Abstract
Purpose
Gantzer’s muscle (GM) is an additional muscle in the forearm, which develops as an accessory head of the flexor pollicis longus or the flexor digitorum profundus. The study aimed to determine the topography of the GM and to define the topographical relationship between the GM and the neurovascular structures surrounding it.
Methods
After confirming the presence of GM, its topography and the neurovascular structures were analyzed to determine the correlation between them in 73 upper limbs.
Results
The incidence of GM was 47.95% (35/73) and the average insertion point of GM was identified at 49.33 ± 7.47‰ (119.82 ± 20.80 mm) on the reference line between the medial epicondyle and the pisiform bone. And the branching points of the median nerve and the ulnar artery were located 19.91 ± 11.23‰ (52.21 ± 24.67 mm), 17.45 ± 8.39‰ (42.53 ± 20.54 mm) on the reference line, respectively. The presence of GM had no significant correlation with the position of the nerve branches. On the other hand, the branching point of the ulnar artery was distally located in the cases with the presence of the GM (17.35 ± 8.65 vs 19.42 ± 10.87, p = 0.031). There was a significant positive correlation between the point of arterial bifurcation and the length of the GM (r = 0.407, p = 0.015).
Conclusions
This study suggested that the GM has a topographical relation with the arterial structures, perhaps for embryological reasons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the anatomical variations of the muscles of the forearm, Gantzer’s muscle (GM) is an additional muscle in the forearm as the accessory head of the flexor pollicis longus (FPL) and accessory head of flexor digitorum profundus (FDP). Its prevalence and morphological variations have been described in the previous studies [7, 13, 15, 16, 20]. Although the prevalence of the accessory head of FPL (aFPL) and FDP (aFDP) varies in these studies, it is consistently reported in 50% of the Asian population [13, 15]. The various origins of the GM have been described in previous studies: the medial humeral epicondyle, the coronoid process or dual origin from the medial epicondyle and the coronoid process [1, 13, 15]. The insertions of the aFPL and aFDP have been consistently reported in the ulnar border of the FPL and in the tendon of the FDP at the wrist level, respectively [1, 16, 19].
The anterior interosseous nerve (AIN) is a branch of the median nerve (MN) and it innervates the FPL, FDP, pronator quadratus, and the GM. Although a variable topological relationship has been reported between the AIN and the aFPL, the more frequent course of the AIN is posterior to the aFPL [13, 15, 20]. Owing to its anatomical position, the aFPL has been assumed as one of the causes of AIN compression, the so-called “AIN syndrome” [8, 21, 22].
Considering the influence of the anatomical variation on the nerve branching in a previous study [10], it has been postulated that the GM may also affect the branching point of the AIN from the MN. However, the influence of the GM on the adjacent anatomical structures remains obscure. In our study, we aimed to determine the topography of the GM and its neurovascular structures.
Materials and methods
The prevalence and length of the Gantzer’s muscle
In this study, 73 upper limbs (from 37 cadavers, 37 right and 36 left) were dissected. Each cadaver was placed in a supine position with the arms extended, and the palms facing up. The skin, superficial fascia, and adipose tissue were removed to expose the flexor compartment of the forearm. The MN and the ulnar nerve branches were dissected from the artery and the fascia. After dissecting the flexor carpi radialis, palmaris longus, flexor digitorum superficialis and flexor carpi ulnaris muscles, the presence of the GM was confirmed. After identifying the GM, the distance between the medial epicondyle and the pisiform bone was measured using digital calipers (NA500-300S, Blue bird, Korea): this line was defined as the reference line [3, 9]. The origin and insertion points of the GM were identified and analyzed. The length of the GM was compared with that of the reference line as a percentile.
Topography of the Gantzer’s muscle and the neurovascular structures
The branching points of the MN and the ulnar artery were determined and defined as the distance from the medial epicondyle to the branching point of the AIN from the MN. The branching point of the ulnar artery was defined as the distance from the medial epicondyle to the branching point into the ulnar and common interosseous arteries. All GMs were originated from the medial epicondyle and crossed the neurovascular structures. The crossing points of the GM over nerve or artery (AIN, MN, and ulnar artery) were also identified and measured as a percentile of the reference line. The SPSS statistical package (SPSS software, version 20.0; SPSS, Inc., Chicago, IL) was used for all statistical analyses. A p value <0.05 was considered significant.
Results
The prevalence and length of the Gantzer’s muscle
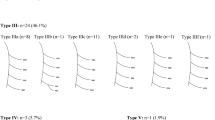
The prevalence of the GM was 47.95% (35 limbs of total 73 limbs). It was bilateral in five cadavers and unilateral in 25 cadavers (19 of the right side and 16 of the left side), respectively. All GMs were originated from the medial epicondyle and inserted into the FDL or the FDS (Fig. 1). This muscle was composed of only muscle tissues not tendinous or fibrous tissues histologically. The mean length of reference line (see “Materials and methods”) was 244.82 ± 17.31 mm. The average of insertion point of the GM was identified at 49.33 ± 7.47‰ (119.82 ± 20.80 mm) of the reference line.
Gantzer’s muscle (one asterisk) originated from the medial epicondyle under the flexor digitorum superficialis (FDS) and inserted into the flexor digitorum profundus (FDP). Small Gantzer’s muscle (two asterisks) originated from FDS and inserted into flexor pollicis longus (FPL). These muscles crossed the branches of the median nerve (MN) and the anterior interosseous nerve (AIN), respectively (arrows)
Topography of the Gantzer’s muscle and the neurovascular structures
The mean values for the location of the neurovascular structures on the reference line are shown in Table 1 as the percentile. The ulnar artery divided into the ulnar and common interosseous arteries slightly distal to the elbow joint, near the origin of the GM (branching point of the ulnar artery). The MN gave off one small branch, the AIN immediately distal to the arterial bifurcation (branching point of the MN). The GM crossed either superficial or deep to the ulnar artery, the MN, and the AIN. The branching points of the MN and the ulnar artery were located 21.13 ± 10.34‰ (52.69 ± 25.57 mm) and 17.35 ± 8.65‰ (47.09 ± 27.05 mm) on the reference line, respectively. The arterial branching point was significantly more proximal than that of the MN (p = 0.001). The branching points of the ulnar artery were more distal in cases with GM (19.42 ± 10.87‰) than in those without the GM (15.34 ± 5.14‰, p = 0.031). However, the branching point of the MN was not significantly associated with the presence of the GM (p = 0.652).
Moreover, the GM crossing points on the MN, AIN and ulnar artery were 34.29 ± 8.47‰ (83.10 ± 21.24 mm), 33.59 ± 5.65‰ (81.45 ± 15.43 mm), and 31.01 ± 6.78‰ (74.38 ± 14.58 mm), respectively (Fig. 2). The GM crossed the ulnar artery more proximally than the nervous structures, the AIN (p = 0.052) and MN (p = 0.010).
Correlation between the Gantzer’s muscle and the neurovascular structures
The association between the bifurcation points of neurovascular structures, the length of the GM, crossing point of the GM over the neurovascular structures is presented in Table 2. Concordant with the above differences, there was a significant positive relationship between the point of the arterial bifurcation and the length of the GM. (Pearson correlation test, r² = 0.165, p = 0.015, Fig. 3). The points of the arterial and nervous bifurcation also showed a positive relationship, statistically (r² = 0.502, p < 0.001). However, the branching points of the MN were not associated with the GM length. Instead, the crossing points of the AIN and the ulnar artery with the GM had a positive relationship (r² = 0.191, p = 0.020). The crossing points of the AIN with the GM were also associated with the GM length, however, it did not get statistical significance (p = 0.076).
Discussion
In our study, conducted on Korean cadavers, we determined the prevalence of the GM and the topological relationships with the GM and the adjacent neurovascular structures. In earlier studies, the prevalenceof the GM was has been reported between 45 and 66.7% [1, 3, 11, 14, 15, 19, 23]. The prevalence of GM in our study is 47.95% in 73 upper limbs; this is concurrent with the findings of most previous studies.
In numerous previous reports, it has been suggested that the presence of the GM may be related to pathological conditions of the AIN [2, 4, 7, 13, 15–18, 22]. Several case studies have also suggested an association of the GM with a high division of the MN within the carpal tunnel [5, 6]. However, there are no anatomical data supporting the correlation between the GM and the AIN or other adjacent neurovascular structures. To our knowledge, this study is the first to demonstrate the anatomical correlation between the GM and the contiguous neurovascular structures, including the AIN, the MN, and the ulnar artery. Based on the simultaneous development of muscles, nerve and vessels during embryogenesis, we hypothesized that the presence of the GM may affect the development of the neurovascular structures, which may possibly manifest as variations of the nerve branch points or the artery bifurcation points. However, our results show that there is no significant topological difference in the anatomy of AIN or the MN with or without the presence of the GM. It is believed that the tendency of neural variation in the carpal tunnel syndrome may be associated with the presence of the GM [6].
However, in the presence of the GM, the origin of the posterior interosseous artery from the ulnar artery was more distal. Quantitative analyses also show a positive correlation between the arterial bifurcation points and the length of the GM. These data suggest that the presence of the GM may induce the development of the brachial and ulnar arteries, thereby delaying their bifurcation. During embryonic forearm formation, an incomplete cleavage of the flexor mass is considered one of the causes of the accessory muscles, such as GM [12]. Further, extensive studies are required to confirm the embryological mechanism and effects.
Our study is the first to demonstrate the topography of the GM, and its anatomical association with the adjacent neurovascular structures. Based on our results, we propose the localization of neurovascular structures according to the presence of the GM (Fig. 4). Knowledge of the frequent variations in the topography may be useful to improve the safety of surgical and clinical procedures, thereby reducing the risk of iatrogenic complications. Although still hypothetical, the strong relation between the GM and the arterial structures may lead to a new direction for anatomical study of muscle variations. These data also improve awareness and encourage further development of the concept of variation studies.
References
Al-Qattan M (1996) Gantzer’s muscle: an anatomical study of the accessory head of the flexor pollicis longus muscle. J Hand Surg Br 21:269–270
Alexandre A, Alexandre AM, Zalaffi A (2011) Considerations on the treatment of anterior interosseous nerve syndrome. Acta Neurochir Suppl 108:247–250. doi:10.1007/978-3-211-99370-5_38
Beekman R, Van Den Berg L, Franssen H, Visser L, van Asseldonk J, Wokke J (2005) Ultrasonography shows extensive nerve enlargements in multifocal motor neuropathy. Neurology 65:305–307
Bilecenoglu B, Uz A, Karalezli N (2005) Possible anatomic structures causing entrapment neuropathies of the median nerve: an anatomic study. Acta Orthop Belg 71:169–176
Dellon AL, Mackinnon SE (1987) Musculoaponeurotic variations along the course of the median nerve in the proximal forearm. J Hand Surg 12:359–363
Eid N, Ito Y, Otsuki Y (2014) Anomalous muscles in carpal tunnel associated with neurovascular variations: case report and brief review. Forens Med Anat Res 2:8–10. doi:10.4236/fmar.2014.21003
El Domiaty MA, Zoair MM, Sheta AA (2008) The prevalence of accessory heads of the flexor pollicis longus and the flexor digitorum profundus muscles in Egyptians and their relations to median and anterior interosseous nerves. Folia Morphol (Warsz) 67:63–71
Gardner-Thorpe C (1974) Anterior interosseous nerve palsy: spontaneous recovery in two patients. J Neurol Neurosurg Psychiatry 37:1146–1150
Grechenig W, Clement H, Egner S, Tesch N, Weiglein A, Peicha G (2001) Musculo-tendinous junction of the flexor carpi ulnaris muscle. An anatomical study. Surg Radiol Anat 22:255–260
Gunther SF, Dipasquale D, Martin R (1993) Struthers’ ligament and associated median nerve variations in a cadaveric specimen. Yale J Biol Med 66:203
Hemmady MV, Subramanya AV, Mehta IM (1993) Occasional head of flexor pollicis longus muscle: a study of its morphology and clinical significance. J Postgrad Med 39:14–16
Jones M, Abrahams P, Sanudo J, Campillo M (1997) Incidence and morphology of accessory heads of flexor pollicis longus and flexor digitorum profundus (Gantzer’s muscles). J Anat 191:451–455
Kara A, Elvan O, Yildiz S, Ozturk H (2012) Accessory head of flexor pollicis longus muscle in fetuses and adult cadavers and its relation to anterior interosseous nerve. Clin Anat 25:601–608. doi:10.1002/ca.21296
Mahakkanukrauh P, Surin P, Ongkana N, Sethadavit M, Vaidhayakarn P (2004) Prevalence of accessory head of flexor pollicis longus muscle and its relation to anterior interosseous nerve in Thai population. Clin Anat 17:631–635. doi:10.1002/ca.20016
Oh CS, Chung IH, Koh KS (2000) Anatomical study of the accessory head of the flexor pollicis longus and the anterior interosseous nerve in Asians. Clin Anat 13:434–438. doi:10.1002/1098-2353(2000)13:6<434::aid-ca7>3.0.co;2-4
Pai MM, Nayak SR, Krishnamurthy A, Vadgaonkar R, Prabhu LV, Ranade AV, Janardhan JP, Rai R (2008) The accessory heads of flexor pollicis longus and flexor digitorum profundus: incidence and morphology. Clin Anat 21:252–258. doi:10.1002/ca.20612
Pham M, Baumer P, Meinck HM, Schiefer J, Weiler M, Bendszus M, Kele H (2014) Anterior interosseous nerve syndrome: fascicular motor lesions of median nerve trunk. Neurology 82:598–606. doi:10.1212/wnl.0000000000000128
Saxena A, Agarwal KK, Parshuram V, Das AR (2013) Gantzer muscles and their applied aspects: an exceptional finding. Singap Med J 54:e102–e104
Shirali S, Hanson M, Branovacki G, Gonzalez M (1998) The flexor pollicis longus and its relation to the anterior and posterior interosseous nerves. J Hand Surg Br 23:170–172
Sunderland S (1978) Nerves and nerve injuries, 2nd edn. Churchill Livingstone, Edinburgh
Tabib W, Aboufarah F, Asselineau A (2001) Compression of the anterior interosseous nerve by Gantzer’s muscle. Chir Main 20:241–246
Ulrich D, Piatkowski A, Pallua N (2011) Anterior interosseous nerve syndrome: retrospective analysis of 14 patients. Arch Orthop Trauma Surg 131:1561–1565. doi:10.1007/s00402-011-1322-5
Uyaroglu FG, Kayalioglu G, Erturk M (2006) Incidence and morphology of the accessory head of the flexor pollicis longus muscle (Gantzer’s muscle) in a Turkish population. Neurosciences (Riyadh) 11:171–174
Acknowledgements
This research was supported by the Keimyung University Research Grant of 2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
K. Yang and S.-J. Jung contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, K., Jung, SJ., Lee, H. et al. Topographical relations between the Gantzer’s muscle and neurovascular structures. Surg Radiol Anat 39, 843–848 (2017). https://doi.org/10.1007/s00276-016-1803-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-016-1803-x