Abstract
We present a patient with a recurrent precaval left renal artery, stemming from a right-sided common trunk renal artery. The patient was a 44-year male who presented with a post-traumatic grade IV renal injury. After 3 months without renal function improvement and repeated urinary tract infection, a laparoscopic nephrectomy of the affected right kidney was performed, without upfront identification of the vascular variation, resulting in ischemia of the remaining left kidney. An anastomosis of the common renal trunk and the distal left renal artery was created in between the abdominal aorta and the inferior vena cava. This case describes the importance of upfront detection of renal vascular variations using the appropriate imaging techniques.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increased use of laparoscopic renal surgery makes it essential to understand and document renal artery anomalies. Renal vascular variations are described in a wide variety. Prevalences of 13.2–37 % have been reported [2, 6, 9]. Most common reported variations are the accessory (hilar) renal arteries and the aberrant (polar) renal arteries [6, 7, 10]. A less common variation is a precaval right renal artery with prevalences reported between 0.8 and 6.0 % [2, 4, 7]. A common trunk renal artery originating from the abdominal aorta is very rare, with only one previous case report [9]. We describe a patient with an undiagnosed common trunk right renal artery feeding a precaval left renal artery. The patient has given his consent to write this article.
Case report
A 44-year male presented, 4 months after grade IV renal injury, with a non-functioning right kidney. A computed tomography (CT) scan performed immediately after the trauma showed a fornix rupture with a dilated ureter, free fluid in the retroperitoneum and a torsion of the left kidney.
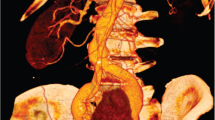
Although present, vascular variations were not detected or mentioned in the radiology report of the CT scan. A renogram was performed which demonstrated a non-functional right kidney. After 3 months without renal function improvement (creatinine 120 umol/L, eGFR 58 ml/min), repeated urinary tract infection and persistent leukocyturia, laparoscopic nephrectomy was planned. Surgery was started as a total laparoscopic procedure. The combination of an organized fibrotic hematoma around a considerably enlarged right kidney made the urologic surgeons decide to convert to a hand-assisted procedure. The right renal artery and vein, as well as the ureter, were identified and clipped with three hemolocks. In addition a second right renal artery was identified and clipped. After clipping the second artery, the surgeons became suspicious that they had clipped a precaval artery which supplied the left kidney. The surgeons decided to review the preoperative CT scan during the procedure. On the preoperative CT scan a left renal artery originating from the abdominal aorta and two right renal arteries, one precaval and the other retrocaval, were thought to be identified. The presumption was made that this second right renal artery may have actually been an anatomic variation of the left renal artery. The right-sided kidney was removed and the procedure was terminated. Immediately postoperatively a computed tomography intravenous urography (CT/IVU) was ordered which confirmed this presumption. Postoperatively the patient was transferred to the intensive care unit. Vascular centers were consulted and the patient was transferred to our hospital. After arriving at our hospital, the patient was immediately reoperated. The retrocaval common trunk renal artery and the precaval renal artery were mobilized. Warm ischemia time had been nine hours. Subsequently an anastomosis of the common renal trunk and the distal left renal artery was created in between the abdominal aorta and the inferior vena cava (Fig. 1). Postoperatively, blood flow to the kidney was controlled by ultrasound and the patient was transferred to the intensive care unit. Renal function showed a slow recovery. A magnetic resonance angiography (MRA) scan confirmed restored blood flow in the kidney (Fig. 2). After 2 weeks of recovery with 10 days of hemodialysis, the patient was dismissed from our hospital without the need for renal replacement therapy. To evaluate the entire procedure, a 3D reconstruction of the preoperative CT scan was ordered which confirmed the common trunk right renal artery and the precaval left renal artery (Fig. 3). The hilum of the left kidney faced anteriorly, suggesting a non-rotational abnormality and the left renal artery penetrated the renal cortex from posterior. To our knowledge this transcortical path has never been described before [Fig. 4]. In retrospect, the failure to diagnose the common renal arterial trunk upfront had almost led to the loss of the patients’ remaining left kidney. Recovery after 6 months to an eGFR of 22 ml/min, and a creatinine clearance after 24-h urine collection of 31 ml/min was measured.
Discussion
This case is the second in the literature presenting a common trunk renal artery originating from the abdominal aorta [9] and the first case describing a transcortical path of a renal artery.
Variations of renal arteries are explained by the development of mesonephric arteries. These arteries form a vascular net feeding the developing kidneys. Over time, this vascular net degenerates, leaving only one artery. Deficiency of the mesonephric arteries results in anomalies of the renal artery [6]. In this case the failure to diagnose the vascular variation of the renal arteries led to an almost total loss of renal function. Although a preoperative CT scan with contrast was preformed and showed the vascular and renal variation, these variations were not detected upfront. The golden standard to detect vascular variations are CT/IVU or MRA [2].
In the literature a possible association between renal arterial and venous variations and renal malformations is described, although exact numbers lack [1, 3]. In retrospect, the torsion anomaly of the left kidney, visible on the preoperative CT scan, should have been a second upfront sign of the renal vascular variation encountered during the described surgical procedure (Fig. 4).
Furthermore, ischemia time is an important factor in determining final renal function outcome. Although the outcome after prolonged ischemia of an in situ-situated kidney is poorly described in literature, ischemia time should be minimized as much as possible, while renal function outcome can be reduced in less than 20 min [5, 8]. In this case, the created anastomosis between the common renal trunk and the distal left renal artery resulted in the excellent recovery of the renal function.
These findings emphasize the importance of awareness and understanding of renal vascular variations and shows their implication in clinical practice, especially in laparoscopic renal surgery. The choice of preoperative imaging techniques, with intravenous contrast, and the careful examination of these images, with the knowledge of vascular and kidney anatomical variations, are the keystones in preventing unforeseen complications and belong to the preoperative workup before laparoscopic renal surgery.
References
Aljabri B, MacDonald PS, Satin R, Stein LS, Obrand DI, Steinmetz OK (2001) Incidence of major venous and renal anomalies relevant to aortoiliac surgery as demonstrated by computed tomography. Ann Vasc Surg 15:615–618. doi:10.1007/s10016-001-0095-7
Arevalo Perez J, Gragera Torres F, Marin Toribio A, Koren Fernandez L, Hayoun C, Daimiel Naranjo I (2013) Angio CT assessment of anatomical variants in renal vasculature: its importance in the living donor. Insights Imaging 4:199–211. doi:10.1007/s13244-012-0217-5
Cocheteux B, Mounier-Vehier C, Gaxotte V, McFadden EP, Francke JP, Beregi JP (2001) Rare variations in renal anatomy and blood supply: CT appearances and embryological background A pictorial essay. Eur Radiol 11:779–7864
Gupta A, Gupta R, Singhal R (2011) Precaval right renal artery: a cadaveric study incidence and clinical implications. Int J Biol Med Res 2:1195–1197
Morris PJ, Johnson RJ, Fuggle SV, Belger MA, Briggs JD (1999) Analysis of factors that affect outcome of primary cadaveric renal transplantation in the UK. HLA Task Force of the Kidney Advisory Group of the United Kingdom Transplant Support Service Authority (UKTSSA). Lancet 354:1147–1152
Ozkan U, Oguzkurt L, Tercan F, Kizilkilic O, Koc Z, Koca N (2006) Renal artery origins and variations: angiographic evaluation of 855 consecutive patients. Diagn Interv Radiol 12:183–186
Parimala NB (2013) Bilateral aberrant renal arteries with abnormal left renal vein: a case report. J Clin Diagn Res 7:1425–1426. doi:10.7860/jcdr/2013/5424.3151
Thompson RH, Frank I, Lohse CM, Saad IR, Fergany A, Zincke H, Leibovich BC, Blute ML, Novick AC (2007) The impact of ischemia time during open nephron sparing surgery on solitary kidneys: a multi-institutional study. J Urol 177:471–476. doi:10.1016/j.juro.2006.09.036
Tulunay G, Ureyen I, Karalok A, Turan T, Boran N (2012) A ptotic kidney with multiple arteries, one from a common renal artery stem. Asian Pac J Reprod 1:318–319. doi:10.1016/S2305-0500(13)60101-3
Yeh BM, Coakley FV, Meng MV, Breiman RS, Stoller ML (2004) Precaval right renal arteries: prevalence and morphologic associations at spiral CT. Radiology 230:429–433. doi:10.1148/radiol.2302021030
Acknowledgments
Figure 1 is designed and created by Roderik van Heijst.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Buisman, W.J., Ünlü, Ç., de Boer, S.W. et al. An undetected common renal arterial trunk: surgical consequences and morbidity analysis. Surg Radiol Anat 38, 1111–1114 (2016). https://doi.org/10.1007/s00276-016-1638-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-016-1638-5