Abstract
Purpose
The aim of our study was to clarify the origin of the inferior vesical artery and determine its existence in women.
Methods
This descriptive study is based on 25 dissections (6 male and 19 female cadavers). We dissected the internal iliac artery and its branches from the iliac bifurcation, bilaterally and comparatively. Each arterial branch supplying the bladder was identified and dissected as far as the bladder.
Results
In total, 50 topographies of the bladder vascularization were visualised. The inferior vesical artery was observed in 92% of the male subjects and in 47.4% of the female subjects. In the male cadavers, it arose from the internal iliac artery in 72.7% of cases and from the umbilical artery in 27.3% of cases. In the female cadavers, it arose from a common trunk with the umbilical artery and the uterine artery in 33.3% of cases and directly from the umbilical artery in 33.3% with one terminal branch supplying the upper part of the vagina. In two female subjects, the inferior vesical artery arose from the first segment of the uterine artery (22.2%), and in one subject from the obturator artery (11.1%).
Conclusions
The inferior vesical artery is not specific to the male sex. The contradictions found in the literature of this artery are due to the variations observed in pelvic vascularization and to the close connections between vaginal and bladder vascularisation in women. However, surgeons should consider these variations, to prevent bladder devascularization by non-selective ligation.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Data from the literature describe wide variations in bladder vascularization, closely dependent on the branching type of the internal iliac artery, which shows the complexity of detailed knowledge of pelvic vascularization. The number of arteries supplying the vesical vascularization and their origin is variable [3, 13].
The principal arteries are the visceral branches of the anterior trunk of the internal iliac artery, arising either directly from the terminal branch (internal pudendal artery) or from the lateral branches (the umbilical, middle rectal, and obturator arteries) [13]. Anatomical works on the subject divide the bladder blood supply into four regions [19]: The upper part of the bladder is supplied by the superior vesical arteries arising from the patent portion of the umbilical artery (UMBA) or the obturator artery. The inferior and lateral walls of the bladder are supplied by the inferior vesical arteries arising either directly from the internal iliac artery (IIA) or from the inferior gluteal artery. They are often described as being specific to men [12]. The anterior and lateral walls are supplied by the anterior vesical arteries which arise from the internal pudendal artery. The posterior and lateral walls are supplied by the vesical branches of the middle rectal arteries, the prostatic branches of the inferior vesical artery (IVA), and by the vesical branches of the deferential arteries in men and of the uterine and vaginal arteries in women. The various works relate many anastomoses between these arteries for bladder. In fact, in men, it is accepted that the IVA usually arise from a vesico-genital artery that vascularise the prostate, the inferior and posterior walls of the bladder, the seminal vesicle, and the vas deferent. In women, the IVA is more controversial, but if it exists, could arise also from genital arteries like UA and some vaginal arteries. The IVA artery is further defined by its target than origin, since they are many in their description.
If the description of the superior vesical artery (SVA) seems consensual between the two genders [9], the description of the IVA is a large variability according to the authors.
In men, the IVA supplies the lower parts of the bladder, and the bladder neck, ureter, seminal vesicles, and prostate [3].
In women, the posterior, lower, and side bladder vascularization seems less systematized, with a participation of the uterine artery (UA) with its vesical and vaginal branches, and its cervical and vaginal branches, including the upper part of the fundus and the bladder neck.
The IVA is often described as being suitable for men [12]. However, an IVA is sometimes described in women, distinctly of the vaginal artery [3].
Accurate knowledge of the bladder blood supply and of its variations is essential in urological and gynecological surgery, but also in interventional radiology [7, 8]. Indeed, surgeons and radiologists should consider the frequency of anatomic variations in arterial embolization or surgical ligation to prevent bladder devascularization by non-selective ligation [18].
The aim of our study was to clarify the origin of the IVA and determine its existence in women.
Methods
This descriptive study is based on dissections of cadavers at the Laboratory of Applied Anatomy, CHU Rangueil, Toulouse.
The pelvises of 25 fresh adult cadavers were macroscopically dissected. We carefully dissected the IIA and its branches from the iliac bifurcation, bilaterally and comparatively, providing 50 visualisations of the topography of the bladder vascularization. Each arterial branch supplying the bladder was identified and dissected as far as the bladder. All cadavers dissected were out of pelvic scars and the previous surgery.
Results (Table 1)
The study population comprised 6 male cadavers and 19 female cadavers.
In all subjects, the anterior trunk of the IIA and its branches supplying the bladder were observed bilaterally. From the 25 dissections performed bilaterally, 50 topographies bladder vascularization were visualised. In all the male cadavers (100% of cases), the UMBA was observed arising directly from the IIA, as the first visceral branch, and in most of the female cadavers (78.9%: n = 30) from a common trunk with the UA. The UMBA born directly from IIA in 21.1% (n = 8) of cases in female cadavers. The SVA appeared from the patent portion of the UMBA in all 25 cadavers studied, (100%, n = 50).
In male subjects, the IVA was identified in all 6 cadavers dissected, but in one of the subjects, it was not observed on the left side (92%, n = 11). It emerged from the IIA in 72.7% of cases (n = 8) and from the UMBA in 27.3% of cases (n = 3) (Figs. 1, 2).
In male subjects: figures showing the various origins of the inferior vesical artery (IVA) identified in our study. a IVA arising from IIA (72.7%), b IVA arising directly from the UMBA (27.3%); PT posterior trunk, EIA external iliac artery, IIA internal iiiac artery, UMBA umbilical artery, OA obturator artery, CIA common iliac artery, MRA middle rectal artery, SVA superior vesical artery, VDA vesico-deferencial artery
In one of the male subjects, a vesiculo-deferential artery was observed arising from the IIA with branches supplying the ureter.
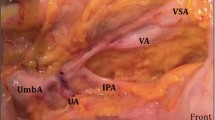
In female subjects, IVA was observed in 9 subjects (47.4%, n = 18) arising from a common trunk with the UMBA and the UA in 33.3% (n = 6) of cases, arising directly from the UMBA in 33.3% (n = 6) with one terminal branch supplying the upper part of the vagina (Fig. 3). In two female subjects, the IVA arose from the first segment of the UA (22.2%, n = 4) and in one subject from the obturator artery (11.1%, n = 2).
The UA was identified in all the females studied (100%, n = 38): in 78.9% of cases (n = 30) it arose from a common trunk with the UMBA, and in four subjects, it arose directly from the IIA (21.1%, n = 8).
The obturator artery was identified in 100% of cases (n = 50) arising from the IIA in both female and male subjects (Fig. 4).
In female subjects: figures showing the various origins of the inferior vesical artery (IVA) identified in our study. a IVA arising from a common trunk with the UMBA and the UA in 33.3% of cases; b IVA arising directly from the UMBA in 33.3% with one terminal branch supplying the upper part of the vagina (VA vaginal artery); c IVA from the obturator artery (11.1%); d IVA from the first segment of the UA (22.2%); PT posterior trunk, EIA external iliac artery, IIA internal iliac artery, UA uterine artery, UMBA umbilical artery, OA obturator artery, CIA common iliac artery, SVA superior vesical artery
Discussion
Our study shows that the IVA is not exclusive to the male bladder vascularization. In female subjects, the IVA was observed in nine subjects (47.4%, n = 18) arising from a genital arterie. However, in women, the inferior vesical vascularization is closely linked with the blood supply to the upper vagina and raises the question of how to distinguish between the IVA and the VA. Some authors describe this IVA in women quite separately from the VA, which suggests that it can be identified [15].
Bichat already described the IVA in 1812 for taking classic origin the IIA, arising either side of the UMBA, or by a common trunk with her and made no distinction in its description between man and woman [3].
However, some descriptions are contradictory: Kamina describes the IVA as a male-specific artery, running to the fundus of the bladder and supplying the seminal vesicle, the prostate, the fundus of the bladder, and the lower part of the ureter [12]. However, in a previous gynecological work dating from 1974, he also describes IVA as arising either from the IIA or most commonly from the UA [11]. The work of Drake (Gray’s) [9] does not describe IVA in women, claiming that the VA (branch of the UA) would be the equivalent of the IVA of man, giving branch to the vagina and adjacent parts of the bladder and rectum. Moore described the IVA only in men, with a sub-peritoneal course in the lateral ligament of the bladder and giving the prostatic artery and occasionally the conduit deferent artery [13]. He described anastomoses with the superior bladder arteries and considers also that the IVA is replaced in women with VA, which gives branches to the posterior and inferior part of the bladder, like Moses, Bouchet, and Cuilleret [4, 14].
Conversely, Schünke described an IVA in women [20]. In addition, Rouviere, repeating a description of Farabeuf, described an IVA in the two sexes, giving some branches to the vagina and ureter in women, and the seminal vesicles and the prostate in men [19].
Netter described an IVA both in men and women, arising from the IIA or its branches, and reproduced in his drawings, an IVA with a vaginal branch and ureteral branch to distal part of the ureter or sometimes a “inferior vesical branch” arising from VA [15].
Abrahams presented in his Atlas of Anatomy, a dissection of the IIA and showed distinctly an IVA artery giving rise to the VA [1].
In 1959, Shafiroff observed in a study of 150 specimens that the IVA was observed only in 78% of specimens [21]. When it was highlighted, it arises in over 10% of cases from the IIA (anterior division) or from umbilical, prostatic, or vaginal arteries. In less than 10% of cases, he observed an origin from the vesico-deferential artery or from the internal pudendal artery.
Our results for the male cadavers are comparable to those found in the literature. The patent portion of the UMBA is systematically observed originating from the IIA and branching to supply the lower bladder.
In the light of our results, there is some discussion to be had about the male-specific nature of the IVA. In nine of the female cadavers, arteries supplying the lower bladder were clearly observed and were distinct from the traditional description of the vaginal arteries.
There is certainly a difference between the genders in terms of the results (47.4% in the females versus 92% in the males), but these data do not confirm that the artery is specific to males. The major difference between the two genders lies in the virtually constant nature of the artery in male subjects (over 90%) [22].
In the literature, the IVA in women, when it is highlighted, is described arising either directly from the IIA, or from one of its branches [15]. Therefore, the question of its existence in females is primarily related to its origin. When it arises directly from the IIA, it is mentioned distinctly as a main visceral branch of the latter [13]. When it arises from a branch of the IIA, it is not usually described in isolation but often as a “vesicovaginal” artery.
In terms of the main branches of the IIA in females, our results agree with the previous works on the matter, observing the UMBA arising from a common trunk with the UA in 78.9% of cases [6].
The existence of this IVA in women should be considered in gynecological surgeries but also during embolisation in interventional radiology. Its variable origin is a major element to be considered during not-selective devascularization. Indeed, UMBA ligated at its origin is likely to cause a devascularization of the uterus [6] but also bladder (six subjects in our study). During hysterectomy for benign cause, linking the UA at its origin must be discussed because of the risk of ligature of the IVA, as shown in our study in two female subjects.
A precise knowledge of the anatomy of this area is also important when treating urological diseases. Just recently, the use of interventional radiology to treat prostate adenoma in men has been introduced. To date, very few studies on the subject exist and only two teams, one Brazilian and one Portuguese, have published works in this area, but bladder vascularization and its variations must again be considered to achieve as possible a more selective devascularization [5, 16].
Furthermore, it is acknowledged that arterial insufficiency represents a significant cause of erectile dysfunction after surgical treatment of localised prostate cancer [2]. If, therefore, during a radical prostatectomy, surgeons are able to preserve the accessory pudendal arteries, which arise most commonly from the inferior vesical arteries and are found in 42% of men, this would significantly improve rates of postoperative erectile function recovery [10, 17]. Therefore, as in women, precise knowledge of the pelvic vascularization, and of the existence of anatomic variations, is essential for preserving perineal functionality during selective devascularisation.
Conclusions
The IVA is not specific to the male gender and is further defined by its target than origin. The contradictions found in the literature of this artery are due to the variations observed in pelvic vascularization and to the close connections between vaginal and bladder vascularisation in women. However, urological and gynecological surgeons should consider these variations, especially when performing the ligation of pelvic vessels at their origin.
References
Abrahams PH, Boon JM Spratt JD (2014) Atlas clinique d’anatomie humaine de McMinn et Abrahams. Elsevier Masson, Issy-les-Moulineaux, pp 267–268, 270, 272
Benoit G, Droupy S, Quillard J, Paradis V, Giuliano F (1999) Supra and infralevator neurovascular pathways to the penile corpora cavernosa. J Anat 195(Pt 4):605–615
Bichat X (1812) Traité d’anatomie descriptive. Brosson Tome IV:294–306
Bouchet ACJ (1983) Anatomie topographique, descriptive et fonctionnelle: l’abdomen, la région rétro-péritonéale, le petit bassin, le périnée. SIMEP SA, MASSON Tome 4, 2ème édition:2227–2233
Carnevale FC, Moreira AM, Antunes AA (2014) The “PErFecTED technique”: proximal embolization first, then embolize distal for benign prostatic hyperplasia. Cardiovasc Intervent Radiol 37:1602–1605. doi:10.1007/s00270-014-0908-z
Chantalat E, Merigot O, Chaynes P, Lauwers F, Delchier MC, Rimailho J (2014) Radiological anatomical study of the origin of the uterine artery. Surg Radiol Anat 36:1093–1099. doi:10.1007/s00276-013-1207-0
Cho CL, Lai MH, So HS, Kwok KK, Chan JC, Velayudhan V (2008) Superselective embolisation of bilateral superior vesical arteries for management of haemorrhagic cystitis. Hong Kong Med J 14:485–488
Delgal A, Cercueil JP, Koutlidis N, Michel F, Kermarrec I, Mourey E, Cormier L, Krause D, Loffroy R (2010) Outcome of transcatheter arterial embolization for bladder and prostate hemorrhage. J Urol 183:1947–1953. doi:10.1016/j.juro.2010.01.003
Drake RL, Duparc F, Duparc J, Mitchell AHG, Vogl AW (2015) Gray’s Anatomie pour les étudiants, 3 edn, Elsevier Masson, Issy-les-Moulineaux, pp 469–474
Droupy S, Benoit G, Giuliano F, Jardin A (1997) Penile arteries in humans. Origin–distribution–variations. Surg Radiol Anat 19:161–167
Kamina P (1974) Traité d’anatomie gynécologique et obstétricale. Maloine, Paris
Kamina PG A (2014) Anatomie clinique: Organes urinaires et génitaux, pelvis, coupes du tronc, 3 edn, Tome 4:54-112-196, Maloine, Paris
Moore KLD, Dalley II AF (2011) Anatomie médicale: aspects fondamentaux et applications cliniques. De Broeck 3ème édition, Paris, pp 349–355
Moses KP, Banks JC Jr, Petersen DK (2015) Le grand manuel illustré d’anatomie générale et clinique. Elsevier Masson, Issy-les-Moulineaux
Netter FH (1997) Atlas d’anatomie humaine. Maloine, Paris
Pisco JM, Rio TintoH, Campos Pinheiro L, Bilhim T, Duarte M, Fernandes L, Pereira J, Oliveira AG (2013) Embolisation of prostatic arteries as treatment of moderate to severe lower urinary symptoms (LUTS) secondary to benign hyperplasia: results of short- and mid-term follow-up. Eur Radiol 23:2561–2572. doi:10.1007/s00330-012-2714-9
Rogers CG, Trock BP, Walsh PC (2004) Preservation of accessory pudendal arteries during radical retropubic prostatectomy: surgical technique and results. Urology 64:148–151. doi:10.1016/j.urology.2004.02.035
Roman H, Zanati J, Friederich L, Resch B, Lena E, Marpeau L (2008) Laparoscopic hysterectomy of large uteri with uterine artery coagulation at its origin. JSLS 12:25–29
Rouvière HD A (2002) Anatomie humaine: descriptive, topographique et fonctionnelle, 15ème édition, Tronc. 2, Elsevier Masson, Paris
Schünke M, Schulte E, Schumacher U, Rude J (2007) Atlas d’anatomie Prométhée: cou et organes internes. Maloine, pp 175, 176, 234, 239, 262, 284, 290
Shafiroff BG, Grillo EB, Baron H (1959) Bilateral ligation of the hypogastric arteries. Am J Surg 98:34–40
Shehata R (1976) The arterial supply of the urinary bladder. Acta Anat (Basel) 96:128–134
Acknowledgements
We would like to thank Gisele Ponsolle, Magalie Ramos, Hubert Desroques, and Philipe Peugeot for their technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest and that they have no financial relationship with the organisation that sponsored the research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Treigny, O.M., Roumiguie, M., Deudon, R. et al. Anatomical study of the inferior vesical artery: is it specific to the male sex?. Surg Radiol Anat 39, 961–965 (2017). https://doi.org/10.1007/s00276-017-1828-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-017-1828-9