inferences that can be made regarding development and growth trajectories of the maxilla. Furthermore, the sample size may be considered to be small.
Conclusion
In conclusion, the dimensions of the infraorbital canal were larger and the walls and bone surrounding the canal were more defined in the postnatal stages of growth. While the width of the external infraorbital canal had achieved approximately 64–67% of its adult size, it appears that the length of the canal will continue to increase relative to viscero-cranial growth following the postnatal period. Furthermore, the branching configuration of the infraorbital canal was better defined in concert with the more developed dentition in post-natal individuals. The description of the morphometry and branching patterns of the infraorbital canal during the late prenatal and early postnatal stages may provide valuable insights to predict aberrant growth patterns and neurovascular distributions in the development of the mid-facial region. The study also may assist with interpreting the normal, complex growth relationships between the craniofacial bones and associated spaces including the maxillary sinuses.
Data availability
Data related to the project is available in accordance with the terms and conditions as outlined by the Human Research Ethics Committee (medical) and the Collections Committee of the School of Anatomical Sciences, University of the Witwatersrand.
References
Açar G, Özen KE, Güler İ, Büyükmumcu M (2017) Computed tomography evaluation of the morphometry and variations of the infraorbital canal relating to endoscopic surgery. Braz J Otorhinolaryngol 84:713–721
Allan JC (1982) Learning about statistics: a primer in simple statistical methods for students of the medical, biological, paramedical, social and behavioural sciences, 1st edn. Macmillan, Johannesburg
Alqahtani SJ, Hector P, Liversidge HM (2010) Brief communication: the London atlas of human tooth development and eruption. Am J Phys Anthropol 142:481–490
Aziz SR, Marchena JM, Puran A (2000) Anatomic characteristics of the infraorbital foramen: a cadaver study. J Oral Maxillofac Surg 58:992–996
Bosenberg AT, Kimble FW (1995) Infraorbital nerve block in neonates for cleft lip repair: anatomical study and clinical application. Brit J Anaesthesia 74:506–508
Caspersen LM, Christensen IJ, Kjær I (2009) Inclination of the infraorbital canal studied on dry skulls expresses the maxillary growth pattern: a new contribution to the understanding of change in inclination of ectopic canines during puberty. Acta Odontol Scand 67:341–345
Caspersen LM, Christensen IJ, Kjær I (2010) Maxillary canine ectopia and maxillary canine premolar transposition are associated with deviations in the maxilla. Dent Anthropol 23:37–41
Chávez-Lomelí ME, Mansilla Lory J, Kjear I (1996) The human mandibular canal arises from three separate canals innervating different tooth groups. J Dent Res 75:1540–1544
Dağıstan S, Miloğlu Ö, Altun O, Umar EK (2017) Retrospective morphometric analysis of the infraorbital foramen with cone beam computed tomography. Niger J Clin Pract 20:1053–1064
Farah G, Faruqi NA (2008) Morphometric analysis of infraorbital foramen and infraorbital canal in human foetuses. Int J Morphol 26:289–29311
Fontolliet M, Bornstein MM, von Arx T (2019) Characteristics and dimensions of the infraorbital canal: a radiographic analysis using cone beam computed tomography (CBCT). Surg Rad Anat 41:169–179
Hansen K, Sung CJ, Huang C (2003) Reference values for second trimester fetal and neonatal organ weights and measurements. Paediatr Devel Pathol 6:160–167
Hutchinson EF, Farella M, Hoffman J, Kramer B (2017) Variations in bone density across the body of the immature human mandible. J Anat 230:679–688
Hwang K, Baik S (1999) Surgical anatomy of Korean adults. J Craniofac Surg 10:129–134
Hwan SH, Kim SW, Park CS, Kim S, Cho JH, Kang JM (2013) Morphometric analysis of the infraorbital groove, canal, and foramen on three-dimensional reconstruction of computed tomography scans. Surg Radiol Anat 35:565–571
Iwanaga J, Watanabe K, Oskouian RJ, Tubbs RS (2017) Previously undescribed palpebral branch from the infraorbital canal: application to surgery of the eyelid and treatment of orbital floor fractures. Clin Anat 30:835–838
Kazkayasi M, Ergin A, Ersoy M, Bengi O, Tekdemir I, Elhan A (2001) Certain anatomical relations and the precise morphometry of the infraorbital foramen, canal and groove: an anatomical and cephalometric study. Laryngoscope 111:609–614
Kramer B, Hutchinson EF, Brits DM, Billings BK (2019) Making the ethical transition in South Africa: acquiring human bodies for training in anatomy. Anat Sci Edu 12:264–271
Lubchenco LO, Hansman C, Boyd E (1966) Intrauterine growth in length and head circumference as estimated from live births at gestational ages from 26 to 42 weeks. Paediatrics 37:403–408
Moss M, Greenberg SN (1967) Functional cranial analysis of the human maxillary bone: I, Basal bone. Angle Orthod 37:151–164
Nowlan NC (2015) Biomechanics of foetal movement. Eur Cell Mater 29:1–21
Nunez-Castruita A, Lopez-Serna N, Lopez GS (2011) Prenatal development of the maxillary sinus: a perspective for paranasal sinus surgery. Otolaryngol Head Neck Surg 146:997–1003
Pearson AA (1977) The early innervation of the developing deciduous teeth. J Anat 123:563–577
Rene C (2006) Update on orbital anatomy. Eye 20:1119–1129
SA-NHA (2004) National Health Act No. 61 of 2003. Government Gazette, Republic of South Africa, Volume 469, No. 35099, 2 March 2012. Pretoria, South Africa: South African Government. 94 p. https://www.gov.za/sites/default/files/gcis_document/201409/a61-03.pdf. Accessed 25 Jan 2019
Scott JH (1959) Further studies on the growth of the human face. Proc Royal Soc Med 52:263–269
Schwartz JH, Langdon LH (1991) Innervation of the human upper primary dentition: implications for understanding tooth initiation and rethinking growth and eruption patterns. Am J Phys Anthropol 86:27–286
Tuncer FB, Jacob D, Papay F (2019) Anatomical location of the infraorbital foramen in infant dry skulls: implications for cleft surgery. J Craniofacial surg 30:e623–e625
Uzun Ç, Șanverdi ȘE, Üstüner E, Gürses MA, Șalvarlı Ș (2016) Evaluation of infraorbital canal anatomy and related anatomical structures with multi-detector Ct. J Ankara University Faculty of Medicine 69:89–93
Yenigun A, Gun C, Uysal II, Nayman A (2016) Radiological classification of the infraorbital canal and correlation with variants of neighboring structures. Eur Arch Otorhinolaryngol 273:139–144
Acknowledgements
Dr Frikkie de Beer, Mr Jakobus Hoffmann and Mr Lunga Bam of the South African Nuclear Energy Corporation (NECSA) are acknowledged for the use of the MIXRAD facilities. The authors also acknowledge that this work is based on research supported in part by the National Research Foundation of South Africa (Grant Number: 180501325183) as well as a grant from the Faculty of Health Sciences Research Committee (University of the Witwatersrand).
Funding
This work is based on research supported in part by the National Research Foundation of South Africa (Grant no. TTK180501325183) as well as a grant from the Faculty of Health Sciences Research Committee (University of the Witwatersrand).
Author information
Authors and Affiliations
Contributions
SS: Data collection and analysis; manuscript writing/editing; funding acquisition (postgraduate student). EFH: Conceptualization; methodology; manuscript writing, review and editing; funding acquisition; co-supervision. BK: Conceptualization; methodology; manuscript writing, review and editing; co-supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
Ethics clearance to utilize the cadavers and skeletonized specimens for this study was obtained from the University of the Witwatersrand’s Human Research Ethics Committee (HREC-medical, W-CJ-140604-1).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Smit, S., Hutchinson, E.F. & Kramer, B. A morphometric analysis of the immature human infraorbital canal. Surg Radiol Anat 43, 201–210 (2021). https://doi.org/10.1007/s00276-020-02563-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-020-02563-y